Abstract
A subset of Down syndrome (DS) (trisomy 21) neonates is born with a unique erythromegakaryocytic myeloproliferative disorder that spontaneously resolves over the first few months of life (DS-transient abnormal myelopoiesis (DS-TAM); previously called DS-transient myeloproliferative disorder (DS-TMD) and DS-transient leukemia (DS-TL)). These infants are at high risk for developing subsequent acute megakaryoblastic leukemia (myeloid leukemia associated with Down syndrome (ML-DS); previously called DS-acute megakaryoblastic leukemia (DS-AMKL)). The molecular basis for DS-TAM/ML-DS remained mysterious for a long period of time. However, new genetic insights have been gained over the past 12 years that have begun to decipher the pathophysiology of this unusual disorder.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A subset of Down syndrome (DS) (trisomy 21) neonates is born with a unique erythromegakaryocytic myeloproliferative disorder that spontaneously resolves over the first few months of life (DS-transient abnormal myelopoiesis (DS-TAM); previously called DS-transient myeloproliferative disorder (DS-TMD) and DS-transient leukemia (DS-TL)). These infants are at high risk for developing subsequent acute megakaryoblastic leukemia (myeloid leukemia associated with Down syndrome (ML-DS); previously called DS-acute megakaryoblastic leukemia (DS-AMKL)). The molecular basis for DS-TAM/ML-DS remained mysterious for a long period of time. However, new genetic insights have been gained over the past 12 years that have begun to decipher the pathophysiology of this unusual disorder.
Epidemiology
The true incidence of DS-TAM has been difficult to discern since not all DS infants receive blood count analysis in the neonatal period and it is likely that many asymptomatic cases go unnoticed. In addition, clear diagnostic criteria for DS-TAM have not been established [1]. The World Health Organization (WHO) defines DS-TAM as “increased” peripheral blasts in neonates with DS but does not provide cutoff values for “increased” blasts [1, 2]. This is particularly problematic since nearly all DS neonates have blasts present in their peripheral blood [1]. Earlier retrospective studies estimated that ∼5–10 % of neonates with DS have clinically apparent DS-TAM [3, 4]. Pine et al. [5] performed PCR amplification and GATA1 gene Sanger sequencing from dried blood spots of 585 neonates with DS. They detected GATA1 mutations (a recently identified molecular hallmark of DS-TAM (see “Genetics” below)) in 22 (3.8 %) of the samples. They also noted a small increased incidence of GATA1 mutations for Hispanic compared to non-Hispanic babies. A recent prospective population-based study in the UK collected clinical data, peripheral blood counts, peripheral blood morphology, and GATA1 mutational analysis on 200 DS neonates [1]. Using the criteria of peripheral blood blasts of >10 % (by morphology) and a GATA1 mutation detected by Sanger sequencing/denaturing high-performance liquid chromatograph (DHPLC) analysis followed by next-generation sequencing, the authors found an incidence of 8.5 %. However, they also detected 18 cases of “silent” DS-TAM in which GATA1 mutations were detected by next-generation sequencing, but no hematologic abnormalities compared to non-GATA1-mutated DS neonates were evident. If one includes these silent DS-TAM cases, then the incidence in this study would be 17.5 %. Thus, the true incidence of DS-TAM depends on how it is defined and what methods are used to detect GATA1 mutations, but it is likely to be in the 4–18 % range. Additional large prospective population-based studies, as well as adoption of strict universal diagnostic criteria and sequencing methods, will be required to better define the true incidence of DS-TAM and whether it differs among ethnic groups.
About 20–25 % of patients with “clinically apparent” DS-TAM that survive the neonatal period subsequently develop ML-DS [6–9]. The mean age for presentation of ML-DS is about 20 months (range, ∼6–38 months) [6, 7]. ML-DS can also develop from silent DS-TAM [1]. Overall, children with DS have ∼500-fold increased risk of developing AMKL compared to the general population [4].
Clinical features
The clinical presentation of DS-TAM is highly variable. The majority of patients are asymptomatic. They are typically diagnosed incidentally after blood counts are obtained for other reasons and reveal abnormalities consistent with DS-TAM. A subset of infants presents with more severe manifestations, which can include fetal hydrops, liver failure, jaundice, coagulation defects, bleeding diasthesis, heart failure, pleural effusions, ascites, and/or respiratory failure. Symptomatic patients present either as stillborns or within the first 3 weeks of life [4]. Hepatomegaly is common. There is frequent megakaryocytic liver infiltration and hepatic fibrosis. Peripheral blood analysis reveals thrombocytopenia or thrombocytosis, elevated white blood cell with excess blasts, and frequently nucleated red blood cells. The full clinical picture may not be apparent until the second or third week of life. Remarkably, the peripheral blasts and other DS-TAM symptoms typically self-resolve by ~3–4 months of age (∼36–49 days from diagnosis) [6–9].
Trisomy 21 is typically constitutional in DS-TAM/AMKL. However, a substantial proportion of patients can have trisomy 21 mosaicism or harbor germ line translocations involving chromosome 21 [7]. Therefore, the absence of typical DS physical features does not exclude the diagnosis of DS-TAM.
Subsequent development of ML-DS is frequently preceded by a myelodysplastic syndrome (MDS)-like phase that can last for months [10]. This is characterized by worsening thrombocytopenia followed by anemia, ineffective erythropoiesis, and dysplastic changes of megakaryocytic and erythroid precursor cells on bone marrow exam.
Morphology
The peripheral blood smear of DS-TAM shows significant polychromasia, abundant nucleated red blood cells, and the presence of blasts (Fig. 1). Large-sized platelets are usually present and sometimes megakaryocyte fragments can be seen. The blasts in ML-DS exhibit typical French–American–British (FAB) M7 morphology [6]. They have a scant deeply basophilic cytoplasm with cytoplasmic blebbing, open chromatin, and prominent nucleoli (Figs. 2 and 3). Bi-nucleated erythroblasts are sometimes observed on bone marrow exam (Fig. 3).
Immunochemistry and expression profiling
The blasts in DS-TAM and ML-DS are positive for CD7, CD33, CD34, CD36, CD38, CD41, C42b, CD45, CD61, CD71, CD117, glycophorin A, TPO receptor (TPO-R), and IL-3 receptor alpha (IL-3Rα) [6, 7, 11] (Table 1). They are negative for CD10, the EPO receptor (EPO-R), and IL-6 receptor alpha (IL-6Rα), and variably positive for CD4 (Dim), CD13, and CD56.
Histochemical staining of DS-TAM blasts is negative for periodic acid–Schiff (PAS) and typically negative or weakly staining for myeloperoxidase and Sudan Black [6] (Table 2). Staining for acid phosphatase and esterase is usually positive.
ML-DS samples have distinct gene expression profiles compared to non-DS-AMKL samples [12]. Many of the key differences are not explained by the simple presence of an extra copy of chromosome 21 genes. These include over expression of c-Kit, c-MYC, and GATA2, known direct GATA1-repressed target genes involved in early progenitor cell survival and proliferation. These findings along with the unique clinical features of ML-DS versus non-DS-AMKL suggest that the two leukemias represent distinct molecular entities.
Genetics
GATA1 mutations in DS-TAM and ML-DS
A major advance in the understanding of the molecular basis of DS-TAM and ML-DS came in 2002 when Wechsler and colleagues [13] identified somatic mutations in the gene encoding the key megakaryocytic/erythroid transcription factor GATA1 in all cases of ML-DS examined. This was rapidly followed by a number of reports identifying similar mutations in DS-TAM as well as in additional cases of ML-DS [14–18]. With rare exceptions, these types of GATA1 mutations have never been reported in non-DS individuals. The GATA1 mutations are not detectable in remission samples from patients treated successfully for ML-DS, indicating their close linkage to the disease [13, 17]. It is now recognized that GATA1 mutations occur in all cases of DS-TAM and ML-DS and that it should be considered a molecular hallmark of the disorder [1].
A wide variety of GATA1 mutations have been described, including missense, splice-site, deletions, insertions, and duplications, but all target exon 2 (or rarely exon 3) [19]. All of the mutations lead to introduction of a premature stop codon. However, in these cases, translation initiates from an in-frame ATG located at codon 84. This produces a short GATA1 isoform (∼40 kDa), called “GATA1s” that lacks an N-terminal transactivation domain (Fig. 4). Low levels of GATA1s are produced normally during development but decrease relative to full-length GATA1 during terminal erythroid differentiation [20, 21]. In contrast to wild-type cells, GATA1s is produced exclusively in the mutant cells (the GATA1 gene is located on the X-chromosome).
Schematic drawing of protein product produced by DS-TAM/ML-DS-related GATA1 mutations. Full-length protein is shown in the top panel with the two carboxyl terminal zinc finger (zf) domains indicated. TA transcriptional activation domain. Asterisks represent mutations in exon 2 (or exon 3) that introduce premature stop codons. The alternate translational start site at codon 84 is indicated, and the protein product (“GATA1s”) generated from translation from this site is shown below. (Adapted from [13, 19])
GATA1 plays essential roles in terminal erythroid [22] and megakaryocyte maturation [23], and contributes to eosinophil [24] and mast cell development [25, 26]. GATA1 deficient murine megakaryocytes markedly hyperproliferate in liquid culture and fail to complete their full maturation [27]. Mice containing megakaryocyte-selective GATA1 deficiency are thrombocytopenic, have impaired megakaryocyte maturation, and develop bone marrow fibrosis as they age [23, 28]. Remarkably, knock-in mice that exclusively express GATA1s have developmental stage-specific effects on megakaryocyte proliferative control [29]. Yolk sac and early fetal liver derived megakaryocytes markedly hyperproliferate. However, late fetal liver and adult bone marrow megakaryocytes proliferate close to normal. These findings indicate that developmental stage-specific factors modulate the effect of GATA1s on megakaryocyte proliferative control and may be involved in the spontaneous remission of DS-TAM. The mechanism(s) underlying these stage-specific effects of GATA1s remain incompletely understood. Involvement of the insulin-like growth factor (IGF) and mTOR signaling pathways [30] and/or type I interferon signaling pathways may be involved [31].
Studies of dried blood spots from neonates who developed DS-TAM or ML-DS [32] as well as prospective studies of infants with DS-TAM/ML-DS [1, 33] have identified the simultaneous presence of multiple distinct GATA1 mutant clones. With rare exceptions, GATA1s-generating mutations have not been detected in large numbers of healthy non-DS newborns or non-DS-AMKL. Collectively, these findings suggest that a trisomy 21 genetic background creates a strong selective pressure for clones containing GATA1s-generating mutations during in utero development. However, the molecular basis for this remains unknown.
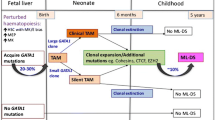
GATA1s-generating mutations have been detected as early as 21 weeks of gestation [34]. In the cases that have been analyzed, GATA1 nucleotide changes in the ML-DS cells are identical to those found in the preceding DS-TAM cells (or a subclone) from the same patient [1, 17, 33]. These findings support a clonal evolution model, in which the GATA1s mutations are early in utero initiating events that predispose to later development of ML-DS [14, 16] (Fig. 5).
Clonal evolution model of ML-DS. Mutant GATA1s progenitor cell clones emerge during early fetal hematopoiesis under strong selective pressure in a trisomy 21 genetic background. Multiple clones may emerge. Megakaryocyte–erythroid progenitor cells from these clones markedly hyperproliferate under the influence of GATA1s. These clones are either extinguished or reverse their hyperproliferative phenotype during the transition to postnatal hematopoiesis, reflecting the spontaneous remission of DS-TAM. In some cases, additional genetic events occur in a subclone(s) that develops into ML-DS. (Adapted from [61])
The role of trisomy 21
GATA1s-generating mutations by themselves appear to be insufficient to cause a transient myeloproliferative disorder or megakaryoblastic leukemia in humans. This is based on the observation of a family containing a germ line GATA1 exon 2 (GATA1s-generating) mutation in the absence of trisomy 21 [35]. Male members from several generations have macrocytic anemia and neutropenia, but normal platelet counts. None of the affected individuals have had clinically evident neonatal transient myeloproliferative disorder or have developed leukemia.
A number of recent studies have documented that trisomy 21, in the absence of GATA1 mutations, leads to perturbed fetal and postnatal hematopoiesis [36–39]. This includes significantly increased number of hematopoietic stem cells (HSCs) and megakaryocyte–erythroid progenitor (MEPs) in the trisomy fetuses compared to chromosome 21 disomy fetuses. Moreover, these cells have significantly enhanced cell-autonomous clonogenic and proliferative potential. There is also impairment of B-cell development. In vitro differentiation of human-induced pluripotent stem cells (iPS) from trisomy 21 individuals largely recapitulates these hematologic abnormalities compared to isogenic disomy 21 control cells [40]. A mouse model of DS, the Ts65Dn mouse, develops a highly penetrant myeloproliferative disorder with significant thrombocytosis, megakaryocyte hyperplasia, megakaryocyte dysmorphology, and bone marrow fibrosis, in the absence of GATA1 mutations [41]. Thus, it seems likely that the underlying genetic background in trisomy 21 contributes to the molecular pathogenesis of DS-TAM and ML-DS beyond the initial selection for GATA1s-generating mutant clones.
A number of candidate genetic factors on chromosome 21 have been investigated for their role in DS-TAM/AMKL. Supporting data has been reported for ERG [42, 43], ETS2 [44], and the microRNA miR-125b [45]. Interestingly, RUNX1, which is also located on chromosome 21 and encodes a key megakaryocytic transcription factor that physically associates with GATA1 and is frequently mutated in human leukemia, does not appear to be involved [12, 41].
Secondary mutations leading to ML-DS
While the combination of GATA1s mutations and trisomy 21 is likely sufficient to produce DS-TAM, they appear to be insufficient to generate ML-DS. Current data suggests that the acquisition of additional genetic perturbations in a preexisting DS-TAM (GATA1 mutant containing) clone is required for development of full blown ML-DS. Acquired cytogenetic aberrations and copy number alterations are common in ML-DS and include trisomy 8, loss of chromosome 5/7 material, gain of chromosome 21, dup(1q), del(16q), and other rarer abnormalities [46, 47]. Somatic point mutations in JAK1 [48], JAK2 [48], JAK3 [48–50], TP53 [51], FLT3, and MPL have been described in small subsets of cases. A recent study of 41 DS-TAM, 49-ML-DS, and 19 non-DS-AMKL cases using genomic profiling, whole exome, and/or whole genome sequencing identified a high proportion of cases with somatic mutations in genes encoding components of the cohesin complex (53 %) or the cohesin-binding insulator factor CTCF (20 %) in ML-DS [33]. Mutations in these genes were not found in any of the DS-TAM samples and only 11 % of non-DS-AMKL. Additional somatic mutations in genes encoding epigenetic regulators (including EZH2 and KANKSL1) and signaling pathway factors (including RAS pathway genes and SH2B3 (LNK)) were also seen in the ML-DS samples. The molecular significance of these findings remains unknown at this time but strongly suggests that additional genetic events, particularly in the cohesin complex, collaborate with GATA1s and trisomy 21 to generate ML-DS.
Prognosis
Most children (∼75–90 %) spontaneously clear their peripheral blasts and normalize their blood counts over the first few months of life [6]. However, despite the transient nature of DS-TAM and its mild presentation in many patients, ∼11–20 % of clinically apparent DS-TAM cases result in perinatal death, usually due to fulminant liver failure, disseminated intravascular coagulation, and/or multiple effusions [6–8, 52]. Treatment with low dose cytarabine (Ara-C; 0.5 to 1.5 mg/kg/day for 3 to 12 days) is effective in severe neonatal presentations [7].
For infants that develop ML-DS, the overall prognosis is considerably better than patients with non-DS-AMKL. Treatments with current chemotherapy protocols (which include Ara-C) produce long-term event-free survival rates of around 80 % [47, 53–55]. Up-front bone marrow transplantation is typically not indicated given the excellent cure rates with conventional chemotherapy. The reason for the strong response to therapy in ML-DS versus non-ML-DS is not known. It has been proposed that low levels the cytarabine metabolizing enzyme cytidine deaminase, whose gene is transcriptionally activated by GATA1, in ML-DS may contribute [56]. Despite the excellent response to therapy, toxic deaths remain a problem and occur in ∼7 % of treated cases [47].
Potential predictive factors
During the neonatal DS-TAM phase of the disease, risk of early death is associated with high white blood cell count (WBC) (>100 × 109/L), ascites, preterm delivery, bleeding diasthesis, direct hyperbilirubinemia, increased liver enzymes, and failure to clear peripheral blasts [6–8]. For ML-DS, a normal karyotype (except trisomy 21) is associated with increased incidence of relapse (∼21 %) compared to cases with aberrant karyotypes (∼9 %) [47]. Other independent predictors of poor event-free survival in ML-DS include WBC ≥20 × 109/L and age >3–4 years at diagnosis [47, 55].
Summary and perspectives
In summary, DS-TAM and ML-DS are characterized at the molecular pathophysiologic level by the constellation of GATA1s-producing mutations, trisomy 21, fetal origin, and acquisition of additional genetic events in the progression to ML-DS. Fortunately, with the exception of certain cases of severe neonatal presentations, the overall prognosis and cure rate for infants with DS-TAM/ML-DS is excellent. Additional investigation will be necessary to: (1) fully understand the strong selective pressure for GATA1s mutant containing clones during fetal hematopoiesis in trisomy 21 individuals; (2) identify the specific genetic component(s) on chromosome 21 that contributes to the disorder; (3) fully uncover the mechanisms underlying the spontaneous remission of DS-TAM; and (4) elucidate the key genetic steps that lead to progression to ML-DS.
Clinical efforts are underway to develop less intensive therapies in order to reduce the toxic death rates and morbidity associated with ML-DS treatment. Important clinical questions yet to be addressed include (1) whether mutational screening for GATA1s mutations in all DS neonates is clinically useful; (2) whether quantitative measurement of GATA1s mutant clone levels is clinically useful in following patients with known GATA1s clones; and, (3) if tumor bulk reduction during the DS-TAM phase of the disease (such as with low-dose AraC) would decrease the frequency and/or severity of subsequent ML-DS development. Additional recent and detailed reviews on DS-TAM/AMKL can be found in ref. [4, 57–60].
References
Roberts I, Alford K, Hall G, Juban G, Richmond H, Norton A, Vallance G, Perkins K, Marchi E, McGowan S, Roy A, Cowan G, Anthony M, Gupta A et al (2013) GATA1-mutant clones are frequent and often unsuspected in babies with Down syndrome: identification of a population at risk of leukemia. Blood 122:3908–3917
Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, Harris NL, Le Beau MM, Hellstrom-Lindberg E, Tefferi A, Bloomfield CD (2009) The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood 114:937–951
Zipursky A (2003) Transient leukaemia—a benign form of leukaemia in newborn infants with trisomy 21. Br J Haematol 120:930–938
Lange B (2000) The management of neoplastic disorders of haematopoiesis in children with Down’s syndrome. Br J Haematol 110:512–524
Pine SR, Guo Q, Yin C, Jayabose S, Druschel CM, Sandoval C (2007) Incidence and clinical implications of GATA1 mutations in newborns with Down syndrome. Blood 110:2128–2131
Massey GV, Zipursky A, Chang MN, Doyle JJ, Nasim S, Taub JW, Ravindranath Y, Dahl G, Weinstein HJ (2006) A prospective study of the natural history of transient leukemia (TL) in neonates with Down syndrome (DS): Children’s Oncology Group (COG) study POG-9481. Blood 107:4606–4613
Klusmann JH, Creutzig U, Zimmermann M, Dworzak M, Jorch N, Langebrake C, Pekrun A, Macakova-Reinhardt K, Reinhardt D (2008) Treatment and prognostic impact of transient leukemia in neonates with Down syndrome. Blood 111:2991–2998
Muramatsu H, Kato K, Watanabe N, Matsumoto K, Nakamura T, Horikoshi Y, Mimaya J, Suzuki C, Hayakawa M, Kojima S (2008) Risk factors for early death in neonates with Down syndrome and transient leukaemia. Br J Haematol 142:610–615
Gamis AS, Alonzo TA, Gerbing RB, Hilden JM, Sorrell AD, Sharma M, Loew TW, Arceci RJ, Barnard D, Doyle J, Massey G, Perentesis J, Ravindranath Y, Taub J et al (2011) Natural history of transient myeloproliferative disorder clinically diagnosed in Down syndrome neonates: a report from the Children’s Oncology Group Study A2971. Blood 118:6752–6759, quiz 6996
Zipursky A, Poon A, Doyle J (1992) Leukemia in Down syndrome: a review. Pediatr Hematol Oncol 9:139–149
Langebrake C, Creutzig U, Reinhardt D (2005) Immunophenotype of Down syndrome acute myeloid leukemia and transient myeloproliferative disease differs significantly from other diseases with morphologically identical or similar blasts. Klin Padiatr 217:126–134
Bourquin JP, Subramanian A, Langebrake C, Reinhardt D, Bernard O, Ballerini P, Baruchel A, Cave H, Dastugue N, Hasle H, Kaspers GL, Lessard M, Michaux L, Vyas P et al (2006) Identification of distinct molecular phenotypes in acute megakaryoblastic leukemia by gene expression profiling. Proc Natl Acad Sci U S A 103:3339–3344
Wechsler J, Greene M, McDevitt MA, Anastasi J, Karp JE, Le Beau MM, Crispino JD (2002) Acquired mutations in GATA1 in the megakaryoblastic leukemia of Down syndrome. Nat Genet 32:148–152
Mundschau G, Gurbuxani S, Gamis AS, Greene ME, Arceci RJ, Crispino JD (2003) Mutagenesis of GATA1 is an initiating event in Down syndrome leukemogenesis. Blood 101:4298–4300
Greene ME, Mundschau G, Wechsler J, McDevitt M, Gamis A, Karp J, Gurbuxani S, Arceci R, Crispino JD (2003) Mutations in GATA1 in both transient myeloproliferative disorder and acute megakaryoblastic leukemia of Down syndrome. Blood Cells Mol Dis 31:351–356
Rainis L, Bercovich D, Strehl S, Teigler-Schlegel A, Stark B, Trka J, Amariglio N, Biondi A, Muler I, Rechavi G, Kempski H, Haas OA, Izraeli S (2003) Mutations in exon 2 of GATA1 are early events in megakaryocytic malignancies associated with trisomy 21. Blood 102:981–986
Hitzler JK, Cheung J, Li Y, Scherer SW, Zipursky A (2003) GATA1 mutations in transient leukemia and acute megakaryoblastic leukemia of Down syndrome. Blood 101:4301–4304
Xu G, Nagano M, Kanezaki R, Toki T, Hayashi Y, Taketani T, Taki T, Mitui T, Koike K, Kato K, Imaizumi M, Sekine I, Ikeda Y, Hanada R et al (2003) Frequent mutations in the GATA-1 gene in the transient myeloproliferative disorder of Down syndrome. Blood 102:2960–2968
Alford KA, Reinhardt K, Garnett C, Norton A, Bohmer K, von Neuhoff C, Kolenova A, Marchi E, Klusmann JH, Roberts I, Hasle H, Reinhardt D, Vyas P (2011) Analysis of GATA1 mutations in Down syndrome transient myeloproliferative disorder and myeloid leukemia. Blood 118:2222–2238
Calligaris R, Bottardi S, Cogoi S, Apezteguia I, Santoro C (1995) Alternative translation initiation site usage results in two functionally distinct forms of the GATA-1 transcription factor. Proc Natl Acad Sci U S A 92:11598–11602
Ludwig LS, Gazda HT, Eng JC, Eichhorn SW, Thiru P, Ghazvinian R, George TI, Gotlib JR, Beggs AH, Sieff CA, Lodish HF, Lander ES, Sankaran VG (2014) Altered translation of GATA1 in Diamond-Blackfan anemia. Nat Med 20:748–753
Fujiwara Y, Browne CP, Cunniff K, Goff SC, Orkin SH (1996) Arrested development of embryonic red cell precursors in mouse embryos lacking transcription factor GATA-1. Proc Natl Acad Sci U S A 93:12355–12358
Shivdasani RA, Fujiwara Y, McDevitt MA, Orkin SH (1997) A lineage-selective knockout establishes the critical role of transcription factor GATA-1 in megakaryocyte growth and platelet development. Embo J 16:3965–3973
Yu C, Cantor AB, Yang H, Browne C, Wells RA, Fujiwara Y, Orkin SH (2002) Targeted deletion of a high-affinity GATA-binding site in the GATA-1 promoter leads to selective loss of the eosinophil lineage in vivo. J Exp Med 195:1387–1395
Zon LI, Gurish MF, Stevens RL, Mather C, Reynolds DS, Austen KF, Orkin SH (1991) GATA-binding transcription factors in mast cells regulate the promoter of the mast cell carboxypeptidase A gene. J Biol Chem 266:22948–22953
Migliaccio AR, Rana RA, Sanchez M, Lorenzini R, Centurione L, Bianchi L, Vannucchi AM, Migliaccio G, Orkin SH (2003) GATA-1 as a regulator of mast cell differentiation revealed by the phenotype of the GATA-1low mouse mutant. J Exp Med 197:281–296
Vyas P, Ault K, Jackson CW, Orkin SH, Shivdasani RA (1999) Consequences of GATA-1 deficiency in megakaryocytes and platelets. Blood 93:2867–2875
Vannucchi AM, Bianchi L, Cellai C, Paoletti F, Rana RA, Lorenzini R, Migliaccio G, Migliaccio AR (2002) Development of myelofibrosis in mice genetically impaired for GATA-1 expression (GATA-1(low) mice). Blood 100:1123–1132
Li Z, Godinho FJ, Klusmann JH, Garriga-Canut M, Yu C, Orkin SH (2005) Developmental stage-selective effect of somatically mutated leukemogenic transcription factor GATA1. Nat Genet 37:613–619
Klusmann JH, Godinho FJ, Heitmann K, Maroz A, Koch ML, Reinhardt D, Orkin SH, Li Z (2010) Developmental stage-specific interplay of GATA1 and IGF signaling in fetal megakaryopoiesis and leukemogenesis. Genes Dev 24:1659–1672
Woo AJ, Wieland K, Huang H, Akie TE, Piers T, Kim J, Cantor AB (2013) Developmental differences in IFN signaling affect GATA1s-induced megakaryocyte hyperproliferation. J Clin Inv 123:3292–3304
Ahmed M, Sternberg A, Hall G, Thomas A, Smith O, O’Marcaigh A, Wynn R, Stevens R, Addison M, King D, Stewart B, Gibson B, Roberts I, Vyas P (2004) Natural history of GATA1 mutations in Down syndrome. Blood 103:2480–2489
Yoshida K, Toki T, Okuno Y, Kanezaki R, Shiraishi Y, Sato-Otsubo A, Sanada M, Park MJ, Terui K, Suzuki H, Kon A, Nagata Y, Sato Y, Wang R et al (2013) The landscape of somatic mutations in Down syndrome-related myeloid disorders. Nat Genet 45:1293–1299
Taub JW, Mundschau G, Ge Y, Poulik JM, Qureshi F, Jensen T, James SJ, Matherly LH, Wechsler J, Crispino JD (2004) Prenatal origin of GATA1 mutations may be an initiating step in the development of megakaryocytic leukemia in Down syndrome. Blood 104:1588–1589
Hollanda LM, Lima CS, Cunha AF, Albuquerque DM, Vassallo J, Ozelo MC, Joazeiro PP, Saad ST, Costa FF (2006) An inherited mutation leading to production of only the short isoform of GATA-1 is associated with impaired erythropoiesis. Nat Genet 38:807–812
Chou ST, Opalinska JB, Yao Y, Fernandes MA, Kalota A, Brooks JS, Choi JK, Gewirtz AM, Danet-Desnoyers GA, Nemiroff RL, Weiss MJ (2008) Trisomy 21 enhances human fetal erythro-megakaryocytic development. Blood 112:4503–4506
Roy A, Cowan G, Mead AJ, Filippi S, Bohn G, Chaidos A, Tunstall O, Chan JK, Choolani M, Bennett P, Kumar S, Atkinson D, Wyatt-Ashmead J, Hu M et al (2012) Perturbation of fetal liver hematopoietic stem and progenitor cell development by trisomy 21. Proc Natl Acad Sci U S A 109:17579–17584
Tunstall-Pedoe O, Roy A, Karadimitris A, de la Fuente J, Fisk NM, Bennett P, Norton A, Vyas P, Roberts I (2008) Abnormalities in the myeloid progenitor compartment in Down syndrome fetal liver precede acquisition of GATA1 mutations. Blood 112:4507–4511
Roberts I, O’Connor D, Roy A, Cowan G, Vyas P (2013) The impact of trisomy 21 on foetal haematopoiesis. Blood Cells Mol Dis 51:277–281
Maclean GA, Menne TF, Guo G, Sanchez DJ, Park IH, Daley GQ, Orkin SH (2012) Altered hematopoiesis in trisomy 21 as revealed through in vitro differentiation of isogenic human pluripotent cells. Proc Natl Acad Sci U S A 109:17567–17572
Kirsammer G, Jilani S, Liu H, Davis E, Gurbuxani S, Le Beau MM, Crispino JD (2008) Highly penetrant myeloproliferative disease in the Ts65Dn mouse model of Down syndrome. Blood 111:767–775
Stankiewicz MJ, Crispino JD (2013) AKT collaborates with ERG and Gata1s to dysregulate megakaryopoiesis and promote AMKL. Leukemia 27:1339–1347
Ng AP, Hyland CD, Metcalf D, Carmichael CL, Loughran SJ, Di Rago L, Kile BT, Alexander WS (2010) Trisomy of Erg is required for myeloproliferation in a mouse model of Down syndrome. Blood 115:3966–3969
Ge Y, LaFiura KM, Dombkowski AA, Chen Q, Payton SG, Buck SA, Salagrama S, Diakiw AE, Matherly LH, Taub JW (2008) The role of the proto-oncogene ETS2 in acute megakaryocytic leukemia biology and therapy. Leukemia 22:521–529
Klusmann JH, Li Z, Bohmer K, Maroz A, Koch ML, Emmrich S, Godinho FJ, Orkin SH, Reinhardt D (2010) miR-125b-2 is a potential oncomiR on human chromosome 21 in megakaryoblastic leukemia. Genes Dev 24:478–490
Blink M, van den Heuvel-Eibrink MM, Aalbers AM, Balgobind BV, Hollink IH, Meijerink JP, van der Velden VH, Beverloo BH, de Haas V, Hasle H, Reinhardt D, Klusmann JH, Pieters R, Calado RT et al (2012) High frequency of copy number alterations in myeloid leukaemia of Down syndrome. Br J Haematol 158:800–803
Blink M, Zimmermann M, von Neuhoff C, Reinhardt D, de Haas V, Hasle H, O’Brien MM, Stark B, Tandonnet J, Pession A, Tousovska K, Cheuk DK, Kudo K, Taga T et al (2014) Normal karyotype is a poor prognostic factor in myeloid leukemia of Down syndrome: a retrospective, international study. Haematologica 99:299–307
Blink M, Buitenkamp TD, van den Heuvel-Eibrink MM, Danen-van Oorschot AA, de Haas V, Reinhardt D, Klusmann JH, Zimmermann M, Devidas M, Carroll AJ, Basso G, Pession A, Hasle H, Pieters R et al (2011) Frequency and prognostic implications of JAK 1-3 aberrations in Down syndrome acute lymphoblastic and myeloid leukemia. Leukemia 25:1365–1368
Walters DK, Mercher T, Gu TL, O’Hare T, Tyner JW, Loriaux M, Goss VL, Lee KA, Eide CA, Wong MJ, Stoffregen EP, McGreevey L, Nardone J, Moore SA et al (2006) Activating alleles of JAK3 in acute megakaryoblastic leukemia. Cancer Cell 10:65–75
Malinge S, Ragu C, Della-Valle V, Pisani D, Constantinescu SN, Perez C, Villeval JL, Reinhardt D, Landman-Parker J, Michaux L, Dastugue N, Baruchel A, Vainchenker W, Bourquin JP et al (2008) Activating mutations in human acute megakaryoblastic leukemia. Blood 112:4220–4226
Malkin D, Brown EJ, Zipursky A (2000) The role of p53 in megakaryocyte differentiation and the megakaryocytic leukemias of Down syndrome. Cancer Genet Cytogenet 116:1–5
Homans AC, Verissimo AM, Vlacha V (1993) Transient abnormal myelopoiesis of infancy associated with trisomy 21. Amn J Pediatr Hematol Oncol 15:392–399
Creutzig U, Reinhardt D, Diekamp S, Dworzak M, Stary J, Zimmermann M (2005) AML patients with Down syndrome have a high cure rate with AML-BFM therapy with reduced dose intensity. Leukemia 19:1355–1360
Rao A, Hills RK, Stiller C, Gibson BE, de Graaf SS, Hann IM, O’Marcaigh A, Wheatley K, Webb DK (2006) Treatment for myeloid leukaemia of Down syndrome: population-based experience in the UK and results from the Medical Research Council AML 10 and AML 12 trials. Br J Haematol 132:576–583
Gamis AS, Woods WG, Alonzo TA, Buxton A, Lange B, Barnard DR, Gold S, Smith FO (2003) Increased age at diagnosis has a significantly negative effect on outcome in children with Down syndrome and acute myeloid leukemia: a report from the Children’s Cancer Group Study 2891. J Clin Oncol : Off J Am Soc Clin Oncol 21:3415–3422
Ge Y, Stout ML, Tatman DA, Jensen TL, Buck S, Thomas RL, Ravindranath Y, Matherly LH, Taub JW (2005) GATA1, cytidine deaminase, and the high cure rate of Down syndrome children with acute megakaryocytic leukemia. J Natl Cancer Inst 97:226–231
Malinge S, Izraeli S, Crispino JD (2009) Insights into the manifestations, outcomes, and mechanisms of leukemogenesis in Down syndrome. Blood 113:2619–2628
Gurbuxani S, Vyas P, Crispino JD (2004) Recent insights into the mechanisms of myeloid leukemogenesis in Down syndrome. Blood 103:399–406
Gamis AS, Smith FO (2012) Transient myeloproliferative disorder in children with Down syndrome: clarity to this enigmatic disorder. Br J Haematol 159:277–287
Hitzler JK, Zipursky A (2005) Origins of leukaemia in children with Down syndrome. Nature reviews. Cancer 5:11–20
Cantor AB (2005) GATA transcription factors in hematologic disease. Int J Hematol 81:378–384
Acknowledgments
This work was supported by the National Institutes of Health grant P01 HL32262.
Conflict of interest
The author declares no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cantor, A.B. Myeloid proliferations associated with Down syndrome. J Hematopathol 8, 169–176 (2015). https://doi.org/10.1007/s12308-014-0225-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12308-014-0225-0