Abstract
The microenvironment plays an important role in tumorigenesis. Fibroblast activation protein alpha (FAP) is overexpressed by fibroblasts present in the microenvironment of many tumors. High FAP expression is a negative prognostic factor in several malignancies, but this has not been investigated in epithelial ovarian cancer (EOC). The aim of this study is to define the value of FAP in EOC. Immunohistochemical staining using an anti-FAP antibody was performed on 338 EOC tissues. mRNA levels in cancer cell lines and FAP silencing using siRNA was also done. FAP immunoexpression by tumor stroma was a significant predictive factor for platinum resistance (p = 0.0154). In survival analysis of days to recurrence, FAP stoma+ was associated with shorter recurrence than those with FAP− stroma (p = 0.0247). In 21.8 % of tumors, FAP protein was expressed by the tumor epithelium, and FAP mRNA was more highly expressed in tumors (n = 489) than in normal tissues (n = 8) (p = 3.88 × 10−4). In vitro, addition of FAP to EOC cells induced a 10–12 % increase in cell viability both in the presence and absence of cisplatin. Conversely, siRNA silencing of FAP resulted in ~10 % reduction in EOC cell proliferation. We have shown that FAP expression in EOC is associated with poorer clinical outcomes. FAP may have novel cell-autonomous effects suggesting that targeting FAP could have pleiotropic anti-tumor effects, and anti-FAP therapy could be a highly effective novel treatment for EOC, especially in cisplatinum-resistant cases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Historically, studies on the underlying biology of most malignancies have focused on the epithelial component of tumors. However, there is increasing awareness of the necessity to understand a tumor within the context of its surroundings, the so called “tumor microenvironment”. The tumor and its microenvironment have dynamic and reciprocal interactions and signaling that affect various processes including survival, growth, proliferation, metastasis, and chemoresistance [1, 2]. Activated fibroblasts, also known as cancer-associated fibroblasts (CAFs), can promote tumorigenesis through multiple mechanisms including increased angiogenesis, proliferation, and invasion [1–4]. Moreover, CAFs regulate the dynamic interactions between tumor cells, extracellular matrix and other cell types in the tumor stroma, including endothelial, adipose tissue, and inflammatory cells. The origins of CAFs have been explored in some detail, and it appears that CAFs may derive from multiple different cellular origins. These include normal fibroblasts, which transdiffentiate into CAFs through genetic and transcriptomic changes induced by adjacent carcinoma cells; from epithelial cells through epithelial-mesenchymal transition (EMT); from bone marrow derived circulating cells; and from endothelial cells through endothelial-mesenchymal transition [for more details see ref 5, 6].
CAFs acquire perpetually activated phenotypes that are identified by the expression of fibroblast activation protein α (FAP) [7]. FAP is a type II integral membrane serine protease of the prolyl oligopeptidase family [8]. FAP expression is not typically expressed in normal tissue, but FAP is expressed by fibroblasts in wound healing and granulation, as well as on mesenchymal stem cells derived from human bone marrow [9]. FAP is expressed in the stroma of more than 90 % of human cancers of epithelial origin, and its overexpression has been associated with poor prognosis in multiple cancer types, including pancreatic, hepatocellular, colon, and gastrointestinal cancers [10–13]. Numerous reports have detailed the non-cell autonomous signaling pathways through which FAP promotes epithelial carcinogenesis, and it is clear that FAP has pleiotropic effects during tumor development and dissemination [for details see review in ref 14]. FAP expression by the tumor stroma can promote tumor cell invasion through its gelatinase activity [15], as well as enhancing tumor cell proliferation and migration [16, 17]. In in vivo models stromal FAP expression is associated with increased tumor incidence, growth and microvessel density [18, 19].
EOC accounts for 80–90 % of all malignant ovarian tumors. It is the leading cause of death in the United States in women diagnosed with gynecologic malignancies, with 22,240 new cases and 14,030 estimated deaths from the disease in 2012 [20]. Primary tumor debulking (cytoreduction) followed by cisplatinum based chemotherapy is considered the standard of care for patients with advanced stage EOC [21]. However, patients usually develop chemoresistant tumor recurrence, which is the main reason for the poor prognosis and high mortality rates associated with ovarian cancer. The supportive stromal network has been speculated to play a role in this ovarian cancer chemoresistance [22]. In the present study, we evaluated the biological and prognostic role of FAP in stromal and epithelial components of EOC using a combination of immunohistochemistry in primary surgical specimens from patients with EOC, and in vitro analysis in in vitro models of ovarian cancer.
Materials and Methods
Patient Population
Three hundred thirty eight cases were available for evaluation from our department. After Institutional Review Board approval was obtained, the pathology archives and medical records were searched for the following: age, clinical stage, time of initial diagnosis, and disease status [alive with evidence of disease (AWED), alive no evidence of disease (ANED) and dead of disease (DOD)] at last follow-up after the initial diagnosis. Three disease outcomes were considered, recurrence, platinum-resistance and death. Recurrence was defined as evidence of disease seen on CT scan or by CT scan and CA125 levels. Platinum-resistance was defined as disease recurrence within a year of surgery or persistent disease despite therapy.
Tumor Histology, Tumor Microarray Construction and Immunohistochemistry
Hematoxylin and eosin slides were reviewed by a pathologist (PMF) to assess tumor histologic subtype, tumor stage and tumor grade. Tumor grade was evaluated based on Silverberg’s grading system [23]. This system is based on architecture, cytologic atypia and mitotic figures. Scores were calculated and the tumors divided into 3 grading categories, well differentiated (G1), moderately differentiated (G2) and poorly differentiated (G3).
For tumor microarray (TMA) construction, paraffin-embedded tissues from EOC cases were used as described previously [24]. Briefly, morphologically representative regions were carefully selected on each individual paraffin-embedded block (donor blocks) and a core tissue biopsy of 1 mm was punched and transferred to the receiver paraffin-embedded block. Taking into account tumor heterogeneity and tissue loss, 3 core punch biopsies taken from different areas of each tumor was obtained. One section was stained with hematoxylin and eosin to evaluate the presence of the tumor by light microscopy. The final array included 338 EOC cases.
For immunohistochemical (IHC) analysis, 4 μm thick sections were deparaffinized with xylene, and washed with ethanol. Sections were cooled for 20 min then incubated 10 min with 3 % H2O2 to quench endogenous peroxidase activity. Blocking was performed using serum-free protein block, Dakocytomation (Carpenteria, CA), for 30 min. The sections were pretreated with an EDTA buffer saline solution, microwaved for 20 min, and then incubated with an anti-FAP α antibody (LS-A8023; polyclonal, 1:100 dilution, Life Span BioSciences, WA-USA) for 1 h at room temperature. The diaminobenzidine complex was used as a chromogen. Breast and colon cancer were used as positive controls for FAP. Negative control slides (incubated with secondary antibody only) were included in all assays. Two pathologists (PMF, MS) evaluated the slides using a double-headed microscope. The activated fibroblasts were defined morphologically as large spindle shaped mesenchymal cells and on IHC as positive for FAP-α as previously defined [25]. This evaluation was performed twice, separated by a one-month period. The percentage was assessed as follows: 0 %, <10 %, 11–50 %, 51–100 % and the intensity as 0, weak (1+), moderate (2+) and strong (3+). Because the percentage did not change between fields, only the intensity was considered for statistical evaluation. Whenever there were discrepancies among the three scores in any given case, the higher intensity was taken as the final score. The score of the first assessment and the second assessment was reviewed and when there was a discrepancy in scoring, a consensus was reached.
TMA Validation Study
Many studies have validated the use of TMA for biomarker evaluation of tumor expression and results showed that 2 to 3 core biopsies were accurate representation of the whole section for epithelial biomarkers [26]. Whether the same number of cores of tumor stroma will also yield results that represent the whole section is not yet known and so for this study, we elected to validate the TMA results of stromal FAP expression. We therefore analyzed FAP expression on whole section of 51 random cases that were also included in our TMA. In total 6/51 cases (11.7 %) were discordant on TMA in comparison to the whole sections. 2/51 (3.9 %) had minor discordance and 4/51 cases (7.8 %) had major discordance. Minor discordance was defined as positive staining on both TMA and whole section but with different degree of staining intensity and major discordance was defined as negative staining on TMA and positive on whole section. Unsurprisingly, we noted that the discordant cases tended to have focal staining within the whole section. These results are similar to what has previously been reported for epithelial cancer biomarkers [26], therefore TMAs are also a reliable approach to the evaluation of tumor stroma markers.
Statistical Analysis
Statistical analyses were performed by R (http://www.r-project.org/). Recurrence-free survival (RFS) was measured as the elapsed time from date of surgery to the time of recurrence or the day of last visit (as right-censored), and overall survival (OS) was measured from the time of last surgery to the time of death or the day of last visit (as right-censored). Logistic regression was performed to test the association between categorical variables and disease outcomes. For the 139 patients with non-missing values of covariates, the Cox proportional hazard regression was used to compare the recurrence time and survival time difference between patients groups. All reported p-values are two sided. P-values were considered significant if < 0.05.
Analysis of the Cancer Genome Atlas Data
Normalized expression data for 489 TCGA (The Cancer Genome Atlas) high-grade serous ovarian cancers and 8 normal fallopian tubes were analyzed using R and packages therein. We selected the samples used in the following report (TCGA, Nature 2011). TCGA survival data were analyzed using the cBioportal, using the “Ovarian Serous adenocarcinoma (TCGA, Nature 2011)” dataset [27, 28]. In TCGA database the control tissues used are fallopian tubes. This is because in vitro and in vivo studies have shown that fallopian tube epithelial cells are common sites of origin for high grade serous ovarian cancer (HGSOC), which is the most common subtype and the only histotype included in TCGA [29, 30].
TMA of Ovarian Cancer Cell Cultures
We have previously described the creation of an ovarian cancer cell line tissue microarray [31]. Briefly, ovarian cancer cell lines were plated in 2D, or in 3D using cell culture dishes that had been pre-coated with polyHEMA (Sigma). 2D and 3D cultured cells were fixed for 30 min with neutral-buffered formalin, washed with PBS, and resuspended in 70 % ethanol. Fixed cells were mixed with 1 % melted agarose and transferred to a 10x10x5mm mold to create uniformly sized samples for use in the construction of a cell line TMA, using the methods described above.
Cell Culture and Proliferation Assays
CaOV3 cells were a kind gift from Dr. G Mills at MD Anderson and were grown in RPMI medium (Sigma) supplemented with 10 % FBS (PAA). For proliferation assays, 1000 cells were plated per well in 96 well plates and FAP (Novus Biologicals) and 0.1 mg/ml cisplatin (Sigma) added the following day. Control cells received vehicle (saline solution) alone. After 48 h, MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) was added at a concentration of 5 mg/mL (Sigma, in PBS). Cells were incubated for 3 h, the media aspirated and cells lysed using 100ul DMSO (Sigma). Absorbance was then read using a Mikrowin plate reader.
Knockdown Assay Protocol
FAP knockdown assays were performed using SHIN-3 epithelial ovarian cancer cells. SHIN-3 cells were cultured in DMEM/F12 media (Lonza) supplemented with 10 % fetal bovine serum (PAA Laboratories). 1000 cells were plated per well, in a 96 well plate. After 24 h, cells were transfected using Dharmafect 3 (Thermo Scientific) with a non-targeting or FAP targeting siRNA pool (ONTARGETplus, Open Biosystems). 48 h later, cell proliferation was assayed using PrestoBlue (Invitrogen).
Results
Patient Population and Tumor Characteristics
This study included of 338 patients diagnosed with primary EOC. The epidemiological and clinical characteristics of this population are given in Table 1 . Stratification of patients according to tumor histology, stage and grade was typical for a patient population diagnosed with primary EOC in the United States.
Expression of FAP in Primary EOC specimens
FAP expression was evaluated in 338 primary EOC specimens on tumor microarrays (TMAs); the results are summarized in Table 1 . Two hundred and seven cases (61 %) expressed FAP in the tumor stroma (FAP+ stroma); the remaining 33 % did not (FAP− stroma) and 6 % unknown. In the tumor epithelium, 70 cases (21 %) expressed FAP (FAP+ epithelium), 251 cases (74 %) stained negative (FAP− epithelium) and 5 % (unknown due to missing cores on the TMA). Almost all cases (n = 66) that expressed FAP in the epithelium also expressed showed FAP expression in the stroma; thus 141 cases (42 %) showed FAP expression only in the stroma. We found no evidence that FAP expression in the stroma only, or in both the stroma and epithelium to be associated with pathologic parameters such as tumor grade, tumor stage, and optimal debulking. Figure 1a–d shows examples of stroma and epithelial tissues exhibiting positive FAP expression.
Association of FAP Expression with Disease Outcomes
Table 2 summarizes the association of FAP stroma expression with clinical outcomes. The p values were computed by fitting the outcomes with univariate logistic regression. The adjusted p-values were computed by multivariate logistic regression controlling for tumor stage, tumor grade, and optimal debulking. Patients with missing values in either the outcome or the covariate are excluded from the analysis, which is why the sum of counts in Table 2 may not exactly match the sum in Table 1 . In multivariate analysis, FAP+ stroma expression was associated with platinum resistance (i.e. cases with FAP+ stroma were more likely to be resistant than FAP− stroma) [adjusted OR = 1.989, 95 % CI (1.141, 3.468), adjusted p = 0.0154]. For the 139 patients with non-missing values of covariates, Cox proportional regression was used for the survival analysis to compare the days to recurrence and days to death between patients groups. After adjusting for stage, grade and optimal debulking, analysis showed that FAP+ stroma was associated with higher recurrence rate (p = 0.0247). In addition, although the result did not reach statistical significance, survival analysis also showed that FAP+ stroma may be associated with shorter overall survival (p = 0.0685) (Fig 2a, b)
a Cox regression analysis revealed that, after adjusting for tumor grade, stage and tumor debulking surgery, patients with FAP stroma+ had significantly shorter recurrence compared to patients with FAP stroma− (p = 0.0247). b Cox regression analysis revealed that, after adjusting for tumor grade, stage and tumor debulking surgery, patients with FAP stroma+ had nearly significant shorter overall survival compared to patients with FAP stroma− (p = 0.0685)
The Role of FAP in Tumor Epithelial Cells
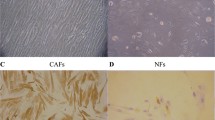
We examined FAP gene expression in The Cancer Genome Atlas data set of 489 high-grade serous EOCs and 8 normal fallopian tubes, and found that FAP expression was significantly higher in tumor tissues compared to normal controls (p = 3.88 × 10−4, Fig. 3a). The FAP locus displayed copy number gain or amplification in 34 % and 4.4 % of tumors respectively, and 4 cases harbored mutations in the FAP gene (2 missense and 2 splice variants). Moreover high expression of FAP was associated with worse overall survival (p = 0.013, Fig. 3b). We tested the ability of FAP to modulate the proliferation of the CaOV3 ovarian cancer cell line. The proliferation of CaOV3 cells increased by an average of 8.9 % when cultured with FAP (P > 0.02, two-tailed paired Student’s T-test). Moreover, in the presence of cisplatin plus FAP, CaOV3 cells were also significantly more proliferative than when incubated with cisplatin alone (P > 0.02, two-tailed paired Student’s T-test) (Fig. 3c) When we knocked-down the expression of FAP in the SHIN3 ovarian cancer cells using a FAP-specific small interfering RNA (siRNA) we observed a reduction in cell proliferation of 9–13 % compared to control cells transfected with a non-targeting siRNA pool (p = 0.013) (Fig. 3d). These data suggest that FAP may also play a role in ovarian cancer epithelial cells and so we sought to examine the function of FAP in in vitro cell biology models of EOC. Firstly, we used a tissue microarray containing 36 two-dimensional and three-dimensional cultured EOC cell lines, to verify that FAP can be expressed endogenously by EOC cells. Twelve (33%) of 2D cultured EOC cell lines and 9 (25%) of 3D cultured cell lines expressed FAP. The cell lines expressing FAP were of various histologies including low-grade serous (OAW42, positive in 2D and 3D) (Fig. 4a–b), mucinous (EFO27, positive in 2D but negative in 3D) (Fig. 4c–d), and high-grade serous (UWB.1289, negative in 2D and positive in 3D).
a Analysis of TCGA gene expression data reveals that the FAP gene is significantly overexpressed in high-grade serous ovarian cancers (HGSOC) compared to normal fallopian tubes (FT). b FAP overexpression was associated with poorer overall survival: median survival for patients with FAP overexpression was 37.91 months, compared to 44.29 months for patients without FAP overexpression. cThe proliferation of CaOV3 cells increased by an average of 8.9 % when cultured with FAP (P > 0.02, two-tailed paired Student’s T-test). Moreover, in the presence of cisplatin (cis-diamminedichloroplatinum(II), CDDP) plus FAP, CaOV3 cells were also significantly more proliferative than when incubated with cisplatin alone. d Transfection of FAP siRNAs in SHIN3 ovarian cancer cells led to a significant reduction in cell proliferation. Si-SCR = scrambled (not targeting) siRNA. * P > 0.05, two-tailed paired Student’s T-test, error bars show mean ± standard deviation
Discussion
CAFs have been implicated in all stages of tumor development, from initiation to progression to metastasis. Fibroblast activation protein alpha (FAP) is a marker of cancer associated fibroblasts (CAFs) and this marker is upregulated when CAF precursor cells are recruited into growing tumors [25, 32]. In our series of 338 ovarian cancer patients, FAP was frequently seen in fibroblasts associated stroma. We report, for the first time, that FAP expression is an independent predictor of platinum resistance and overall survival, suggesting that FAP expression could be used in the clinic to identify patients at risk for platinum resistance and poor prognosis. Since therapeutic options for EOC are so limited, these patients may be the best candidates for enrollment on clinical trials testing novel, potentially life-saving, experimental therapeutic agents.
We also report, for the first time, that ~20 % of tumors display expression of FAP in tumor epithelial cells; we were able to confirm this finding in EOC cell line monocultures, which demonstrates that FAP staining of the epithelium represents bona fide expression of FAP by tumor epithelial cells, rather than diffusion or uptake of FAP secreted by the stroma. While the finding of FAP expression in tumor epithelial cells is novel for EOC, this phenomenon is not unique to this disease, as previous studies have found FAP expression in subsets of cancer epithelial cells of the stomach, colorectum, and breast [33–35]. It is not clear how cancer cells come to express FAP, but since it has been proposed that some CAFs may originate from epithelial cells that transdifferentiate to give rise to tumor stroma through an epithelial-to-mesenchymal transition [14, 32], we postulate that FAP could be indicative of more mesenchymal phenotypes in EOC cells. We also observed a trend for reduced FAP expression in 3D EOC cell models compared to 2D cultures, which is consistent with our previous reports describing that culturing normal and transformed ovarian cells as 3D models enhances epithelial phenotypes [36, 37]. Whether the positive tumor epithelial cells we observed are CAFs in evolution is unclear, but FAP since expression was both prognostic in TCGA data, and in vitro studies showed that FAP plays a role in cell autonomous signaling during ovarian cancer progression, it is clear that further in vitro and in vivo studies into the cell-autonomous roles of FAP are warranted.
Tumor-associated stromal expression of FAP has been found to be associated with a worse prognosis in other tumor types too: in colon and pancreatic cancers FAP + stroma is associated with more aggressive disease progression, potential development of metastasis, recurrence, and death [10–13]. However FAP expression is not universally associated with poorer outcomes. FAP expression reduced tumorigenicity in mouse models of melanoma, and is also associated with longer survival in patients with invasive breast carcinoma [38]. These conflicting observations suggest that the physiologic responses to FAP may depend not only on the in vivo tumor microenvironment but also on the different microenvironments of FAP expression and the somatic aberrations found in tumor epithelium [39]. Previous studies in ovarian cancers treated with standard surgery followed by chemotherapy showed an association of FAP with advanced stage disease, lymph node metastasis, omental involvement, lymphovascular disease, and increased angiogenesis, but no association of FAP with patient outcomes was noted [13]. Here, in larger cohorts, we found that FAP expression by cancer-associated stroma was significantly associated with platinum resistance in patients with EOC. In addition, FAP stroma positive expression was associated with shorter recurrence after adjusting to tumor grade, stage and tumor debulking surgery. Furthermore, in vitro studies suggest that addition of FAP significantly increases chemoresistance even in the presence of platinum agent, which perhaps suggest that FAP is involved in microenvironment-induced drug resistance (MIDR) in EOC. This observation is particularly significant as stromal cells can promote resistance to many anti-tumor therapies [40], which is a major clinical challenge for physicians managing patients with EOC. Therefore, it is possible that the development of chemoresistance is in part driven by changes in the tumor stroma of EOCs, and that the co-targeting of tumor epithelium and stroma in advanced EOCs could potentially reduce rates of EOC-associated mortality by inhibiting the development of chemoresistance.
The tumor microenvironment has only recently emerged as a novel chemotherapeutic target in the treatment of cancer patients. The data we have presented above, in addition to the absence of FAP in normal ovaries and its presence in ovarian cancer, makes FAP a very attractive therapeutic target for the treatment of EOC. Initial research revealed that attempting to inhibit FAP with small molecules is particularly challenging, in part due to the difficulties in designing inhibitors that do not affect other closely related dipeptidyl peptidases. While targeting FAP directly may not be possible, the highly restricted, tumor-specific expression of FAP has enabled other innovative therapeutic strategies to be developed. One approach has been to target CAFs through the use of a novel FAP-activated prodrug that specifically activates a cytotoxic compound in the tumor stroma, a therapy that has shown encouraging results in phase I and II clinical trials for metastatic colon cancer and melanoma [41, 42]. Alternatively, Liao et al. demonstrated that, in a murine breast cancer model, cancer associated fibroblasts can be eliminated using a DNA vaccine targeted to FAP, and a reduction in the CAF population was accompanied by increased chemoresponse by the tumor epithelial cells and a concomitant increase in the immune response to the tumor [43]. Thus targeting FAP could have anti-cancer effects through multiple different mechanisms including potentiation of the effects of chemotherapy, suggesting anti-FAP therapy for EOC is well worth pursuing.
In summary, FAP may play an important role in ovarian cancer chemoresponse, via cell autonomous and non-cell autonomous mechanisms. FAP may be a promising therapeutic target that is worthy of investigation in patients with EOC, due to the limited therapeutic options and the frequent occurrence of chemoresistance .
References
Kalluri R, Zeisberg M (2006) Fibroblasts in cancer. Nat Rev Cancer 6:392–401
Franco OE, Shaw AK, Strand DW, Hayward SW (2010) Cancer associated fibroblasts in cancer pathogenesis. Semin Cell Dev Biol 21:33–39
Servais C, Erez N (2013) From sentinel cells to inflammatory culprits: cancer-associated fibroblasts in tumour-related inflammation. Pathology 229:198–207
Otsman A, Augsten M (2009) Cancer-associated fibroblasts and tumor growth-bystanders turning into key players. Curr Opin Genet Dev 19:67–73
Xing F, Saidou J, Watabe K (2011) Cancer associated fibroblasts (CAFs) in tumor microenvironment. Front Biosci 15:166–179
Anderberg C, Pietras K (2009) On the origin of cancer-associated fibroblasts. Cell Cycle 8:1561–1465
Scanlan MJ, Raj BK, Calvo B et al (1994) Molecular cloning of fibroblast activation protein α, a member of the serine protease family selectively expressed in stromal fibroblasts of epithelial cancers. Proc Natl Acad Sci U S A 91:5657–5661
Polgar L (2002) The prolyl oligopepetidase family. Cell Mol Life Sci 59:349–362
Bae S, Park CW, Son HK et al (2008) Fibroblast activation protein alpha identifies mesnechymal stromal cells from human bone marrow. Br J Haematol 142:827–830
Cohen SJ, Alpaugh K, Palazzo I et al (2008) Fibroblast activation protein and its relationship to clinical outcome in pancreatic adenocarcinoma. Pancreas 37:154–158
Shi M, Yu DH, Chen Y et al (2012) Expression of fibroblast activation protein in human pancreatic adenocarcinoma and its clinicopathological significance. World J Gasterol 18:840–846
Henry LR, Lee HO, Lee JS et al (2007) Clinical implications of fibroblast activation protein in patients with colon cancer. Clin Cancer Res 13:1736–1741
Zhang Y, Tang H, Cai J et al (2011) Ovarian cancer-associated fibroblasts contribute to epithelial ovarian carcinoma metastasis by promoting angiogenesis, lymphangiogenesis and tumor cell invasion. Cancer Lett 303:47–55
Kelly T, Huang Y, Simms AE, Masur A (2013) Fibroblast activation protein-α: a key modulator of the microenvironment in multiple pathologies. Int Rev Cell Mol PathoL 297:83–116
O’Brien P, O’Connor BF (2008) Seprase: an overview of an important matrix serine protease. Biochem Biophys Acta 1784:1130–1145
Lai D, Wang F (2012) Fibroblast activation protein regulates tumor-associated fibroblasts and epithelial ovarian cancer cells. Int J Oncol 41:541–50
Yang W, Han W, Ye S et al (2013) Fibroblast activation protein-α promotes ovarian cancer cell proliferation and invasion via extracellular and intracellular signaling mechanisms. Exp Mol Pathol 95:105–110
Spaeth EL, Dembinski JL, Sasser AK et al (2009) Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PLoS One 4:e4992
Monsky WL, Lin CY, Aoyama A et al (1994) A potential marker protease of invasiveness, seprase, is localized on invadopodia of human malignant melanoma cells. Cancer Res 54:5702–5710
The National Cancer Institute’s Surveillance, Epidemiology and End Results Program. The Center for Disease Control and Prevention’s National Program of Cancer Registries 2012. SEER.cancer.gov
du Bois A, Quinn M, Thigpen T et al (2005) 2004 consensus statements on the management of ovarian cancer: final document of the 3rd International gynecologic Cancer Intergroup Ovarian Cancer Consensus Conference. Ann Oncol 16(Suppl 8):viii7–viii12
Ali AY, Farrand L, Kim JY et al (2012) Molecular determinants of ovarian cancer chemoresistance: new insights into an old conundrum. Ann N Y Acad Sci 1271:58–67
Shimizu Y, Kamoi S, Amada S, Hasumi K, Akiyama F, Silverberg SG (1998) Toward the development of a universal grading system for ovarian epithelial carcinoma. I. Prognostic significance of histopathologic features–problems involved in the architectural grading system. Gynecol Oncol 70:2–12
Mhawech-Fauceglia P, Fischer G, Alvarez V Jr, Ahmed A, Herrmann FR (2007) Predicting outcome in minimally invasive (T1A and T1B) urothelial bladder carcinoma using a panel of biomarkers; a high throughput tissue microarray analysis. BJU Int 100:1182–7
Garin-Chesa P, Old LJ, Rettig WJ (1990) Cell surface glycoprotein of reactive stromal fibroblasts as a potential antibody target in human epithelial cancers. Porc Natl Acad Sci USA 87:7235–7239
Batistatou A, Televantou D, Bobos M et al (2013) Evaluation of current prognostic and predictive markers in breast cancer: a validation study of tissue microarrays. Anticancer Res 33:2139–45
Cerami E, Gao J, Dogrusoz U et al (2012) The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2:401
Gao J, Aksoy BA, Dogrusoz U et al (2013) Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 6:11
Karst AM, Levanon K, Drapkin R (2011) Modeling high-grade serous ovarian carcinogenesis from the fallopian tube. Proc Natl Acad Sci U S A 108:7547–7552
Perets R, Wyant GA, Muto KW (2013) Transformation of the fallopian tube secretory epithelium leads to high-grade serous ovarian cancer in brca;tp53;pten models. Cancer Cell 24:751–765
Lee JM, Mhawech-Fauceglia P, Lee N et al (2013) A three-dimensional microenvironment alters protein expression and chemosensitivity of epithelial ovarian cancer cells in vitro. Lab Invest 93:528–542
Radisky DC, Kenny PA, Bissell MJ (2007) Fibrosis and cancer: do myofibroblasts come also from epithelial cells via EMT? J Cell Biochem 101:830–839
Mori Y, Kono K, Matsumoto Y et al (2004) The expression of a type II transmembrane serine protease (Seprase) in human gastric carcinoma. Oncology 67:411–419
Kelly T, Kechelava S, Rozypai TL, West KW, Korourian S (1998) Seprase, a membrane-bound protease is overexpressed by invasive ductal carcinoma cells of human breast cancers. Mod Pathol 11:855–863
Iwasa S, Okada K, Chen WT et al (2005) Increased expression of seprase, a membrane-type serine protease, is associated with lymph node metastasis in human colon cancer. Cancer Lett 227:229–236
Lawrenson K, Benjamin E, Turmaine M, Jacobs I, Gayther A, Dafou D (2009) In vitro three-dimensional modelling of human ovarian surface epithelial cells. Cell Prolif 42:385–93
Lawrenson K, Grun B, Benjamin E, Jacobs IJ, Dafou D, Gayther SA (2010) Senescent fibroblasts promote neoplastic transformation of partially transformed ovarian epithelial cells in a three-dimensional model of early stage ovarian cancer. Neoplasia 12:317–25
Ariga N, Sato E, Ohuchi N, Nagura H, Ohtani H (2001) Stromal expression of fibroblast activation protein/seprase, a cell membrane serine proteinase and gelatinase, is associated with longer survival in patients with invasive ductal carcinoma of breast. Int J Cancer 95:67–72
Mueller MM, Fusenig NE (2004) Friends or foes-bipolar effects of the tumor stroma in cancer. Nat Rev Cancer 4:839–849
Straussman R, Morikawa T, Shee K et al (2012) Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature 487:500–504
Brennen WN, Rosen DM, Wang H, Isaacs JT, Denmeade SR (2012) Targeting carcinoma-associated fibroblasts within the tumor stroma with a fibroblast activation protein-activated prodrug. J Natl Cancer Inst 104:1320–34
Brennen WN, Isaacs JT, Denmeade SR (2012) Rationale behind targeting fibroblast activation protein-expressing carcinoma-associated fibroblasts as a novel chemotherapeutic strategy. Mol Cancer Ther 11:257–266
Liao D, Luo Y, Markowitz D, Xiang R, Reisfeld RA (2009) Cancer associated fibroblasts promote tumor growth and metastasis by modulating the tumor immune microenvironment in a 4T1 murine breast cancer model. Plos One 4(11):e7965
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mhawech-Fauceglia, P., Yan, L., Sharifian, M. et al. Stromal Expression of Fibroblast Activation Protein Alpha (FAP) Predicts Platinum Resistance and Shorter Recurrence in patients with Epithelial Ovarian Cancer. Cancer Microenvironment 8, 23–31 (2015). https://doi.org/10.1007/s12307-014-0153-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12307-014-0153-7