Abstract
For more than 15 years, angiotropism in melanoma has been emphasized as a marker of extravascular migration of tumor cells along the abluminal vascular surface, unveiling an alternative mechanism of tumor spread distinct from intravascular dissemination. This mechanism has been termed extravascular migratory metastasis (EVMM). During EVMM, angiotropic tumor cells migrate in a ‘pericytic-like’ manner (pericytic mimicry) along the external surfaces of vascular channels, without intravasation. Through this pathway, melanoma cells may spread to nearby or more distant sites. Angiotropism is a prognostic factor predicting risk for metastasis in human melanoma, and a marker of EVMM in several experimental models. Importantly, analogies of EVMM and pericytic mimicry include neural crest cell migration, vasculogenesis and angiogenesis, and recent studies have suggested that the interaction between melanoma cells and the abluminal vascular surface induce differential expression of genes reminiscent of cancer migration and embryonic/stem cell state transitions. A recent work revealed that repetitive UV exposure of primary cutaneous melanomas in a genetically engineered mouse model promotes metastatic progression via angiotropism and migration along the abluminal vascular surface. Finally, recent data using imaging of melanoma cells in a murine model have shown the progression of tumor cells along the vascular surfaces. Taken together, these data provide support for the biological phenomenon of angiotropism and EVMM, which may open promising new strategies for reducing or preventing melanoma metastasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metastasis is defined by end points, (metastatic lesions detected in specific organs distant from a primary tumor), but the complex in vivo mode of this process is not fully understood [1]. Melanoma cells can use different migratory strategies depending on varying environments in order to exit the primary tumor mass, invade surrounding tissues and metastasize to distant sites [2] and such a plasticity seems to be linked to their embryonic and/or stem cell like cellular properties [3–6]. After considerable debate about the potential mechanisms of cancer metastasis continuing until the end of the 19th century [7], the intravascular dissemination of cancer via the blood and/or lymph was finally imposed and is still widely accepted as a central paradigm [8, 9]. Therefore the interaction of tumor cells with the tumor vasculature is mainly studied for its role in tumor blood supply (tumor angiogenesis) [8], and intravascular metastasis of circulating tumor cells (CTC) [9, 10]. However “the acquired capability for invasion and metastasis represents the last great frontier for exploratory cancer research” [11] and opening new fields of basic investigation concerning cancer metastasis is of singular importance.
For more than 15 years, Lugassy and Barnhill have opened the field of extravascular migratory metastasis (EVMM) in melanoma [12, 13]. In EVMM, tumor cells migrate without intravasation, as opposed to the intravascular dissemination of tumor cells. During EVMM along vessels, tumor cells become adherent to the abluminal vascular surface, defining angiotropism, and use selective motility cues on their abluminal surfaces in order to migrate without intravasation (EVMM).
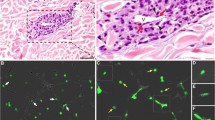
Angiotropic melanoma cells are defined histologically as melanoma cells closely associated with the endothelium of vessels in a pericytic location, generally detected at the advancing front of the tumor, and without intravasation (Fig. 1a) [14]. Angiotropism promotes pericytic mimicry, the spreading of tumor cells along the abluminal vascular surfaces (Fig. 1b) [14]. The continuous migration of melanoma cells along the abluminal vascular surface toward secondary sites represents extravascular migratory metastasis (EVMM), an alternative mechanism of melanoma spread (Fig. 1c) versus intravascular tumor cell dissemination via circulating tumor cells (CTC) [13, 14]. Specifically, the term angiotropism represents a histopathological image; the term pericytic mimicry emphasizes the replacement of pericytes by these angiotropic tumor cells; and the term EVMM describes this extravascular mechanism of tumor spread toward secondary sites without entering inside the lumina of vascular channels (Fig. 1). Notably, in EVMM, tumor cells can also migrate along other anatomical tracks such as nerves (neurotropism or neurotropic EVMM) [13–15], however in the present review we will focus on angiotropic EVMM along vessels.
Angiotropism, pericytic mimicry and EVMM A. Angiotropism. Definition: tumor cells closely associated with the abluminal vascular surfaces without intravasation. A1. Human sample of melanoma showing angiotropism of tumor cells about the abluminal surface of a microvessel some distance from the primary melanoma (about 1 mm) constituting a microscopic satellite in the nearby dermis (arrow). A2. Higher magnification. Melanoma cells (dark purple cells) are cuffing the abluminal surface of the microvascular channel (V). B. Pericytic mimicry. Definition: replacement of pericytes (or SMC) by tumor cells. The diagram depictst umor cells migrating in a pericytic location along the abluminal vascular surface at the advancing front of the tumor. The tumor mass (black) gives raise to angiotropic melanoma cells (green with black nucleus) migrating outside the tumor along the abluminal vascular surface. C. EVMM. Definition: extravascular migration of tumor cells toward secondary site. EVMM is schematically depicted in the diagram by the five following steps: 1) single migrating (cancer stem cell?) tumor cells at the advancing front of the primary tumor; 2) angiotropism; 3) epithelial mesenchymal transition (EMT) /pericytic mimicry; 4) migration along vessels without intravasation; 5) secondary tumor formation (metastasis). Metastasis can then give rise to the same sequence of five steps and to the development of more distant metastases
Utilizing EVMM, melanoma cells may spread to nearby, or to more distant sites, without entering the vascular channel. Interestingly, tumor cells have been reported to migrate at rates of 0.1 to 2 μm/min, culminating in distances of 5.2 to 105 cm/year [15]. These distances may theoretically be even greater via the more rapid amoeboid migration of cancer cells [16]. Such velocities are compatible with the time intervals between the recognition of the primary cancer and the formation of obvious metastases in human cancer progression. EVMM may recapitulate embryonic migration in order to reach secondary sites.
These new concepts of angiotropism, pericytic mimicry and EVMM are now recognized as an important alternative means of melanoma dissemination [17–21]. Pertinent to EVMM are the origin of melanocytes from the neural crest (NC) and the strong analogies of EVMM with NC cell (NCC) migration [22]. In addition, analogies of EVMM with vasculogenesis and angiogenesis [15] suggest that this pericytic-like spread or pericytic mimicry exists in solid tumors from other origins than the neural crest.
In this paper we will review the various scientific areas of interest leading to our current state of knowledge and understanding about angiotropism, pericytic mimicry and EVMM in melanoma.
Neural Crest Cell Migration, Vasculogenesis and Angiogenesis Represent Mechanisms Potentially Deregulated During the Pericytic Mimicry of Angiotropic Melanoma Cells
-
1.
Neural crest cell (NCC) migration
Given the correlations between cancer and embryogenesis [23] it is important to emphasize the analogy of EVMM with the mechanisms of migration during embryogenesis, particularly during the migration of neural crest cells in the developing embryo. The neural crest provides a useful paradigm for cellular migration during morphogenesis. The embryonic pathways of highly migratory NCC and their regulation during development eventuate, among other phenotypes, in the establishment of melanocytes in the skin. Neural crest cells (NCCs) arise from the lateral edges of the neural folds at the end of gastrulation. Subsequently, NCCs delaminate from the neural tubes and migrate along specific routes to a number of sites where NCC derivatives arrest and fully differentiate into a wide variety of derivatives. These include melanocytes, neurons and glial cells of the peripheral nervous system, smooth muscle cells, connective tissue, and cartilaginous and skeletal elements in the head. It is generally admitted that melanoblasts arise from the dorsal neural tube and follow the so-called dorsolateral pathway to populate the skin [24]. “Once neural crest differentiation occurs, cells occupying the dorsal portion of the neural tube are disrupted in their cadherin-mediated cell-cell contacts, acquire epithelial-mesenchymal transition (EMT) and motile properties, migrate away from the neural tube and embark upon an extensive continuous migration through the embryo to reach their ultimate phenotype-specific sites” [25]. It is possible to paraphrase the latter process in cancer as follows: “Once a solid tumor becomes invasive, cells occupying the advancing front of the tumor disrupt their cadherin-mediated cell-cell contacts, acquire EMT and motile properties, migrate away from the primary tumor and embark upon an extensive continuous migration through the body to reach their metastatic secondary sites”.
With respect to angiotropic migration along vessels, recent studies examining mice in embryonic development have confirmed the migration of NCCs along the external surfaces of microvascular channels during the course of the development [26]. In addition, it has been demonstrated that enteric NCCs follow endothelial cells during their migration within the gut of embryos, and the images from this study demonstrate striking angiotropism of migrating NCCs [27] (Fig. 2a).
Fig. 2 Analogies of angiotropism with neural crest cell (NCC) migration and pericytes recruitment A. Quail hindgut stained with antibodies to QH1 staining endothelial cells (red), and HNK-1 staining NCC (blue). Note the angiotropism of the NCC migrating along a vessel (v) and the analogy with melanoma angiotropism (Fig. 1). From [27]: Nagy N, Mwizerwa O, Yaniv K, Carmel L, Pieretti-Vanmarcke R et al. (2009) Endothelial cells promote migration and proliferation of enteric neural crest cells via beta1 integrin signaling. Dev Biol 330 (2):263–72. B. Scanning electron microscopy showing pericytes along the abluminal surface of a microvessel (stars). Note the analogy with angiotropism and pericytic mimicry of tumor cells (Fig. 1). Pictures from pathologyoutlines.com: http://www.pathologyoutlines.com/topic/softtissue3vascnormal.html Author: Nat Pernick; Copyright: (c) 2002–2009, PathologyOutlines.com, Inc.
Interestingly, it has been demonstrated that human metastatic melanoma cells, when transplanted into the chick embryonic neural crest microenvironment, respond to cues from surrounding host tissues and emigrate along stereotypical migratory routes traveled by neural crest cells [28, 29]. Furthermore, recent data have specified that “melanoma revives an embryonic migration program to promote plasticity and invasion” and that aberrant regulation of neural crest developmental genes may promote plasticity and invasiveness in melanoma [6]. It is therefore possible that some angiotropic melanoma cells use embryonic migratory properties in order to migrate along vessels and even other cellular surfaces, for example migration along nerves in neurotropism. Such mechanisms of migration could represent an alternative metastatic pathway to “the inefficient intravascular metastatic process” [30–32]. In addition, such a recapitulation of embryonic migration could be related to the “seed and soil” hypothesis, since melanoma cells may migrate to reach their “ultimate phenotype-specific sites” [25, 33, 34]. Finally, neural crest cells migrate at rates of about 0.5 to 2 μm/min or more [35, 36], and are therefore comparable to migrating tumor cells.
-
2.
Vasculogenesis and angiogenesis
Vessel formation can occur by a number of different processes. Early in embryonic development, vessel formation occurs by a process referred to as vasculogenesis in which endothelial cells differentiate and proliferate in situ within a previously avascular tissue. Angiogenesis involves the sprouting from existing vessels into a previously avascular tissue. Angiogenesis is responsible for vascularizing certain structures during normal development and for most new vessel formation in the adult [37]. Concerning the embryonic formation of vessels, it has been observed that the primordial endothelium, once assembled into vascular tubes, can recruit undifferentiated cells with mesenchymal morphology and direct their differentiation into pericytes and smooth muscle cells (SMCs) [38, 39]. Similarly, during angiogenesis, pericytes are recruited and begin to migrate along the abluminal side of vessel to stabilize neovessels [39] (Fig. 2b). Importantly, pericytes have recently been recognized as mesenchymal stem cells (MSC) [40]. Invasive tumor cells are known to exhibit morphologic and biologic properties characteristic of embryonic/stem cells particularly during EMT [23]. It is therefore conceivable that invasive melanoma cells are recruited instead of pericytes in microvessels (and/or SMC in larger vessels), for the external vascular surfaces, exhibiting EMT and pericytic mimicry (or mural cell mimicry) in order to migrate along the abluminal vascular surface (Fig. 1). Although EMT processes are documented in many in vitro cancer cell models, the significance of EMT during cancer progression is still controversial. Very interestingly, it has been demonstrated in several solid tumors that EMT occurs at the invasive front and produces single migratory cells [23], as observed with angiotropic melanoma cells. Our recent molecular data [15] suggests that angiotropic melanoma cells exhibit cancer stem cell properties and specifically express EMT and mesenchymal stem cell (pericytic) associated genes (see below).
In non-lesional tissue, pericytes and endothelial cells are surrounded by a basement membrane exhibiting a normal architecture. At the ultrastructural level, the basement membranes possess a distinctive electron-lucent layer (lamina lucida) sandwiched between the plasma membrane and an outer electron-dense layer (lamina densa) [41]. However, the interaction of melanoma cells with endothelial cells results in an amorphous ultrastructural basement membrane zone, not assembled into the usual organized supramolecular structure recognized as conventional basement membrane. It is of interest that immunohistochemical analysis has confirmed the presence of laminin in this amorphous matrix. It has been suggested that such an amorphous matrix in tumors could expose cryptic promigratory sites on laminin that might trigger increased cellular motility [12, 42] (see below).
Pericytes are increasingly studied for their role in tumor formation, growth and invasion. The respective role of pericytes and angiotropic tumor cells in tumor invasion and metastasis requires much more detailed investigation.
Given the analogies of EVMM with NCC migration and angiogenic pathways, it is quite possible that the dysregulation of these mechanisms result in pericytic mimicry and mesenchymal stem cell or embryonic-like migration of angiotropic melanoma cells along the abluminal vascular surface, or EVMM.
Angiotropism as a Prognostic Factor for Melanoma
Several studies have demonstrated that angiotropism is a prognostic factor in melanoma.
-
1.
Angiotropism as a prognostic factor of loco-regional and distant melanoma metastases
We investigated the prognostic significance of angiotropism in a series of 40 patients with primary cutaneous melanoma and confirmed metastasis matched with a similar group of 40 patients with non-metastasizing primary melanoma and long-term disease-free survival [43]. Metastases included loco-regional and distant melanoma metastases. Angiotropism was found exclusively in patients with metastasizing melanoma, while vascular/lymphatic invasion was absent.
-
2.
Angiotropism as a prognostic factor for local and in transit melanoma metastases
In a subsequent independent study utilizing the same microscopic criteria, angiotropism has been confirmed as a prognostic factor for primary melanomas associated with local and in transit metastases [18]. In this study, angiotropism was found more often in patients who developed local or in-transit recurrence (cases) as compared to patients who did not. In addition, the median disease-free survival was shorter for patients with angiotropism versus for those without.
-
3.
It has also been demonstrated that angiotropism of melanoma cells correlates with microscopic satellite (MS) formation in cutaneous melanomas and thus is likely to explain the development of MS [44]. Patients with MS and controls without MS were evaluated for the presence or absence of angiotropism. MS was defined as a dermal/subcutaneous tumour nodule >0.05 mm, separated from the primary tumour by at least 0.3 mm. Forty four cases and controls were matched for tumour thickness, mitotic rate, ulceration, age, gender and primary site. Angiotropism was significantly more frequent in melanomas with MS than in those without MS.
-
4.
We have also utilized a large bank of frozen primary melanomas for the specific evaluation of angiotropism as a differential marker in melanoma specimens [45]. From the statistical analysis of clusters, this study demonstrated the correlation between angiotropism and distant metastasis at 4 years yielded, while the correlation between angiotropism and Breslow melanoma thickness was highly significant.
-
5.
Finally, in a recent study we have shown that ulcerated primary human melanomas with abundant neutrophils and reactive angiogenesis frequently show angiotropism and a high risk for metastases [17]
Angiotropism as a Microscopic Marker of Migration Along the Abluminal Vascular Surface
Angiotropism has been shown to be a marker of migration along the abluminal Vascular Surface.
-
1.
In vitro and ex vivo studies using co-cultures of melanoma cells with capillary-like structures
In order to study the relationships between human melanoma cells and vascular endothelium, melanoma cells have been cultured with endothelial cells forming endothelial tubules or capillary-like structures on a tumor basement membrane (Matrigel [BD] or BME [(Basement Membrane Extract) Trevigen]). After 24 h, five human melanoma cell lines isolated from metastatic melanoma from different patients were localized along the external surface of the vascular tubules, occupying a pericytic-like location in a pattern analogous to angiotropic melanoma cells in vivo [46]. Similar results have also been obtained using co-cultures of melanoma cells with rat aortic rings ex vivo [47]. More recently, time-lapse images of human C8161 migrating melanoma cells in vitro toward and along the abluminal surface of endothelial tubules were obtained post-seeding using the Nikon Eclipse Ti live-cell imaging microscope [48]. Real time imaging of melanoma cancer cell migratory behaviour exhibited a migration toward and along endothelial cells. We also observed unique changes in the structural physiology of migrating melanoma cells suggesting epithelial to mesenchymal cell state transition (EMT), not seen in stationary melanoma cells. Live cell imaging of this co-culture EVMM assay enabled quantitative analysis of migration in real time in single cells (Figs. 3a, b). Single cell tracking of individual melanoma cells engaging in EVMM over a course of 24 h post-seeding provided cell path length and velocity of EVMM migration during the 24 h time course. An average velocity of cell EVMM was approximately at 0.3 μm/min was observed, which is within the range of both tumor cell and neural crest migration average velocities as mentioned above.
Fig. 3 Single cell analysis of melanoma cells engaging in EVMM along the abluminal surfaces of endothelial tubules A and B. Time lapse images of melanoma cells (marked as melanoma cell 1 and melanoma cell 2) engaging in EVMM and obtained between 5 and 9 h post-seeding, reveal two melanoma cells having moved along the endothelial tubule from points 1a to 1b (melanoma cell 1) and points 2a to 2b (melanoma cell 2). C and D. Single cell tracking monitoring path length and velocity of migration. C. Melanoma Cell 1: Path length: 54.64 μm, Average Velocity: 0.004 μm/s or 0.25 μm/min. D. Melanoma Cell 2: Path Length: 66.31 μm, Average Velocity: 0.0046 μm/s or 0.3 μm/min. Solid lines indicate Velocity (μm/s) while broken lines indicate Acceleration (μm/s2). Scale Bars = 60 μM. Video available [52]. From [52]: Zadran S, McMickle R, Shackelford D, Kleinman H, Barnhill R, Lugassy C. Monitoring extra-vascular migratory metastasis (EVMM) of migrating cancer cells using an in vitro co-culture system. Protoc exch. 2013 Nov 22;2013. D. A modified shell-less chick CAM assay as an in vivo real-time model of angiotropic EVMM The GFP-expressing cells on the CAM viewed with a stereo fluorescence microscope after the passage of 3 days. Melanoma cells (fluorescent) are spreading along microvascular channels, exhibiting pericytic mimicry and EVMM. Histopathology confirmed melanoma angiotropism without intravasation [25]. Bars = 1 mm. From [25]: Lugassy, C., Kleinman, H.K., Vernon, S.E., Welch, D.R., and Barnhill, R.L. (2007). C16 laminin peptide increases angiotropic extravascular migration of human melanoma cells in a shell-less chick chorioallantoic membrane assay. Br. J. Dermatol. 157,780–782.6
-
2.
In vivo real-time model of angiotropic melanoma spread in a modified shell-less chick chorioallantoic membrane assay (CAM)
An in vivo model to monitor angiotropic melanoma migration was in fertilized chicken eggs. Briefly, fertilized chicken eggs were opened into glass dishes, and C8161 GFP human melanoma cells were applied to the surfaces of the open CAM without traumatizing the CAM [49]. With stereo fluorescence microscopy, it was possible to observe directly in vivo and over time the growth and spread of melanoma cells along the vasculature. The rate of migration of angiotropic human melanoma cells along the abluminal surface of vessels was approximately 1.2 μm/min, which is in agreement with the data mentioned above. (Fig. 3c). Histopathology confirmed the angiotropism of melanoma cells without intravasation, as observed with human angiotropic melanoma.
Studies on Gene Expression Profiling of Human Angiotropic Primary Melanoma
Using a previously constructed melanoma gene expression microarray, we have identified 15 genes potentially critical to EVMM [45, 50]. A large bank of frozen primary melanomas was employed in a previous study to correlate gene expression profiles with either metastasis or death, or distant metastasis-free survival at 4 years. 254 genes were identified that distinguished primary melanomas with and without metastases. We have also utilized the same bank of melanomas for the specific evaluation of angiotropism as a differential marker in melanoma specimens (see above). By combining genomic databases and published work from the scientific literature, we identified among the 128 differentially expressed genes, 15 genes potentially involved in EVMM. These 15 genes were classified according to their function (Table 1). Because of the apparently close and mechanistically important relationship between EVMM and NCC migration, we have undertaken immunohistochemical studies of TCOF1 and ANHAK, two of the identified genes directly implicated in NCC migration. Indeed strong Treacle (the protein product of the TCOF1 gene) expression has been observed in migrating neural crest cells in the craniofacial mesenchyme [51]. On the other hand, AHNAK is one of the genes whose expression is typically repressed in neuroblastoma, which represents the most primitive neoplasm originating from migratory neural crest cells [45]. Our results have specifically shown in angiotropic melanoma cells the increased expression of Treacle (Fig. 4a) and the down regulation of ANHAK (the protein product of the AHNAK gene).
Immunohistochemical studies of melanoma angiotropism in human melanoma samples A. Frozen-section immunohistochemistry of an angiotropic primary melanoma stained with TCOF1/Treacle antibody. At the advancing front of the tumor, the angiotropic melanoma cells arrayed about a vascular channel show nuclear immunoreactivity with the treacle antibody. (Purple arrows: angiotropic treacle-positive melanoma cells). B. Angiotropic melanoma metastatic to a lymph node stained with a laminin polyclonal antibody. Laminin expressed by angiotropic melanoma cells forms a striking lattice (red) about vessels with angiocentric projections into the tumor mass. C. Primary cutaneous human melanoma stained with PDGFRB antibody. Note the specific immunostaining of angiotropic melanoma cells (and pericytes) around vascular lumina in the main tumor mass, and the absence of staining of the other melanoma cells. D. Metastatic recurrence of BRAF melanoma resistant to anti-BRAF therapy stained with PDGFRB antibody. PDGFRB is expressed in angiotropic melanoma cells (and pericytes) around vascular lumina, versus the absence of such expression before the onset of resistance (not shown)
In addition, this study confirmed from the statistical analysis of clusters, the correlation between angiotropism and distant metastasis at 4 years yielded, while the correlation between angiotropism and Breslow melanoma thickness was highly significant.
Laminin, Angiotropism and EVMM
Cell to cell interactons via cell surface receptors, such as integrins and other laminin-binding proteins, regulate gene expression and significantly influence cell fate. Laminins are cell adhesion molecules that comprise a family of glycoproteins found predominantly in basement membranes. Laminins are essential for early embryonic development and organogenesis, and during the course of neural crest cell migration, specific laminins are expressed by the basal surfaces of the epithelia lining these pathways [25, 52–55]. Laminin are also implicated in a wide variety of biological processes including cell adhesion, differentiation, migration, signaling, neurite outgrowth, and metastasis [25, 52–55].
As mentioned above, it has been demonstrated that angiotropic melanoma cells are linked to the endothelium by an amorphous matrix confirmed by immunohistochemistry to contain laminin (Fig. 4b) [12, 56, 57] . Given the role of laminin in NCC migration, tumor migration and metastasis, the presence of laminin between melanoma cells and endothelial cells raises the possibility that exposed cryptic pro-migratory sites on laminin triggers tumor cell motility along the abluminal surface [12, 42].
In addition, biologically active sites on laminin-1 have been identified using proteolytic fragments and synthetic peptides [58]. The C16 (KAFDITYVRLKF) peptide from the γ1 laminin chain has previously been shown to significantly enhance pulmonary metastases associated with B16-F10 mouse melanoma cells [59]. The effect of this peptide on melanoma spread has been tested in our modified shell-less chick chorioallantoic membrane (CAM) model [48], where GFP human melanoma cells were applied to the surface of the open CAM incubated with the C16 laminin peptide. The C16 laminin peptide significantly lengthened the distances of extravascular cellular migration by angiotropic melanoma cells. Histopathology confirmed the angiotropism of melanoma cells without intravasation, as observed with human angiotropic melanoma. The results of this study suggest that the C16 laminin γ1 chain peptide has angiotropic, extravascular migration-promoting activity on human melanoma cells, and might represent a possible molecular target for preventing melanoma metastasis.
Finally, it has been shown that angiotropic human melanoma cells over-expressed some laminins and laminin receptors in our shell-less CAM assay. Indeed we have tested the hypothesis that expression levels of some genes related to laminin and metastasis are differentially expressed in vascularized angiotropic melanoma areas versus avascular melanoma areas from the same tumor [60]. C8161 human melanoma cells in our shell-less chick CAM assay were used to study EVMM associated with the presence of vascularized angiotropic melanoma areas. Using laser capture microdissection, angiotropic melanoma areas were microdissected, as well as avascular areas. Using quantitative Real Time PCR, six genes have been studied: LAMC2 (laminin γ2 chain), LAMA4 (laminin α4 chain), ITGB1 (integrin β1), ITGB3 (integrin β3), RSPA (ribosomal protein) and MMP2 (matrix metallopeptidase 2). All genes were up regulated in angiotropic melanoma areas versus avascular melanoma areas, especially LAMC2, LAMA4 and ITGB3. Taken together, these data suggest that some laminins and laminin receptors play a substantial role in pericytic mimicry and EVMM.
Stem Cell Properties of Angiotropic Melanoma Cells Interacting With the Abluminal Vascular Surface
We have used our co-culture model of human melanoma cells with capillary-like structures in vitro to determine whether the interaction between the abluminal surface of endothelial cells and angiotropic melanoma cells could trigger the expression of particular genes. Differential gene expression triggered specifically by the interaction between angiotropic melanoma cells and the vascular tubules was analyzed by microarray analysis and Ingenuity Pathways Analysis (IPA). In addition, immunostaining of 10 human melanoma samples explored the expression of several pericytic/MSC markers by angiotropic melanoma cells. These studies provided data suggesting migratory embryonic/stem cell properties of angiotropic melanoma cells, as well as the pericytic mimicry of these cells [61].
Endothelial cells (EC) alone can form endothelial tubules or capillary-like structures. Indeed, ECs are capable of aggregating in vitro to form endothelial tubules or capillary-like structures, which contain a lumen surrounded by endothelial cells attached to one another by junctional complexes. Notably ECs exhibit a normal polarization [62]. Melanoma cells (MCs) cultivated alone displayed “vasculogenic mimicry”, i.e., formed structures mimicking endothelial channels [63]. MC co-cultivated with endothelial cells demonstrated “pericytic mimicry”, specifically where MCs began spreading along the abluminal surfaces of the endothelial tubules in a pericytic location (Figs. 3a, b).
We analyzed gene expression triggered by the co-culture of MCs and ECs versus simultaneously MCs alone and ECs alone in the similar conditions defined above. Such interaction between MCs and ECs triggered novel differential gene expression when compared to MCs alone and ECs alone. Interestingly, from the co-culture of EC and MC versus simultaneously MC alone and EC alone, 20 from the 28 known differentially expressed genes have demonstrated properties linked to (i) cell migration; (ii) cancer progression and metastasis; (iii) EMT; (iv) embryonic and/or cancer stem cell properties; (v) pericyte recruitment; (vi) inflammation (table 2). Very interestingly, SERPINB2 was among the overexpressed genes linked to inflammation. Indeed, it has been shown very recently that serpins (in particular SERPINB2) promote cancer cell survival and vascular co-option in brain metastasis [64]. Vascular cooption, a phenomenon potentially related to angiotropism and pericytic mimicry, is until now regarded as an alternative tumor blood supply [65].
Furthermore, the 15 most significantly enriched functional groups analyzed by IPA included: development, cell movement, cancer, and embryonic development. These data suggest that the interaction between angiotropic MCs and ECs up regulates specific NC/embryonic/MSC programs during EVMM.
Relevant to “pericytic mimicry” of angiotropic melanoma cells was the pericytic location of angiotropic melanoma cells. In addition, PDGFB was up regulated during the co-culture of endothelial cells and melanoma cells, and the ligand/receptor pair platelet-derived growth factor β (PDGFB/PDGFRB) is essential for pericyte recruitment. Pericytes express PDGFRB, and in normal angiogenesis, the recruitment of pericytes will stabilize neovessels [39]. Melanoma cells are known to be able to express this same receptor, and in the present study, PDGFRB was expressed by angiotropic melanoma cells in several human samples [61] (Fig. 4c). Cancer stem cells are more resistant to therapeutics, and interestingly resistance to (V600E) B-RAF kinase inhibitor, this resistance emerges in part by melanoma cells upregulating PDGFRB [66]. Preliminary results have recently suggested that angiotropic melanoma cells expressing PDGFRB may represent a subpopulation of metastatic melanoma cells having a role in BRAF resistance and disease progression [67] (Fig. 4d).
Two other markers of pericytes/MSC, CD146 and NG2 were expressed by melanoma cells, including angiotropic melanoma cells, but were not specific for angiotropism in the examined samples. Finally, flow cytometry demonstrated the expression of pericytes/MSC markers (CD44, CD73, CD105 and CD144) by the C8161 melanoma cell line.
Taken together, these data support the concept of “pericytic mimicry” by angiotropic melanoma cell, and suggest that the interaction between MCs and the abluminal vascular surface induces the expression of genes linked to cancer migration, embryonic/stem cell properties and inflammation.
Vasculogenic Mimicry Versus Pericytic Mimicry
As described above in our 3D in vitro culture and co-culture studies, we observed a switch between vasculogenic mimicry when the melanoma cells were cultivated alone, and pericytic mimicry when the same melanoma cells were cultivated with endothelial cells. Vasculogenic mimicry is a well-studied and well-recognized phenomenon of melanoma (and other tumors) plasticity, which is independent of tumor angiogenesis [63]. Tumor cells capable of vasculogenic mimicry share a plastic, transendothelial phenotype, which may be induced by hypoxia and may provide a perfusion pathway, transport fluid from leaky vessels, and/or connect with traditional, endothelial-lined vasculature. On human samples, the histopathological recognition of vasculogenic mimicry in human specimens is still difficult due to the lack of specific markers [68]. Alternatively, pericytic mimicry may represent a different type of tumor plasticity, potentially leading to the migration of angiotropic melanoma cells along the abluminal surfaces of vessels or EVMM. As opposed to vasculogenic mimicry, pericytic mimicry imply contact of melanoma cells with the abluminal surface of vessels [15] (Figs. 1, 3 and 4), which may constitute a vascular niche inducing and/or sustaining stemness properties of melanoma cells. It is conceivable that vasculogenic mimicry precedes pericytic mimicry in order to reach the vessel surfaces in some microenvironment circumstances. Histologically, pathologist can recognize angiotropism [69] (Fig. 1), but the identification of specific markers, such as PDGFRB or Treacle, is needed to facilitate its reliable recognition.
Ultraviolet Radiation-Induced Inflammation Promotes Angiotropism and Metastasis in Melanoma
Recently we emphasized the presence of angiotropism in immunocompetent mice HGF-CDK4 (R24C) with melanoma skin transplants that spontaneously develop lung metastases [17]. Repetitive UV exposure of the back skin comprising the tumor transplantation site of HCmel12 melanomas did not affect local growth kinetics but increased angiotropic invasion and lung metastases when compared to non-irradiated controls. Similar results were obtained with a second, independently generated serial skin transplant HCmel31. It was shown that UV irradiation not only causes tumor-initiating genomic alterations in melanocytes but also promotes pericytic mimicry and metastatic dissemination through TLR4/MyD88-driven neutrophilic inflammation initiated by HMGB1 release from UV-damaged keratinocytes. These results indicate that neutrophil-rich inflammatory response increases angiotropism, pericytic mimicry and metastasis. These data also suggest that other sources of inflammation may as well increase angiotropism, such as the presence of ulceration [17] and the development of recurrent melanoma after resistance to anti-BRAF therapy [67] (Fig. 4d).
In addition, our data on genes differentially expressed during the interaction of angiotropic melanoma cells with the abluminal vascular surface have shown several genes directly linked to inflammation [61] (Table 2). This new data raises important questions about whether inflammation is cause or the result of such gene expression in angiotropic melanoma.
Angiotropism and Migration Along Vessels in Other Tumors
-
1.
Glioma and glioblastoma
Glial cells like melanocytes have the same embryonic origin, both deriving from the NCC. Invading glioma cells are known to follow distinct anatomic structures within the central nervous system, including the abluminal surface of blood vessels, exhibiting the same phenotypic pericytic mimicry as angiotropic melanoma cells [70, 71]. Glioma cells intercalate their processes between the endothelial cells and the perivascular astrocyte end feet, but do not invade into the blood vessel lumen cells [70]. Glioma cells can migrate considerable distances and this migration does not correlate with hematogenous tumor spread [71]. In previous studies using a murine brain tumor model we have shown that melanoma cells and glioblastoma cells exhibited the same pattern of invasion along vessels [72]. Interestingly, several authors have suggested that this highly infiltrative nature of human gliomas may recapitulate the migratory behavior of glial progenitors [73, 74]. In addition, a recent study demonstrated that “glioblastoma stem cells generate vascular pericytes” [75], further supporting the concept of pericytic mimicry [76].
-
2.
Detection of EVMM in pancreatic cancer
Notably, the perivascular localization of malignant tumor cells along the celiac trunk in patients with pancreatic carcinoma has been demonstrated [77]. This study described extension of pancreatic cancer along major vessels to sites remote from the primary pancreatic neoplasm. The presence of pancreatic carcinoma cells along the abluminal surfaces of the celiac trunk without intravasation was confirmed by endoscopic ultrasound fine-needle aspiration. It is important to note that pancreatic cells do not originate from NCC. The authors concluded that some cancer cells might travel along the external surface of vessels as a mechanism of dissemination consistent with EVMM.
-
3.
Angiotropism and neurotropic EVMM of human prostate cancer cells
Perineural invasion is emerging as an important pathologic feature of many malignancies, including melanoma, and malignant tumor of pancreas, colon and rectum, prostate, head and neck, biliary tract, and stomach [78]. We have investigated angiotropism in human prostate cancers, and we have used the PC-3 prostate cancer cells in our 3D co-culture model as well as in the CAM assay [79]. Histologically, angiotropism was detected in all human tumors at least focally within the tumor or at the advancing front of the tumor. In vitro, the PC-3 cells spread along the external surface of the vascular tubules; in vivo, PC-3 cells formed a cuff around some vessels a few millimeters beyond the tumor, and angiotropism was confirmed by histopathology. These data raises the possibility that some prostate tumor cells may migrate along the external surface of vessels as a mechanism of spread or EVMM.
-
4.
EVMM in gynecological carcinosarcomas
In a recent independent study, angiotropism (called EVMM by the authors) was observed in an ovarian carcinosarcoma during routine diagnostic assessment [80]. Twenty-three additional, randomly selected gynaecological carcinosarcomas (11 tubo-ovarian and 12 endometrial) were examined retrospectively and angiotropism was identified in 3 of these. Angiotropic malignant cells appeared sarcomatoid and were distributed abluminally, partly or completely surrounding the endothelium. Affected vessels often showed mural fibrin deposition. Immunohistochemistry for α-smooth muscle actin (SMA), CD31, CD34, D2-40, laminin and type IV collagen was performed on the EVMM-positive cases. The perivascular malignant cells showed more consistent SMA and laminin immunoreactivity than the non-vascular tumor elements.
-
5.
Pericytes and Perivascular tumors
The 2002 WHO classification of Soft Tissue Tumors recognizes two perivascular (pericytic) tumor types: glomus tumor and myopericytoma. Hemangiopericytoma (HPC) has also been considered to be a perivascular tumor. However, because of overlap with solitary fibrous tumor (SFT), HPC is now classified as a ’fibroblastic / myofibroblastic tumor. Both glomus tumor and myopericytoma are for the most part benign, subcutaneous tumors with a prominent perivascular growth pattern. In contrast, hemangiopericytoma / extrapleural SFT is comprised of more immature appearing ovoid to spindled stromal cells, and are most commonly found in the deep soft tissues [81].
Finally, neoplasms with perivascular epithelioid cell differentiation (PEComas) are defined by The World Health Organization as “a mesenchymal tumor composed of histologically and immunohistochemically distinctive perivascular epithelioid cells” [82], which have no known normal tissue counterpart. It has been suggested that PEComa may originate from (i) undifferentiated cells of the neural crest; (ii) myoblastic, smooth muscle cells; and (iii) pericytes.
Recent Data Using Imaging of Melanoma Cells in a Murine Model
We began imaging studies using a murine melanoma ear skin model. The murine ear is a very thin organ, and represents an interesting model to observe the relationships between tumor cells and vessels [83].
In this model, human GFP-expressing melanoma cells were transplanted subcutaneously into the pinnae of immune-deficient nude mouse ears. The cells were allowed to grow and to expand for 6 days. Before the recipient mice were sacrificed, a red-fluorescently labeled Tomato Lectin conjugate (Dylight 594) was injected in the circulatory system to visualize the overall ear vasculature. Because the ear skin is very thin, the engrafted cells were imaged directly using a confocal microscope to visualize the cellular interactions of the melanoma cells with the ear vasculature.
Our preliminary results have shown the perivascular cuffing of spreading tumor cells (Figure xx). Moreover, at the advancing front of the main perivascular cuffing, isolated melanoma cells were observed along tumor vessels (Fig. 5). These observations strongly suggest the propensity of tumor cells to spread along the abluminal surface of vessels.
Imaging of human melanoma cells in nude mice 6 days post-implantation in the ear a Representative confocal image of clusters of GFP melanoma cells (green) spreading along vessels 6 days post-implantation inside the nude mouse ear. Blood vessels and ear capillaries are stained red with a 594-lectin conjugate. b Close-up of the area shown in the white box in (A). Note isolated melanoma cells in a pericytic location (white arrows). c Schematic representing migrating melanoma cell (green) interactions (white arrows in B) with ear blood vessels (red). Scale bar is 100 μm
We are actively pursuing this approach in a live animal to follow the long-term behavior of melanoma cells longitudinally (i.e. over time) at the single-cell level in a morphologically intact organ’s microenvironment (manuscript in preparation).
Conclusions and Objectives
As described in this review, accumulating evidence supports angiotropism, pericytic mimicry and EVMM as an important biological phenomenon and means of melanoma (and other tumor) metastasis. Our objectives in pursuing this research are (i) to elucidate the molecular mechanisms underlying this migratory phenomenon; (ii) to facilitate the reliable recognition of angiotropism, particularly via the development of more specific biomarkers; (iii) to identify new molecules implicated in pericytic mimicry and EVMM, with the ultimate goal of targeting this specific association of tumor cells with endothelial cells, which represents a promising strategy for reducing or preventing melanoma and potentially other tumor metastasis.
References
Talmadge JE, Fidler IJ (2010) AACR centennial series: the biology of cancer metastasis: historical perspective. Cancer Res 70:5649–5669
Orgaz JL, Sanz-Moreno V (2013) Emerging molecular targets in melanoma invasion and metastasis. Pigment Cell Melanoma Res 26:39–57
Fang D, Nguyen TK, Leishear K, Finko R, Kulp AN, Hotz S, Van Belle PA, Xu X, Elder DE, Herlyn M (2005) A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res 65:9328–9337
Fukunaga-Kalabis M, Roesch A, Herlyn M (2011) From cancer stem cells to tumor maintenance in melanoma. J Invest Dermatol 131:1600–1604
Girouard SD, Murphy GF (2011) Melanoma stem cells: not rare, but well done. Lab Invest 91:647–664
Bailey CM, Morrison JA, Kulesa PM (2012) Melanoma revives an embryonic migration program to promote plasticity and invasion. Pigment Cell Melanoma Res 25:573–583
Wilder RJ (1956) The historical development of the concept of metastasis. J Mt Sinaï Hosp 23:728–734
Sleeman JP, Cady B, Pantel K (2012) The connectivity of lymphogenous and hematogenous tumor cell dissemination: biological insights and clinical implications. Clin Exp Metastasis 29:737–746
Talmadge JE, Fidler IJ (2010) AACR centennial series: the biology of cancer metastasis: historical perspective. Cancer Res 70(14):5649–69, Jul 15
Paterlini-Bréchot P. (2011). Organ-specific markers in circulating tumor cell screening: an early indicator of metastasis-capable malignancy. Future Oncol. Jul;7 (7):849–71. doi: 10.2217/fon.11.32. Review. PubMed PMID: 21732757.
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100:57–70
Lugassy C, Eyden BP, Christensen L, Escande JP (1997) Angio-tumoral complex in human malignant melanoma characterised by free laminin: ultrastructural and immunohistochemical observations. J Submicrosc Cytol Pathol 29(1):19–28
Lugassy C, Barnhill RL, Christensen L (2000) Melanoma and extravascular migratory metastasis. J Cutan Pathol 27(9):481
Lugassy C, Barnhill RL (2004) Angiotropic malignant melanoma and extravascular migratory metastasis: description of 36 cases with emphasis on a new mechanism of tumour spread. Pathology 36:485–490
Lugassy C, Péault B, Wadehra M, Kleinman HK, Barnhill RL (2013) Could pericytic mimicry represent another type of melanoma cell plasticity with embryonic properties? PigmentCell Melanoma Res 26(5):746–54
Friedl P, Wolf K (2003) Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer 3:362–374
Bald T, Quast T, Landsberg J, Rogava M, Glodde N, Lopez-Ramos D, Kohlmeyer J, Riesenberg S, van den Boorn-Konijnenberg D, Hömig-Hölzel C, Reuten R, Schadow B, Weighardt H, Wenzel D, Helfrich I, Schadendorf D, Bloch W, Bianchi ME, Lugassy C, Barnhill RL, Koch M, Fleischmann BK, Förster I, Kastenmüller W, Kolanus W, Hölzel M, Gaffal E, Tüting T (2014) Ultraviolet radiation-induced inflammation promotes angiotropism and metastasis in melanoma. Nature 507(7490):109–13
Van Es SL, Colman M, Thompson JF, McCarthy SW, Scolyer RA (2008) Angiotropism is an independent predictor of local recurrence and in-transit metastasis in primary cutaneous melanoma. Am J Surg Pathol 32(9):1396–403
Levy MJ, Gleeson FC, Zhang L (2009) Endoscopic ultrasound fine-needle aspiration detection of extravascular migratory metastasis from a remotely located pancreatic cancer. Clin Gastroenterol Hepatol 7(2):246
Zbytek B, Carlson JA, Granese J, Ross J, Mihm MC, Slominski A (2008) Current concepts of metastasis in melanoma. Expert Rev Dermatol 3(5):569–585
Arias AM (2001) Epithelial mesenchymal interactions in cancer and development. Cell 105:425–431
Lugassy C, Barnhill RL. Angiotropic melanoma and extravascular migratory metastasis: a review. Adv Anat Pathol. May;14 (3):195–201 Review
Thiery JP, Acloque H, Huang RY, Nieto MA (2009) Epithelial-mesenchymal transitions in development and disease. Cell 139(5):871–90
Le Douarin NM, Creuzet S, Couly G, Dupin E (2004) Neural crest cell plasticity and its limits. Development 131:4637–4650
Perris R, Perissinotto D (2000) Role of the extracellular matrix during neural crest cell migration. Mech Dev 95:3–21
Schwarz Q, Maden CH, Vieira JM, Ruhrberg C (2009) Neuropilin 1 signaling guides neural crest cells to coordinate pathway choice with cell specification. PNAS 106:6164–6169
Nagy N, Mwizerwa O, Yaniv K, Carmel L, Pieretti-Vanmarcke R, Weinstein BM, Goldstein AM (2009) Endothelial cells promote migration and proliferation of enteric neural crest cells via beta1 integrin signaling. Dev Biol 330:263–272
Hendrix MJ, Seftor EA, Seftor RE, Kasemeier-Kulesa J, Kulesa PM, Postovit LM (2007) Reprogramming metastatic tumour cells with embryonic microenvironments. Nat Rev Cancer 7(4):246–55
Kulesa PM, Kasemeier-Kulesa JC, Teddy JM, Margaryan NV, Seftor EA, Seftor RE, Hendrix MJ (2006) Reprogramming metastatic melanoma cells to assume a neural crest cell-like phenotype in an embryonic microenvironment. Proc Natl Acad Sci U S A 103:3752–3757
Weiss L (1986) Metastatic inefficiency: causes and consequences. Cancer Rev 3:1–24
Poste G, Fidler IJ (1980) The pathogenesis of cancer metastasis. Nature 283:139–146
Talmadge JE, Fidler IJ (2010) AACR centennial series: the biology of cancer metastasis: historical perspective. Cancer Res 70:5649–5669
Paget S (1889) The distribution of secondary growths in cancer of the breast. Lancet 133:571–573
Fidler IJ (2002) The organ microenvironment and cancer metastasis. Differentiation 70:498–505
Drerup CM, Wiora HM, Topczewski J, Morris JA (2009) Disc1 regulates foxd3 and sox10 expression, affecting neural crest migration and differentiation. Development 136:2623–2632
Li A, Ma Y, Yu X et al (2011) Rac1 drives melanoblast organization during mouse development by orchestrating pseudopod-driven motility and cell-cycle progression. Dev Cell 21:722–734
Hirschi KK, Rohovsky SA, D’Amore PA (1998) PDGF, TGF-beta, and heterotypic cell-cell interactions mediate endothelial cell-induced recruitment of 10 T1/2 cells and their differentiation to a smooth muscle fate. J Cell Biol 141:805–814
Risau W (1997) Mechanisms of angiogenesis. Nature 386:671–674
Armulik A, Abramsson A, Betsholtz C (2005) Pericyte recruitment during angiogenesis. Circ Res 97:512–523
Crisan M, Yap S, Casteilla L et al (2008) A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3:301–313
Yurchenco PD, Patton BL (2009) Developmental and pathogenic mechanisms of basement membrane assembly. Curr Pharm Des 15:1277–1294
Giannelli G, Falk-Marzillier J, Schiraldi O, Stetler-Stevenson WG, Quaranta V (1997) Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science 277:225–228
Barnhill RL, Dy K, Lugassy C (2002) Angiotropism in cutaneous melanoma: a prognostic factor strongly predicting risk for metastasis. J Invest Dermatol 119:705–706
Wilmott J, Haydu L, Bagot M, Zhang Y, Jakrot V, McCarthy S, Lugassy C, Thompson J, Scolyer R, Barnhill R (2012) Angiotropism is an independent predictor of microscopic satellites in primary cutaneous melanoma. Histopathology 61:889–898
Lugassy C, Lazar V, Dessen P, van den Oord JJ, Winnepenninckx V, Spatz A, Bagot M, Bensussan A, Janin A, Eggermont AM, Barnhill RL (2011) Gene expression profiling of human angiotropic primary melanoma: selection of 15 differentially expressed genes potentially involved in extravascular migratory metastasis. Eur J Cancer 47:1267–1275
Lugassy C, Kleinman HK, Fernandez PM, Patierno SR, Webber MM, Ghanem G, Spatz A, Barnhill RL (2002) Human melanoma cell migration along capillary-like structures in vitro: a new dynamic model for studying extravascular migratory metastasis. J Invest Dermatol 119:703–704
Lugassy C, Kleinman HK, Engbring JA, Welch DR, Harms JF, Rufner R, Ghanem G, Patierno SR, Barnhill RL (2004) Angiotropism and pericyte-like location of GFP melanoma cells: ex vivo and in vivo studies of extravascular migratory metastasis. Am J Pathol 164:11911198
Zadran S, McMickle R, Shackelford D, Kleinman H, Barnhill R, Lugassy C (2013) Monitoring extra-vascular migratory metastasis (EVMM) of migrating cancer cells using an in vitro co-culture system. Protoc exch 22:2013
Lugassy C, Kleinman HK, Vernon SE, Welch DR, Barnhill RL (2007) C16 laminin peptide increases angiotropic extravascular migration of human melanoma cells in a shell-less chick chorioallantoic membrane assay. Br J Dermatol 157:780–782.6
Winnepenninckx V, Lazar V, Michiels S et al (2006) Gene expression profiling of primary cutaneous melanoma and clinical outcome. J Natl Cancer Inst 98(7):472–82
Dixon J, Jones NC, Sandell LL et al (2006) Tcof1/Treacle is required for neural crest cell formation and proliferation deficiencies that cause craniofacial abnormalities. Proc Natl Acad Sci U S A 103(36):13403–8
Duband JL, Thiery JP (1987) Distribution of laminin and collagens during avian neural crest development. Development 101(3):461–78
Engbring JA, Kleinman HK (2003) Extracellular matrix and malignancy. J Pathol 200:465–470
Tzu J, Marinkovich MP (2008) Bridging structure with function: structural, regulatory, and developmental role of laminins. Int J Biochem Cell Biol 40(2):199–214
Durbeej M (2010) Laminins. Cell Tissue Res 339(1):259–68
Lugassy C, Shahsafaei A, Bonitz P, Busam KJ, Barnhill RL (1999) Tumor microvessels in melanoma express the beta-2 chain of laminin Implications for melanoma metastasis. J Cutan Pathol 26:222–226
Lugassy C, Dickersin GR, Christensen L et al (1999) Ultrastructural and immunohistochemical studies of the periendothelial matrix in human melanoma: evidence for an amorphous matrix containing laminin. J Cutan Pathol 26:78–83
Nomizu M, Kuratomi Y, Song SY et al (1997) Identification of cell binding sequences in mouse laminin gamma1 chain by systematic peptide screening. J Biol Chem 272:32198–32205
Kuratomi Y, Nomizu M, Tanaka K et al (2002) Laminin gamma 1 chain peptide, C-16 (KAFDITYVRLKF), promotes migration, MMP-9 secretion, and pulmonary metastasis of B16-F10 mouse melanoma cells. Br J Cancer 86:1169–1173
Lugassy C, Torres-Muñoz JE, Kleinman HK, Ghanem G, Vernon SE, Barnhill RL (2009) Over-expression of malignancy-associated laminins and laminin receptors by angiotropic human melanoma cells in a chick chorioallantoic membrane model. J Cutan Pathol 36(12):1237–43
Lugassy C, Wadehra M, Li X, Corselli M, Akhavan D, Binder SW, Péault B, Cochran AJ, Mischel PS, Kleinman HK, Barnhill RL (2013) Pilot study on “pericytic mimicry” and potential embryonic/stem cell properties of angiotropic melanoma cells interacting with the abluminal vascular surface. Cancer Microenviron 6:19–29
Kubota Y, Kleinman HK, Martin GR, Lawley TJ (1988) Role of laminin and basement membrane in the morphological differentiation of human endothelial cells into capillary-like structures. J Cell Biol 107:1589–1598
Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, Pe’er J, Trent JM, Meltzer PS, Hendrix MJ (1999) Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol 155:739–752
Valiente M, Obenauf AC, Jin X, Chen Q, Zhang XH, Lee DJ, Chaft JE, Kris MG, Huse JT, Brogi E, Massagué J (2014) Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell 156(5):1002–16
Donnem T, Hu J, Ferguson M, Adighibe O, Snell C, Harris AL, Gatter KC, Pezzella F (2013) Vessel co-option in primary human tumors and metastases: an obstacle to effective anti-angiogenic treatment? Cancer Med 2(4):427–36
Shi H, Kong X, Ribas A, Lo RS (2011) Combinatorial treatments that overcome PDGFRb-driven resistance of melanoma cells to V600EB-RAF inhibition. Cancer Res 71:5067–5074
Lugassy C, Scolyer R, Long G, Menzies A, Mischel P, Barnhill RL (2014) PDGFBR expression in anti-BRAF resistant melanoma: are angiotropic melanoma cells a source of BRAF resistance and disease progression? J Cutan Pathol 41:159–160
Seftor RE, Hess AR, Seftor EA, Kirschmann DA, Hardy KM, Margaryan NV, Hendrix MJ (2012) Tumor cell vasculogenic mimicry: from controversy to therapeutic promise. Am J Pathol 181:1115–1125
Barnhill RL, Busam KJ, From L, Bagot M, Lugassy C, Berwick M (2011) Inter-observer concordance for the recognition of angiotropism in human melanoma. Pigment Cell Melanoma Res 24(3):582–3
Farin A, Suzuki SO, Weiker M, Goldman JE, Bruce JN, Canoll P (2006) Transplanted glioma cells migrate and proliferate on host brain vasculature: a dynamic analysis. Glia 53:799–808
Wesseling P, Ruiter DJ, Burger PC (1997) Angiogenesis in brain tumors: pathobiological and clinical aspects. J Neurooncol 32:253–265
Lugassy C, Haroun RI, Brem H, Tyler BM, Jones RV, Fernandez PM, Patierno SR, Kleinman HK, Barnhill RL (2002) Pericytic-like angiotropism of glioma and melanoma cells. Am J Dermatopathol 24(6):473–8
Dirks PB (2001) Glioma migration: clues from the biology of neural progenitor cells and embryonic CNS cell migration. J Neurooncol 53:203–212
Suzuki SO, Kitai R, Llena J, Lee SC, Goldman JE, Shafit-Zagardo B (2002) MAP-2e, a novel MAP-2 isoform, is expressed in gliomas and delineates tumor architecture and patterns of infiltration. J Neuropathol Exp Neurol 61:403–412
Cheng L, Huang Z, Zhou W et al (2013) Glioblastoma stem cells generate vascular pericytes to support vessel function and tumor growth. Cell 153:139–152
Lugassy C, Barnhill RL, Kleinman HK. (2013) Comment on line: Omission of references to prior work related to Cell publication: “Cheng et al. Glioblastoma stem cells generate vascular pericytes to support vessel function and tumor growth”. Cell. July http://www.cell.com/abstract/S0092-8674(13)00210-9#Comments.
Levy MJ, Gleeson FC, Zhang L (2009) Endoscopic ultrasound fine-needle aspiration detection of extravascular migratory metastasis from a remotely located pancreatic cancer. Clin Gastroenterol Hepatol 7:246–248
Liebig C, Ayala G, Wilks JA, Berger DH, Albo D (2009) Perineural invasion in cancer: a review of the literature. Cancer 115(15):3379–91
Lugassy C, Vernon SE, Warner JW, Le CQ, Manyak M, Patierno SR, Barnhill RL (2005) Angiotropism of human prostate cancer cells: implications for extravascular migratory metastasis. BJU Int 95(7):1099–103
Dyke JM, Crook ML, Platten M, Stewart CJ (2014) Extravascular migratory metastasis in gynaecological carcinosarcoma. Histopathology 65(3):363–70
Mravic M, Asatrian G, Soo C, Lugassy C, Barnhill RL, Dry SM, Peault B, James AW (2014) From pericytes to perivascular tumors: correlates between pathology, stem cell biology, and tissue engineering. Int Orthop 38(9):1819–24
Martignoni G, Pea M, Reghellin D, Zamboni G, Bonetti F (2008) PEComas: the past, the present and the future. Virchows Arch 452(2):119–32
Hirayama R, Sato K, Hirokawa K, Chang MP, Mishima Y, Makinodan T (1984) Different metastatic modes of malignant melanoma implanted in the ear of young and old mice. Cancer Immunol Immunother 18(3):209–14
Acknowledgments
In vivo confocal imaging shown in Fig. 5 was performed at the California NanoSystems Institute (CNSI) Advanced Light Microscopy/Spectroscopy Shared Resource Facility at UCLA with support from a NIH/National Center for Advancing Translational Science UCLA CTSI Grant (UL1TR000124).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lugassy, C., Zadran, S., Bentolila, L.A. et al. Angiotropism, Pericytic Mimicry and Extravascular Migratory Metastasis in Melanoma: An Alternative to Intravascular Cancer Dissemination. Cancer Microenvironment 7, 139–152 (2014). https://doi.org/10.1007/s12307-014-0156-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12307-014-0156-4