Abstract
Lung cancer is one of the most commonly reported cancers, and is known to be associated with a poor prognosis. The function of tumour-associated macrophages (TAMs) in lung cancer patients is multifaceted and the literature shows conflicting roles. (I) To analyze the Th1 and Th2 cytokine levels that contribute to the differentiation of M1 and M2 macrophage populations in the serum of patients with NSCLC versus non-cancer controls; and (II) To characterize the M1 and M2 macrophage populations within TAMs in different subtypes of NSCLC compared to non-tumour tissue. The Th1 and Th2 cytokine levels were analyzed in serum using the Bio-Plex assay. In addition, TAMs subsets from non-tumour and tumour tissues were analyzed using immunohistochemistry (IHC). The level of IL-1β, IL-4, IL-6 and IL-8 was found to be increased in the serum of patients with large cell carcinoma but not in other NSCLC subtypes compared to non-cancer controls. In addition, the expression of CD68 and M2 marker CD163 was found to be increased (P ≤ 0.0001) in all NSCLC subtypes compared to non-tumour tissues. In contrast, the expression of iNOS (M1 marker) was decreased in the tumour tissue of patients with adenocarcinoma (P ≤ 0.01) and squamous carcinoma (P ≤ 0.05) but not in large cell carcinoma compared to non-tumour tissue. The results of this study indicate that NSCLC might have the ability to alter phenotype within the lung tumour areas in the local environment (TAMs) but not in the bloodstream in the systemic environment (serum) except for large cell carcinoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The progression of lung cancer is a complex and multistep process where several mechanisms such as transformation, hypoxia, invasion, migration and metastasis are known to be the main hallmarks [1, 2]. Improving our understanding of these mechanisms is a fundamental approach to control the aggressiveness of lung cancer and eventually overcome the obstacles to successful lung cancer treatment. Macrophages within the tumour microenvironment termed tumour associated macrophages (TAMs) are known to be crucial cells in lung cancer as they are in close proximity to tumour cells compared to other stromal cells [1]. They are known to be responsible for releasing several growth factors, cytokines, chemokines, inflammatory mediators and other molecules [3]. Many of these factors are well known and have been associated with tumour growth, poor prognosis and metastasis including VEGF, PDGF and IL-10 [3]. In addition, the presence of high numbers of TAMs has been connected with the invasion, angiogenesis, hypoxia and early occurrence of metastasis in different tumour types including lung cancer [2, 4–6].
TAMs are a type of cell that belong to the monocyte-macrophage lineage and like other macrophages have been described as a heterogeneous population [6]. The activation of TAMs in response to cytokines, pro- (e.g., IFN-γ, TNF and IL-12) or anti-inflammatory (e.g., IL-4 and IL-10) molecules and microbial agents such as LPS (lipopolysaccharide) are well known [2, 7, 3]. There are two main phenotypes of macrophages: M1 and M2. The M1 phenotype is activated by IFN-γ, LPS and TNF-α [8, 9, 3, 7]. The M1 macrophage phenotype has been connected to the expression of IL-1, IL-12, TNF-α, and inducible nitric oxide synthase (iNOS) and also has been correlated with extended survival time in patients with NSCLC [3, 9]. On the other hand, the M2 macrophages have been correlated with tumour initiation and progression and have also been described as inhibitors of inflammation [8, 9]. The M2 macrophages produce anti-inflammatory cytokines such as IL-10 and reduce the expression of iNOS, inhibit antigen presentation and T cell proliferation [10, 7, 11]. The M2 macrophages have been found to encourage the growth of various tumour cells in vitro [12] and to increase tumour cell survival [13]. They also play a vital role in promoting angiogenesis via VEGF, which is a prominent mediator of angiogenesis [9, 14].
There are many examples of macrophage-polarizing events during tumour progression, including the secretion of tumour-derived mediators and hypoxic tissue damage, as well as influences from other immune cells and stromal components [9, 15–17]. The exact characterization of macrophage populations within M1 and M2 subtypes can perhaps be overgeneralized, as macrophages have been described as highly plastic cells that can demonstrate a variety of phenotypes [15]. However, the markers of M1 and M2 phenotype can still be used to categorize the phenotype and function of macrophages [15]. A small number of macrophages express both M1 and M2 markers and this leads to the suggestion that a mixed phenotype occurs [17]. A study previously verified that the M1 and M2 markers differentiate macrophage populations, although about 5 % of the cells stained for both M1 and M2 markers using immunohistochemistry (IHC) [9].
In this study, the Th1 and Th2 cytokine levels were analyzed in the serum of patients with NSCLC versus non-cancer controls. Also lung tissues from patients with NSCLC were used to determine the possible phenotypic changes in TAM phenotype and these were compared to non-tumour tissue from the same patient. TAM phenotype was determined using immunohistochemistry (IHC). The TAM phenotype was determined using CD68 (macrophage marker), iNOS (M1) and CD163 (M2) antibodies, respectively. This study aims to provide a better understanding of the effect of NSCLC on TAM phenotype and is an important aspect of macrophage investigation since TAMs are the macrophages most likely to come in direct contact with lung tumour cells.
Materials and Methods
Lung Specimen Collection and Sectioning
Staging was applied in this study using the new TNM (tumour, node, metastases) staging system (seventh edition) for lung cancer [18]. Inclusion criteria of lung cancer (tissue and serum samples) were proven by histopathological examination of lung biopsy, without diseases of immune system or previous chemotherapy, radiotherapy or immunomodulating treatment. All tissue samples were purchased from the Victorian Cancer Bio-Bank (Victoria, Australia). Human Ethics approval was received from RMIT University Human Research Ethics Committee ASEHAPP 15–13. All lung specimens were fixed with 4 % formaldehyde, followed by dehydration through graded alcohols, paraffin embedding and preparation of 4-μm sections. For H&E staining, sections were rehydrated, stained with haematoxylin for 2 min and rinsed in running tap water for 2 min. The sections were then blued in Scott’s tap water for 1 min, rinsed with tap water for 2 min, stained with eosin for 2 min, dehydrated in alcohols cleared in xylene and coverslipped using DePeX mounting media.
Immunohistochemical Staining
Lung sections were heated at 60 °C for 1 h, hydrated and rinsed in tap water for 2 min. The sections were then boiled in 10 mM citrate buffer, pH = 6 for 10 min for antigen retrieval followed by cooling at room temperature for 20 min. The sections were then incubated with peroxidase incubator 0.3 % H2O2 for 15 min at room temperature and then protein blocker (2 % goat serum, 1 % BSA, 0.1 % cold fish gelatin, 0.1 % Triton X-100, 0.05 % Tween 20 and 0.05 % sodium azide) to block nonspecific staining for 30 min at room temperature. Primary antibodies to CD68 (monoclonoal mouse anti-human CD68 Clone KP1, ready-to-use) (Dako, Carpinteria, CA, USA), NCL-CD163 (1:100; Novocastra™ liquid mouse monoclonal antibody CD163 clone 10D6) (Leica Biosystems, UK) and iNOS (inducible nitric oxide synthase) (1:200; rabbit polyclonal anti-human iNOS, Abcam, UK) were incubated for 1 h at room temperature and then washed three times using washing buffer for 5 min. The CD68 sections were incubated with EnVision™+ FLEX+ mouse linker for 15 min and then washed three times using washing buffer for 5 min. All sections were then incubated with secondary antibody Dako EnVision™+ Dual Link system-HRP (Dako, Glostrup, Denmark). The sections were incubated at room temperature for 30 min and washed with washing buffer three times for 5 min. The sections were then incubated with DAB solution (Dako, Glostrup, Denmark) for 1–3 min and washed in the washing buffer three times for 5 min. The sections counterstained with haematoxylin while being observed under a microscope, dehydrated, cleared and coverslipped using DePeX mounting media.
Quantitative Analysis of Immunohistochemical Staining
All slides were scanned at an absolute magnification of 20× using the Aperio Scanscope XT pathology digital imaging systems at Austin Health, Heidelberg, Victoria, Australia (Aperio Technologies, USA). TAMs were analysed based on CD68+ expression and then further analysed for expression of defined M1 and M2 markers. The background illumination levels were calibrated using a prescan procedure. The acquired digital images representing whole tissue sections were evaluated for image quality. All acquired images were labeled, placed in dedicated project folders, and stored in a designated external hard drive. The slides were viewed and analyzed using ImageScope analysis software (version 12; Aperio Technologies, USA). The colocalisation algorithm (version 11; Aperio Technologies, USA) was applied to quantify IHC staining. The algorithm calculated the percentage area of positive staining based on the deconvolution method to separate the stains and classify each pixel according to the number of stains present. The threshold for each stain was specified and the algorithm reports the percentage of area for which each stain combination is detected: 1, 2, 3, 1 + 2, 1 + 3, 2 + 3, 1 + 2 + 3 or none.
Cytokine and Chemokine Measurement by Bio-Plex Multiplex System
Serum samples were purchased from the Victorian Cancer Bio-Bank (Victoria, Australia) to analyze the Th1 and Th2 cytokines profile using the Bio-Plex, MAGPIX-Luminex assay. The Bio-Plex assay kit from Bio-Rad was applied to detect the Th1/ Th2 cytokines including IL-1β, IL-4, IL-6, IL-8, IL-10, IL-12 (p70), IP-10, TNF-α, MCP-1 and VEGF in serum samples of patients with NSCLC (adenocarcinoma, squamous cell lung carcinoma and large cell lung carcinoma) and non-cancer controls. The serum samples were diluted fourfold (1:4) by adding 12.5 μl serum and 37.5 μl sample diluent. The method was performed by the same operator according to manufacturers’ instructions. The kit supplied standards that were reconstituted and diluted at seven serial concentrations following manufacturer’s instructions (standard curves). Standards included all recombinant cytokines tested and were considered as positive controls for the procedure. Bead fluorescence readings were analyzed using the Bio-Plex MAGPIX multiplex reader (Bio-Rad, USA) and cytokine levels were determined using the Bio-Plex Manager Software (Bio-Rad, USA) within RMIT University, Bundoora, Melbourne.
Statistical Analysis
The results of TAMs and cytokines are shown as % of positive area or mean values ± standard error (SEM) as error bars, respectively. Experiments were performed in triplicate. The statistical analysis was performed using GraphPad Prism-6. One-way ANOVA multiple comparison test (as a post-test analysis) was performed with the Tukey test (multiple comparison test comparing every group with every other group).
Results
Expression of CD68, iNOS (M1 Marker) and CD163 (M2 Marker) in NSCLC Tumour Tissue Compared to Non-Tumour Tissue
In this study, immunohistochemistry (IHC) was used to determine the possible phenotypic changes in TAM phenotype of NSCLC compared to non-tumour tissue from the same patient. The TAM phenotype was determined using CD68 (macrophage marker), iNOS (M1) and CD163 (M2) antibodies, respectively.
The demographic details of tissue samples are presented in Table 1. CD68 staining was used to evaluate TAMs in the NSCLC lung tissue compared to non-cancer tissue. The presence of TAMs (CD68 positive staining) was significantly higher in all NSCLC subtypes (adenocarcinoma P ≤ 0.0001, squamous cell lung carcinoma P ≤ 0.0001 and large cell lung carcinoma P ≤ 0.0001) compared to non-tumour tissue. This result was expected as previous studies suggested that more TAMs are recruited to the tumour area and are associated with pulmonary disorders such as lung cancer [19, 20]. All NSCLC subtypes (adenocarcinoma P ≤ 0.0001, squamous cell lung carcinoma P ≤ 0.0001 and large cell lung carcinoma P ≤ 0.0001) were found to have significantly higher CD68 and CD163-positive cells when compared to non-tumour tissues (Figs. 1, 2, and 3). The % area of positive staining for CD163 was greatly increased in all NSCLC tissues (adenocarcinoma P ≤ 0.0001, squamous cell lung carcinoma P ≤ 0.0001 and large cell lung carcinoma P ≤ 0.0001) compared to non-tumour tissues (Fig. 4).
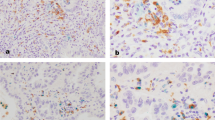
Expression of CD68, iNOS and CD163 in tumour (adenocarcinoma) and non-tumour tissue by immunohistochemistry. a and b CD68 (macrophage marker), c and d iNOS (M1 macrophage marker) and e and f CD163 (M2 macrophage marker) were used to stain tumour (adenocarcinoma) and non-tumour tissue. Black arrows indicate the expression of CD68/iNOS/CD163, ×20. The slides were observed and all photos were taken using Leica DMD108 (Leica Microsystems, Germany)
Expression of CD68, iNOS and CD163 in tumour (squamous cell lung carcinoma) and non-tumour tissue by immunohistochemistry. a and b CD68 (macrophage marker), c and d iNOS (M1 macrophage marker) and e and f CD163 (M2 macrophage marker) were used to stain tumour (squamous cell lung carcinoma) and non-tumour tissue. Black arrows indicate the expression of CD68/iNOS/CD163, ×20. The slides were observed and all photos were taken using Leica DMD108 (Leica Microsystems, Germany)
Expression of CD68, iNOS and CD163 in tumour (large cell lung carcinoma) and non-tumour tissue by immunohistochemistry. a and b CD68 (macrophage marker), c and d iNOS (M1 macrophage marker) and e and f CD163 (M2 macrophage marker) were used to stain tumour (large cell lung carcinoma) and non-tumour tissue. Black arrows indicate the expression of CD68/iNOS/CD163, ×20. The slides were observed and all photos were taken using Leica DMD108 (Leica Microsystems, Germany)
Percentage area of positive staining of CD68, iNOS and CD163 in lung tissue of different NSCLC subtypes (adenocarcinoma, squamous cell lung carcinoma and large cell lung carcinoma) compared to non-tumour tissue. The graphs show % area of positive staining ± SEM of a CD68, b iNOS and c CD163 on TAMs from non-tumour and tumour tissues from the same patient. Results expressed as % area of positive staining ± SEM, (n = 30 non-tumour tissues, 10 adenocarcinoma, 10 squamous cell lung carcinoma and 10 large cell lung carcinoma). Slides were viewed and analyzed using the ImageScope analysis software and co-localisation algorithm were applied to quantify IHC staining. One-way ANOVA multiple comparison test (as a post-test analysis) was performed with the Tukey test (multiple comparison test comparing every group with every other group). *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 and ****P ≤ 0.0001 indicates statistical significance
For iNOS-stained M1 TAMs, staining was found to be decreased in the tissues of patients with adenocarcinoma P ≤ 0.01 and squamous cell lung carcinoma P ≤ 0.05 but not in large cell lung carcinoma compared to non-tumour tissue (Figs. 1, 2, and 3). Surprisingly, the result of the multiple comparison tests demonstrated that the % area of positive staining for iNOS was significantly decreased in adenocarcinoma P ≤ 0.01 and squamous cell lung carcinoma P ≤ 0.01 compared to large cell lung carcinoma (Fig. 4).
Th1/Th2 Cytokine Levels in Serum of Patients with NSCLC Compared to Non-Cancer Controls Using Bio-Plex Assay
The potential impact of NSCLC on the Th1/Th2 cytokines levels in serum was investigated in patients with NSCLC compared to non-cancer controls using Bio-Plex, MAGPIX-Luminex assay and expressed as median fluorescence intensity (FI). All serum samples were purchased from the Victorian Cancer Bio-Bank (Victoria, Australia) (Table 2). Th1/Th2 cytokine serum analysis using the Bio-Plex, MAGPIX-Luminex assay indicated no significant difference in patients with adenocarcinoma and squamous cell lung carcinoma compared to non-cancer controls. However, patients with large cell lung carcinoma showed significant increases in the level of IL-1β, IL-4, IL-6 and IL-8 in serum (Figs. 5 and 6). IL-1β levels in serum were found to be significantly increased in patients with large cell lung carcinoma (FI = 15.53) compared to non-cancer controls (FI = 8.6, P ≤ 0.01) and to patients with squamous cell lung carcinoma (FI = 9.77, P ≤ 0.05). The IL-4 levels in serum were significantly increased in patients with large cell lung carcinoma (FI = 17.97) compared to non-cancer controls (FI = 12.38, P ≤ 0.01) and to patients with adenocarcinoma (FI = 13.43, P ≤ 0.05) as well as squamous cell lung carcinoma (FI = 12.24, P ≤ 0.01). Similarly, the IL-8 levels in serum were significantly increased in patients with large cell lung carcinoma (FI = 37.34) compared to non-cancer controls (FI = 21.11, P ≤ 0.01) and to patients with adenocarcinoma (FI = 25.3, P ≤ 0.05) as well as squamous cell lung carcinoma (FI = 24.58, P ≤ 0.05). Finally, patients with large cell lung carcinoma showed a significant increase (FI = 55.62) in the level of IL-6 compared to non-cancer controls (FI = 13.68, P ≤ 0.01) and adenocarcinoma patients (FI = 23.75, P ≤ 0.05).
Th1 cytokine secretion profiles in serum of patients with NSCLC (lung adenocarcinoma, squamous cell lung carcinoma, large cell lung carcinoma) compared to non-cancer controls. Serum was analyzed for a IL-1β, b TNF-α, c MCP-1, d IP-10 and e IL-12 (p70) by Bio-Plex assay using the MAGPIX-Luminex instrument. Data was analyzed using the Bio-Plex Manager Software (Bio-Rad) and results are expressed as median fluorescence intensity (FI) ± SEM, (n = 10 controls, 10 adenocarcinoma, 10 squamous cell lung carcinoma and 10 large cell lung carcinoma). One-way ANOVA multiple comparison test (as a post-test analysis) was performed with the Tukey test (multiple comparison test comparing every group with every other group). *P ≤ 0.05 and **P ≤ 0.01 indicates statistical significance
Th2 cytokine secretion profiles in serum of patients with NSCLC (adenocarcinoma, squamous cell lung carcinoma, large cell lung carcinoma) compared to non-cancer controls. Serum was analyzed for a IL-4, b IL-6, c IL-8, d IL-10 and e VEGF by Bio-Plex assay using the MAGPIX-Luminex instrument. Data was analyzed using the Bio-Plex Manager Software (Bio-Rad) and results are expressed as median fluorescence intensity (FI) ± SEM, (n = 10 controls, 10 adenocarcinoma, 10 squamous cell lung carcinoma and 10 large cell lung carcinoma). One-way ANOVA multiple comparison test (as a post-test analysis) was performed with the Tukey test (multiple comparison test comparing every group with every other group). *P ≤ 0.05 and **P ≤ 0.01 indicates statistical significance
Discussion
TAMs have been suggested to represent M2 macrophage-like phenotype in lung cancer and other tumour types, however, it has become clear that TAMs consist of multiple distinct populations with different features [6, 3]. Factors that contribute to altering TAMs towards a M2 phenotype include the location of TAMs within the tumour microenvironment, tumour stage and type of cancer. Nevertheless, it is still not fully defined whether the diversity within the TAM population is due to the maturation of unique monocytic precursors or from the various factors within the local tumour microenvironment [6]. In addition, further clarification regarding TAM phenotype within NSCLC subtypes is needed. Here, lung tissues from patients with NSCLC (adenocarcinoma, squamous cell lung carcinoma and large cell lung carcinoma) were used to investigate M1 and M2 marker expression in TAM populations within tumour and non-tumour tissue using immunohistochemistry (IHC).
Various studies have demonstrated increased TAM infiltration in NSCLC using the CD68 macrophage marker [21, 22, 20, 23, 19] with some suggesting the presence of increased macrophage numbers is a powerful predictor of survival in NSCLC [19, 24, 9]. In our study, all NSCLC subtypes were found to have significantly more CD68-positive cells when compared to non-tumour tissues. Other studies have shown extensive TAM infiltration of lung tumour tissue and linked this with poor prognosis [25, 2]. Taken together, these results suggest that TAMs contribute to tumour growth and lung cancer progression rather than supporting an effective host anti-tumour response. Interestingly, other studies have not supported this correlation between TAMs and good and/or poor prognosis in human lung cancer, as they reported no correlation with prognosis [26, 27]. Differences between study results may relate to the examination of different lung cancer histological subtypes, different tumour stages or examination of macrophages from different lung segments. Other factors may also contribute to differences such as patient demographics (e.g., smoking status and gender) and the presence or absence of comorbidities such as COPD.
TAMs that express iNOS have been found to be associated with extended survival in patients with NSCLC [9, 28]. Ohri et al. (2009) used phenotypic markers including iNOS and CD163 to study the association of TAM subsets with prognosis [9]. They looked at NSCLC patients with extended survival versus NSCLC patients with poor survival and established that M1 macrophages (CD68+ and iNOS+) within tumour islets were greatly increased in patients with extended survival compared to poor survival group [9]. Also, the ratio of M1 macrophages in tumour islets and stroma was significantly increased compared to M2 (CD68+ and CD163+) macrophages in patients with extended survival but not the reduced survival cohort [9]. All these results tend to validate the association of M1 TAMs with better lung cancer prognosis. In our study, M1 TAMs were identified using CD68 and iNOS marker in tumour compared to non-tumour tissue in NSCLC patients. Our results indicate that iNOS expression is decreased in tissue from patients with adenocarcinoma and squamous cell carcinoma compared to non-tumour tissues but surprisingly this was not the case in large cell lung carcinoma. Similarly, decreased expression of iNOS in TAMs has been demonstrated in previous studies [29, 30]. A previous study showed reduced iNOS expression in TAMs that were directly isolated from the tumour in tumour-bearing mice [30]. Reduced iNOS expression has also been associated with defective NF-kB signalling, which eventually may lead to incorrect regulation of the immune response [29]. Overall these results suggest iNOS as an important mediator that may be targeted in future studies to alter the TAM phenotype and to be able eventually to manipulate these cells to improve tumour suppressing function. Also, the differences between the NSCLC subtypes expression of iNOS might be a possible explanation to recent suggestions that different lung cancer subtypes present different behaviour and respond differently to treatment [31].
TAMs that express M2 marker CD163 can stimulate tumour growth by producing cytokines to induce proliferation of tumour cells directly or indirectly through increasing endothelial cell proliferation and angiogenesis [3]. In addition, the percentage of TAMs within a tumour microenvironment has been linked with tumour metastasis [32]. Our results showed that the expression of CD163 was significantly increased in all NSCLC subtypes (adenocarcinoma, squamous cell lung carcinoma and large cell lung carcinoma) compared to non-tumour tissues. A study that investigated TAMs in advanced NSCLC found that more than 95 % of CD68+ TAMs were located in the tumour stroma and were positively co-stained with CD163 [33]. Also, the CD68+ and CD163+ TAMs count was found to be significantly increased in patients with progressive disease [33]. Furthermore, other studies have shown that the expression of the M2 marker in TAMs was significantly correlated to poor prognosis, p-TNM staging and lymph node metastasis in patients with advanced adenocarcinoma [22, 32].
Despite the relatively comprehensive and high sensitivity and specificity methodology, further techniques worth considering adding to future studies in order to strengthen study outcomes include Western blot and immunofluorescence. For example, immunofluorescence (IF) method would be used to generate high-resolution images, quantitate the fluorescence signal and perform multiple staining. Other lung cancer subtypes should be considered to inspect if they have any potential role in altering TAM functions and phenotypes. Other M1 makers (e.g., IL-12) are worth adding to support this study outcomes and also the number of tissue samples needs to be expanded to confirm these results in a larger cohort.
Our study also analyzed the Th1 and Th2 cytokine levels that contribute to the differentiation of M1 and M2 macrophage populations in the serum of patients with NSCLC versus non-cancer controls. The presence of cytokines is essential for initiation of immune responses [34, 35]. Th1 cells have been found to play a major role in anti-tumour immunity and stimulation of cell-mediated responses. Pro-inflammatory cytokines such as TNF-α and IFN-γ are known to stimulate Th1 cells. In contrast, Th2 cells are known to act as the helper cells that influence B-cell development and produce anti-inflammatory cytokines such as IL-4 and IL-10 [36, 37]. Analysis of Th1 and Th2 cytokines in the serum revealed no differences in NSCLC patients overall compared to non-cancer controls. Similarly, Gursel et al. (1995) also observed no differences in TNF-α concentration between pleural effusion and serum in patients with cancer [38]. Although many studies have not looked at specific cytokine profiles in lung cancer, it has been shown that freshly prepared monocytes do not show any differences in pro-inflammatory and anti-inflammatory cytokine responses except IL-12 (p70) in endometrial cancer patients when compared to controls [39]. In our study, IL-1β, IL-4, IL-6 and IL-8 cytokine levels were found to be up-regulated in the serum of large cell lung carcinoma patients. IL-6 levels in serum was found to be significantly increased in patients with large cell lung carcinoma compared to non-cancer controls and to patients with adenocarcinoma. Different studies have demonstrated the ability of IL-6 to promote lung tumour growth and it has an association with a poor prognosis. Also, the IL-6 levels in serum were investigated in patients with lung cancer before and during radiotherapy (RT). They found that IL-6 levels were higher compared to controls and were further elevated during RT [40–43]. In addition, IL-1β and IL-8 both are known to promote tumour progression through regulating tumour growth and invasion [44, 45]. IL-1β promotes matrix metalloproteinase secretion and angiogenic factors in the tumour microenvironment [46]. The elevation of IL-1β gene expression in normal lung tissue was also shown to be related to increased risk of developing lung cancer [46]. In this study, IL-1β levels in serum were significantly increased in patients with large cell lung carcinoma compared to non-cancer controls and to patients with squamous cell lung carcinoma. Moreover, increased IL-8 expression was shown to be associated with poor lung cancer patient survival [47]. Elevated circulating IL-8 levels have been shown to be associated with lung cancer models [48]. Similarly, our results indicated that the IL-4 and IL-8 levels in serum were highly increased in patients with large cell lung carcinoma compared to non-cancer controls and to patients with adenocarcinoma as well as squamous cell lung carcinoma. Taken together, IL-1β, IL-4, IL-6 and IL-8 levels have been found to be elevated in most patients suffering from different common cancers [49, 50]. Our results indicate that large cell lung carcinoma is associated with a systemic alteration in cytokines (IL-1β, IL-4, IL-6 and IL-8) and these cytokines have been shown to promote tumour growth and metastasis. However, all non-cancer controls were female and 76.6 % of NSCLC patients were male so this may influence the accuracy of this outcome. Gender difference was described to have the ability to influence the Th1/Th2 production pathways in health and some disease states [51]. For example, sex steroids have been shown to influence the regulation of TH cell network balance and to alter the response type of Th1 and/or Th2 [52]. Although the majority of NSCLC serum samples were collected from male patients in this study, only large cell lung carcinoma was shown to influence Th1/Th2 cytokine level and not the other NSCLC subtypes compared to non-cancer controls, which might exclude the effect of gender difference in this case.
In Conclusion
The results of this study indicate that all NSCLC subtypes (adenocarcinoma, squamous cell lung carcinoma and large cell lung carcinoma) express significantly more CD68 and CD163 compared to non-tumour tissues. Also, the expression of iNOS in patients with adenocarcinoma and squamous cell lung carcinoma was significantly decreased compared to non-tumour tissues but not to that in the large cell lung carcinoma. These results indicated that TAMs express more M2 phenotype in NSCLC patients compared to non-tumour tissues. This study also indicated that the Th1/Th2 cytokine levels were not affected by the presence of NSCLC (except large cell lung carcinoma) compared to non-cancer controls. The expression of some Th1 and Th2 cytokines (IL-1β, IL-4, IL-6 and IL-8) was altered in patients with large cell lung carcinoma, which indicates the ability of this lung cancer subtype to manipulate cytokine expression in the systemic environment. Serum biomarkers are a practical and non-invasive method of diagnosing disease, and predicting prognosis and possibly treatment response. In this study, elevated cytokines IL-1, IL-4, IL-6 and IL-8 were detected only in serum from large cell carcinoma patients and this might therefore hold some promise as a potential diagnostic and/or prognostic biomarker in the serum of these patients. Taken together, the results of this study indicate that NSCLC might have the ability to alter phenotype and function within the lung tumour areas in the local environment (TAMs) but not in the bloodstream in the systemic environment (serum). The study outcomes support the previous suggestion regarding the importance of potentially targeting M2 macrophages for future therapeutic agents and aim to skew macrophage populations back to M1 subsets to stimulate anti-tumour effects within the tumour microenvironment.
References
Shih J-Y, Yuan A, Chen JJ-W, Yang P-C (2006) Tumor-associated macrophage: its role in cancer invasion and metastasis. J Cancer Mol 2(3):101–106
Lewis CE, Pollard JW (2006) Distinct role of macrophages in different tumor microenvironments. Cancer Res 66(2):605–612. doi:10.1158/0008-5472.CAN-05-4005
Mantovani A, Sozzani S, Locati M, Allavena P, Sica A (2002) Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 23(11):549–555
Dirkx AE, Oude Egbrink MG, Wagstaff J, Griffioen AW (2006) Monocyte/macrophage infiltration in tumors: modulators of angiogenesis. J Leukoc Biol 80(6):1183–1196, 10.1189/jlb.0905495
Lamagna C, Aurrand-Lions M, Imhof BA (2006) Dual role of macrophages in tumor growth and angiogenesis. J Leukoc Biol 80(4):705–713. doi:10.1189/jlb.1105656
Quatromoni JG, Eruslanov E (2012) Tumor-associated macrophages: function, phenotype, and link to prognosis in human lung cancer. Am J Transl Res 4(4):376–389
Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M (2004) The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25(12):677–686
Lopez-Gonzalez JS, Avila-Moreno F, Prado-Garcia H, Aguilar-Cazares D, Mandoki JJ, Meneses-Flores M (2007) Lung carcinomas decrease the number of monocytes/macrophages (CD14+ cells) that produce TNF-[alpha]. Clin Immunol 122(3):323–329
Ohri CM, Shikotra A, Green RH, Waller DA, Bradding P (2009) Macrophages within NSCLC tumour islets are predominantly of a cytotoxic M1 phenotype associated with extended survival. Eur Respir J 33(1):118–126. doi:10.1183/09031936.00065708
Redente EF, Dwyer-Nield LD, Merrick DT, Raina K, Agarwal R, Pao W, Rice PL, Shroyer KR, Malkinson AM (2010) Tumor progression stage and anatomical site regulate tumor-associated macrophage and bone marrow-derived monocyte polarization. Am J Pathol 176(6):2972–2985. doi:10.2353/ajpath.2010.090879
Zea AH, Rodriguez PC, Atkins MB, Hernandez C, Signoretti S, Zabaleta J, McDermott D, Quiceno D, Youmans A, O’Neill A, Mier J, Ochoa AC (2005) Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res 65(8):3044–3048
Chang CI, Liao JC, Kuo L (2001) Macrophage arginase promotes tumor cell growth and suppresses nitric oxide-mediated tumor cytotoxicity. Cancer Res 61(3):1100–1106
Biswas SK, Sica A, Lewis CE (2008) Plasticity of macrophage function during tumor progression: regulation by distinct molecular mechanisms. J Immunol 180(4):2011–2017
Gorrin-Rivas MJ, Arii S, Mori A, Takeda Y, Mizumoto M, Furutani M, Imamura M (2000) Implications of human macrophage metalloelastase and vascular endothelial growth factor gene expression in angiogenesis of hepatocellular carcinoma. Ann Surg 231(1):67–73
Edin S, Wikberg ML, Dahlin AM, Rutegard J, Oberg A, Oldenborg PA, Palmqvist R (2012) The distribution of macrophages with a M1 or M2 phenotype in relation to prognosis and the molecular characteristics of colorectal cancer. PLoS One 7(10), e47045. doi:10.1371/journal.pone.0047045
Edin S, Wikberg ML, Oldenborg PA, Palmqvist R (2013) Macrophages: good guys in colorectal cancer. OncoImmunology 2(2), e23038. doi:10.4161/onci.23038
Sica A, Mantovani A (2012) Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 122(3):787–795. doi:10.1172/jci59643
UyBico SJ, Wu CC, Suh RD, Le NH, Brown K, Krishnam MS (2010) Lung cancer staging essentials: the new TNM staging system and potential imaging pitfalls. Radiographics 30(5):1163–1181. doi:10.1148/rg.305095166
Welsh TJ, Green RH, Richardson D, Waller DA, O’Byrne KJ, Bradding P (2005) Macrophage and mast-cell invasion of tumor cell islets confers a marked survival advantage in non-small-cell lung cancer. J Clin Oncol Off J Am Soc Clin Oncol 23(35):8959–8967. doi:10.1200/jco.2005.01.4910
Wang R, Zhang J, Chen S, Lu M, Luo X, Yao S, Liu S, Qin Y, Chen H (2011) Tumor-associated macrophages provide a suitable microenvironment for non-small lung cancer invasion and progression. Lung Cancer (Amsterdam, Netherlands) 74(2):188–196. doi:10.1016/j.lungcan.2011.04.009
Takanami I, Takeuchi K, Kodaira S (1999) Tumor-associated macrophage infiltration in pulmonary adenocarcinoma: association with angiogenesis and poor prognosis. Oncology 57(2):138–142
Ohtaki Y, Ishii G, Nagai K, Ashimine S, Kuwata T, Hishida T, Nishimura M, Yoshida J, Takeyoshi I, Ochiai A (2010) Stromal macrophage expressing CD204 is associated with tumor aggressiveness in lung adenocarcinoma. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer 5(10):1507–1515. doi:10.1097/JTO.0b013e3181eba692
Zeni E, Mazzetti L, Miotto D, Lo Cascio N, Maestrelli P, Querzoli P, Pedriali M, De Rosa E, Fabbri LM, Mapp CE, Boschetto P (2007) Macrophage expression of interleukin-10 is a prognostic factor in nonsmall cell lung cancer. Eur Respir J 30(4):627–632. doi:10.1183/09031936.00129306
Ohri CM, Shikotra A, Green RH, Waller DA, Bradding P (2011) The tissue microlocalisation and cellular expression of CD163, VEGF, HLA-DR, iNOS, and MRP 8/14 is correlated to clinical outcome in NSCLC. PLoS One 6(7), e21874. doi:10.1371/journal.pone.0021874
Bingle L, Brown N, Lewis C (2002) The role of tumour‐associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol 196(3):254–265
Toomey D, Smyth G, Condron C, Kelly J, Byrne AM, Kay E, Conroy RM, Broe P, Bouchier-Hayes D (2003) Infiltrating immune cells, but not tumour cells, express FasL in non-small cell lung cancer: No association with prognosis identified in 3-year follow-up. Int J Cancer J Int Cancer 103(3):408–412. doi:10.1002/ijc.10836
Tataroglu C, Kargi A, Ozkal S, Esrefoglu N, Akkoclu A (2004) Association of macrophages, mast cells and eosinophil leukocytes with angiogenesis and tumor stage in non-small cell lung carcinomas (NSCLC). Lung Cancer (Amsterdam, Netherlands) 43(1):47–54
Ma J, Liu L, Che G, Yu N, Dai F, You Z (2010) The M1 form of tumor-associated macrophages in non-small cell lung cancer is positively associated with survival time. BMC Cancer 10:112. doi:10.1186/1471-2407-10-112
Biswas SK, Gangi L, Paul S, Schioppa T, Saccani A, Sironi M, Bottazzi B, Doni A, Vincenzo B, Pasqualini F, Vago L, Nebuloni M, Mantovani A, Sica A (2006) A distinct and unique transcriptional program expressed by tumor-associated macrophages (defective NF-kappaB and enhanced IRF-3/STAT1 activation). Blood 107(5):2112–2122. doi:10.1182/blood-2005-01-0428
Dinapoli MR, Calderon CL, Lopez DM (1996) The altered tumoricidal capacity of macrophages isolated from tumor-bearing mice is related to reduce expression of the inducible nitric oxide synthase gene. J Exp Med 183(4):1323–1329
Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, Gatzemeier U, Digumarti R, Zukin M, Lee JS, Mellemgaard A, Park K, Patil S, Rolski J, Goksel T, de Marinis F, Simms L, Sugarman KP, Gandara D (2008) Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol Off J Am Soc Clin Oncol 26(21):3543–3551. doi:10.1200/jco.2007.15.0375
Zhang B, Yao G, Zhang Y, Gao J, Yang B, Rao Z, Gao J (2011) M2-polarized tumor-associated macrophages are associated with poor prognoses resulting from accelerated lymphangiogenesis in lung adenocarcinoma. Clinics 66(11):1879–1886. doi:10.1590/S1807-59322011001100006
Chung FT, Lee KY, Wang CW, Heh CC, Chan YF, Chen HW, Kuo CH, Feng PH, Lin TY, Wang CH, Chou CL, Chen HC, Lin SM, Kuo HP (2012) Tumor-associated macrophages correlate with response to epidermal growth factor receptor-tyrosine kinase inhibitors in advanced non-small cell lung cancer. Int J Cancer J Int Cancer 131(3):E227–E235. doi:10.1002/ijc.27403
Belardelli F, Ferrantini M (2002) Cytokines as a link between innate and adaptive antitumor immunity. Trends Immunol 23(4):201–208
Matanic D, Beg-Zec Z, Stojanovic D, Matakoric N, Flego V, Milevoj-Ribic F (2003) Cytokines in patients with lung cancer. Scand J Immunol 57(2):173–178
Romagnani S (1995) Biology of human TH1 and TH2 cells. J Clin Immunol 15(3):121–129
Romagnani S (1996) Th1 and Th2 in human diseases. Clin Immunol Immunopathol 80(3 Pt 1):225–235
Gursel G, Gokcora N, Elbeg S, Samurkasoglu B, Ekim N (1995) Tumor necrosis factor-alpha (TNF-alpha) in pleural fluids. Tuber Lung Dis 76(4):370–371
Brooks N, Stojanovska L, Grant P, Apostolopoulos V, McDonald CF, Pouniotis DS (2012) Characterization of blood monocyte phenotype in patients with endometrial cancer. Int J Gynecol Cancer 22(9):1500–1508. doi:10.1097/IGC.0b013e3182249273
Takahashi H, Ogata H, Nishigaki R, Broide DH, Karin M (2010) Tobacco smoke promotes lung tumorigenesis by triggering IKKbeta- and JNK1-dependent inflammation. Cancer Cell 17(1):89–97. doi:10.1016/j.ccr.2009.12.008
Yi H, Cho HJ, Cho SM, Jo K, Park JA, Kim NH, Amidon GL, Kim JS, Shin HC (2012) Blockade of interleukin-6 receptor suppresses the proliferation of H460 lung cancer stem cells. Int J Oncol 41(1):310–316. doi:10.3892/ijo.2012.1447
De Vita F, Orditura M, Auriemma A, Infusino S, Roscigno A, Catalano G (1998) Serum levels of interleukin-6 as a prognostic factor in advanced non-small cell lung cancer. Oncol Rep 5(3):649–652
Crohns M, Saarelainen S, Laine S, Poussa T, Alho H, Kellokumpu-Lehtinen P (2010) Cytokines in bronchoalveolar lavage fluid and serum of lung cancer patients during radiotherapy—association of interleukin-8 and VEGF with survival. Cytokine 50(1):30–36. doi:10.1016/j.cyto.2009.11.017
Juarez E, Nunez C, Sada E, Ellner JJ, Schwander SK, Torres M (2010) Differential expression of toll-like receptors on human alveolar macrophages and autologous peripheral monocytes. Respir Res 11:2
Waugh DJ, Wilson C (2008) The interleukin-8 pathway in cancer. Clin Cancer Res 14(21):6735–6741. doi:10.1158/1078-0432.CCR-07-4843
Apte RN, Dotan S, Elkabets M, White MR, Reich E, Carmi Y, Song X, Dvozkin T, Krelin Y, Voronov E (2006) The involvement of IL-1 in tumorigenesis, tumor invasiveness, metastasis and tumor-host interactions. Cancer Metastasis Rev 25(3):387–408. doi:10.1007/s10555-006-9004-4
Chen JJ, Yao PL, Yuan A, Hong TM, Shun CT, Kuo ML, Lee YC, Yang PC (2003) Up-regulation of tumor interleukin-8 expression by infiltrating macrophages: its correlation with tumor angiogenesis and patient survival in non-small cell lung cancer. Clin Cancer Res 9(2):729–737
Millar HJ, Nemeth JA, McCabe FL, Pikounis B, Wickstrom E (2008) Circulating human interleukin-8 as an indicator of cancer progression in a nude rat orthotopic human non-small cell lung carcinoma model. Cancer Epidemiol Biomarkers Prev 17(8):2180–2187. doi:10.1158/1055-9965.EPI-07-2915
Culig Z (2011) Cytokine disbalance in common human cancers. Biochim Biophys Acta 1813(2):308–314. doi:10.1016/j.bbamcr.2010.12.010
Albulescu R, Codrici E, Popescu ID, Mihai S, Necula LG, Petrescu D, Teodoru M, Tanase CP (2013) Cytokine patterns in brain tumour progression. Mediat Inflamm 2013:7. doi:10.1155/2013/979748
Pellegrini P, Contasta I, Del Beato T, Ciccone F, Berghella AM (2011) Gender-specific cytokine pathways, targets, and biomarkers for the switch from health to adenoma and colorectal cancer. Clin Dev Immunol 819724. doi:10.1155/2011/819724
Schuurs AH, Verheul HA (1990) Effects of gender and sex steroids on the immune response. J Steroid Biochem 35(2):157–172
Acknowledgments
This work was supported by the Institute for Breathing and Sleep project Grant and the School of Medical Sciences, RMIT University Grant Scheme.
Ethical Approval
Human Ethics approval was received from RMIT University Human Research Ethics Committee ASEHAPP 15-13 and the informed consent of all participants was obtained.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Almatroodi, S.A., McDonald, C.F., Darby, I.A. et al. Characterization of M1/M2 Tumour-Associated Macrophages (TAMs) and Th1/Th2 Cytokine Profiles in Patients with NSCLC. Cancer Microenvironment 9, 1–11 (2016). https://doi.org/10.1007/s12307-015-0174-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12307-015-0174-x