Abstract
Osteochondral lesions of the knee (OLK) are a common cause of knee pain and associated diseases. A new bone-marrow-derived mesenchymal stem cells technique has been developed for the treatment of OLK. 30 patients with OLK underwent arthroscopic one-step procedure. The bone marrow was harvested from the patients’ posterior iliac crest and arthroscopically implanted with a scaffold into the lesion site. Clinical inspection and MRI were performed. Mean International Knee Documentation Committee (IKDC) score before surgery was 29.9 ± 13.2 and 85.4 ± 4.2 at 29 ± 4.1 months (p < 0.0005), while Knee injury and Osteoarthritis Outcome Score (KOOS) before surgery was 35.1 ± 11.9 and 87.3 ± 7.3 at 29 ± 4.1 months (p < 0.0005). Control MRI and bioptic samples showed an osteochondral regeneration of the lesion site. The one-step technique appears to be a good and reliable option for treatment of OLK at three years of follow-up.
Level of evidence Case series, Level IV.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteochondral lesions of the knee (OLK) are lesions of the cartilaginous layer and the subchondral bone below, most frequently traumatic in origin [1].
In most cases, these defects can be found at the level of medial femoral condyle, and associated ligamentous or meniscal pathology is reported in 40 % of cases [2, 3].
It was found through careful biomechanical studies that intense action of localized stress on surface of the osteochondral defect may have significant implications for cartilage health [4].
It is well recognized that the cartilage has limited regenerative capacity so the surgeon must face the problem of treating cartilage OLK lesions before they can lead to osteoarthritis [5–8].
Various surgical options have been proposed for osteochondral repair [6–9] but among them, only few have shown the ability to provide repair of the lesion site with hyaline cartilage [5, 10, 11, 46–49].
Traditionally, hyaline cartilage repair may be reached by cartilage replacement (osteoarticular transfer system OATS, mosaicplasty) [13] or cartilage regeneration through autologous chondrocyte implantation (ACI) [6, 14]. Cartilage replacement procedures have the advantage of repairing cartilage defects by already mature autologous cartilage cells but donor site pathology, difficulties in the orienting of the cartilage plugs, and fibrocartilage in the gaps are limits of these techniques [13]. On the other hand, cartilage regeneration by ACI provides continuous cartilage repair with minimal donor site pathology. ACI has been used for the past 16 years, and several studies have reported successful treatment of lesions, with stable and satisfactory results over time [6, 7, 15–18].
The limit of ACI treatment is that the surgeon needs to run two surgical procedures to complete it properly and requires high costs, and that is the main reason why new methods of cartilage regeneration have been searched.
In the last years, a new bone-marrow-derived mesenchymal stem cells (BMDC) technique has been developed for the treatment of osteochondral defects, and a new one-step technique for BMDC transplantation was proposed.
Following the excellent results achieved in the ankle [19], the same technique has been transferred to the knee joint.
The aim of this study was to investigate the validity of the “one-step” technique in OLK repair and to present the results of a series of 30 patients consecutively treated.
Materials and methods
From March 2006 to February 2007, 20 patients (12 males, 8 females) with OLK underwent the one-step procedure. In 18 cases the lesions were post-traumatic in origin, 2 were due to osteochondritis dissecans, and 4 were secondary to malalignments overload. The treatment was indicated in patients with grade III and IV osteochondral lesions (International Cartilage Repair Society ICRS classification) [20] of the femoral condyle, with clinical symptoms of pain, swelling, locking, or giving way.
In 14 cases, the lesions involved the medial femoral condyle, and in 4 cases, the lateral femoral condyle was involved. In 2 cases, both the medial and the lateral femoral condyles had cartilage defects.
Associated comorbidities were as follows: meniscal injury in 7 (4 medial and 3 lateral meniscus), anterior cruciate ligament injuries in 2, femoro-tibial malalignments in 3, and osteophytosis in 3.
A partial meniscectomy was performed in 6 cases, while in 1 case, the meniscus was repaired. In the patients with ACL injuries, ACL reconstruction was performed with semitendinosus and gracilis tendon grafts and “over the top” technique. In the 3 femoro-tibial malalignments cases, a high tibial osteotomy was performed, while the osteophytes were tangentially resected.
Two patients had previous surgical procedures for cartilage repair by microfractures.
Exclusion criteria were as follows: age <15 years and >50 years, diffuse osteoarthritis, and comorbidities with general medical conditions (diabetes, rheumatoid arthritis), hematological disorders, and infections.
The study protocol was approved by an independent Ethical Committee, and signed informed consent was obtained from all patients participating in this study.
Surgical technique
Step 1: Platelet gel production
About 120 mL of the patient’s venous blood was taken and treated the day before surgery with the Vivostat System® (Vivolution, Denmark) in order to provide 6 mL of platelet-rich fibrin gel (PRF) [19, 21, 22].
Step2: Bone marrow aspiration
A total of 60 mL bone marrow aspirate was taken from the posterior iliac crest (Fig. 1), with the patient prone under spinal or general anesthesia. The bone marrow harvesting was performed with a marrow needle (size 11 G × 100 mm) inserted 3 cm deep into the iliac crest marrow. 5 mL of bone marrow was aspirated into a 20-mL plastic syringe internally coated with calcium-heparin solution, repeating the procedure with several perforations into the iliac crest through the same skin opening, until a total of 60 mL of bone marrow aspirate was collected. The marrow was aspirated in small fractions from different points to maximize the harvesting of the marrow stromal cells and to reduce dilution by peripheral blood.
Step 3: Bone marrow concentration
The harvested bone marrow was treated directly in the operating room, by removing most of the erythrocytes and plasma. A cell separator (Smart PReP®, Harvest Technologies Corp., USA or IOR-G1, Novagenit, Mezzolombardo, TN, Italy), (Figs. 2, 3) provided 6 mL of concentrate containing nucleated cells after 15 min of multiple centrifugation cycles.
Step 4: Arthroscopic BMDC transplantation
After the bone marrow harvesting phase, a standard knee arthroscopy was performed, with the patient in the supine position.
The cartilage lesion was identified (Fig. 4), and a flipped cannula was inserted into the portal ipsilateral to the lesion to enable insertion of the surgical instrumentations and to retract the fat pad from the operative field [14].
A specifically designed low profile drill (Fig. 5) was used to debride the lesion, resulting in a circular area with regular healthy cartilage margins for biomaterial implantation (Fig. 6).
A hyaluronic acid membrane (Hyalofast®, Fidia Advanced Biopolymers, Italy) or collagen membrane (IOR-G1, Novagenit, Mezzolombardo, TN, Italy) was used for cell support. The scaffold was filled with 2 mL of bone marrow concentrate and loaded onto the delivery device (Fig. 7), which was used to position the biomaterial within the defect (Fig. 8). Multiple stamp-sized pieces of membrane can be overlapped in order to cover the whole area.
A layer of PRF was placed onto the implanted material in order to provide growth factors (Fig. 9). Under arthroscopic control, the stability of implanted stamps was evaluated with knee flexions and extensions (Fig. 10).
Postoperative treatment
The rehabilitation was articulated in different stages:
-
1.
The day after surgery: gradual passive and active mobilization of the knee with no weight-bearing.
-
2.
4 weeks after the surgery: muscular reinforcement exercises, closed kinetic chain proprioceptive rehabilitation, static and walking exercises with partial and gradual weight-bearing, swimming.
-
3.
10 weeks after the surgery: open kinetic chain rehabilitation exercises recovery of muscular function, and walking with full weight-bearing, cycling.
-
4.
6 months after the surgery: light running.
-
5.
12 months after the surgery: high impact sports.
Follow-up evaluation
Clinical
Assessment was performed before surgery and at 6, 12, 18, and 24 months and after 3 years postoperatively, following the International Knee Documentation Committee (IKDC) score (subjective) and Knee injury and Osteoarthritis Outcome Score (KOOS) questionaries [23, 24].
MRI
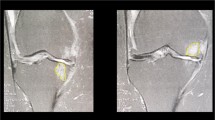
A magnetic resonance imaging scan was performed on all of the patients in the study preoperatively (Fig. 11) and at 6 months, 12 months, and at the final follow-up. Imaging sequences were carried out following the cartilage repair tissue grading scale (Mocart) [25].
Biopsy
After obtaining approval from the local ethics committee and informed consent from the patients, 4 patients underwent a second-look arthroscopy with a biopsy 12 months after surgery. Cartilage specimens were evaluated by hematoxylin/eosin and Safranin-O stainings and collagen type I and II immunohistochemical analyses.
Statistical analysis
The Wilcoxon test, the Mann–Whitney test, and the Student’s paired t test were used to test for significant differences between baseline and various follow-up measurements. A p value of <0.05 was considered significant.
Results
Clinical
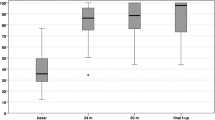
Mean IKDC score before surgery was 29.9 ± 13.2 and 85.4 ± 4.2 at 29 ± 4.1 months (p < 0.005), while KOOS score before surgery was 29.9 ± 13.2 and 85.4 ± 4.2 at 29 ± 4.1 months (p < 0.0005).
Patients’ age at the time of surgery, gender, and size of the cartilage defect did not affect the results.
The clinical improvement was statistically significant both for IKDC and KOOS scores at each follow-up, with increased clinical improvement over time.
Associated procedures significantly affected IKDC score at 12 months (p < 0.005), while no differences were found at final follow-up.
Imaging
The control MRI at 12 and 24 months follow-up showed regeneration of the subchondral bone and the cartilaginous tissue in different parameters of the MOCART score (Fig. 12).
A significative relationship was found between KOOS score at 24 months and signal intensity (p < 0.05). Patients with a hyperintense signal (11 patients) had a KOOS score of 87 ± 7 at 24 months follow-up, while patients with an Isointense signal (19 patients) had a KOOS score of 95 ± 5.
No other significative relationships were found between clinical score and MOCART parameters.
Histological analysis
Safranin-O staining showed a biopsy of the regenerated tissue obtained at 12 months showed in the two biopsies a cartilaginous tissue characterized by the presence of a proteoglycan rich matrix particularly in the middle and deep zone and high collagen layer in the superficial zone. The superficial layer was almost regular. The subchondral bones and the tide marks were well evident. Hematoxylin and eosin staining showed the presence of cells homogenously distributed throughout the tissue, regularly columnarized in the deep one.
Immunohistochemical analysis showed in both specimens a positivity for collagen type II throughout the entire thickness of the biopsies, while collagen type I was negative (data not shown).
Complications
Neither intraoperative nor postoperative complications were observed in this series.
Discussion and conclusions
ACI technique, introduced in 1994 by Brittberg et al. [26–33], has been showed to regenerate cartilage tissue with similar biomechanical properties to the surrounding healthy cartilage, and biomechanically superior to regenerated cartilage induced by other techniques.
In various studies, reliable and durable clinical results have been reported both with the use of the open field surgery and more recently matrix-based techniques permitted an entirely arthroscopic technique [6–18]. The necessity of performing two surgical procedures and high cost, associated with cell expansion, are the weaknesses of ACI [34, 35].
Mesenchymal stem cells represent 2–3 % of the total mononuclear cells in bone marrow and have the ability to differentiate into various lineages, including osteoblasts and chondroblasts [36–41]. The rationale of the “one-step technique” is based on the idea to transfer into the lesion site not only mesenchymal stem cells, but the entire bone marrow cellular pool [19]. This allows not to loose “regenerative potential” present in the bone marrow and cells to be processed directly in the operating room without the need for a laboratory phase and allows BMDC transplantation to be performed in “one step” instead of the two required for ACI [42–44].
The results obtained in this study with the one-step technique in OLK repair are comparable to those achieved with treatment ACI for similar lesions in OLK disease [6, 7, 13, 45].
A significant improvement in both IKDC and KOOS score from preoperative to each follow-up considered was found (p < 0.005). Patients with associated procedures experienced a delay in their clinical improvement between 6 and 12 months, though the findings at final follow-up were not affected.
Magnetic resonance imaging examination showed promising growth of bone and cartilage, nearly complete defect filling and satisfactory integration of the graft at follow-up in 80 % of cases. Among all the considered MRI parameters, only signal intensity was significantly correlated with the KOOS score at 24 months follow-up.
Both biopsy specimens showed a regenerated cartilage tissue in an advanced remodeling phase. Immunohistochemical analysis confirmed the presence of proteoglycans and type-II collagen, well-recognized markers of hyaline cartilage. The number of cases treated is still limited, but the results to date are promising in a high percentage of patients from the clinical, imaging, and histological perspectives, making the one-step technique a concrete part of the “cartilage repair paradigm”.
References
Widuchowski W, Widuchowski J, Trzaska T. Knee. Articular cartilage defects: study of 25,124 knee arthroscopies. Jun;14(3):177–182. [Epub 2007 Apr 10. 2007]
Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG (1997) Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy 13(4):456–460
Hjelle K, Solheim E, Strand T, Muri R, Brittberg M (2002) Articular cartilage defects in 1,000 knee arthroscopies. Arthroscopy 18(7):730–734
Papaioannou G, Demetropoulos CK, King YH (2010) Predicting the effects of knee focal articular surface injury with a patient-specific finite element model. Knee 17(1):61–68. [Epub 2009 May 23]
Hangody L, Vasarhelyi G, Hangody LR, Sukosd Z, Tibay G, Bartha L et al (2008) Autologous osteochondral grafting— technique and long-termresults. Injury 39(Suppl 1):S32–S39
Ferruzzi A, Buda R, Faldini C, Vannini F, Di Caprio F, Luciani D, Giannini S (2008) Autologous chondrocyte implantation in the knee joint: open compared with arthroscopic technique. Comparison at a minimum follow-up of five years. J Bone Joint Surg Am 90(Suppl 4):90–101
Marcacci M, Kon E, Delcogliano M, Filardo G, Busacca M, Zaffagnini S (2007) Arthroscopic autologous osteochondral grafting for cartilage defects of the knee: prospective study results at a minimum 7-year follow-up. Am J Sports Med 35(12):2014–2021. [Epub 2007 Aug 27]
Gobbi A (2009) Bathan L Biological approaches for cartilage repair. J Knee Surg. 22(1):36–44
Gobbi A, Nunag P, Malinowski K (2005) Treatment of full thickness chondral lesions of the knee with microfracture in a group of athletes. Knee Surg Sports Traumatol Arthrosc 13(3):213–221. [Epub 2004 May 14]
Peterson L, Brittberg M, Kiviranta I, Akerlund EL, Lindahl A (2002) Autologous chondrocyte transplantation. Biomechanics and long-term durability. Am J Sports Med 30(1):2–12
Magnussen RA, Dunn WR, Carey JL, Spindler KP (2008) Treatment of focal articular cartilage defects in the knee: a systematic review. Clin Orthop Relat Res 466(4):952–962. [Epub 2008 Jan 12]
Basad E, Ishaque B, Bachmann G, Stürz H, Steinmeyer J (2010) Matrix-induced autologous chondrocyte implantation versus microfracture in the treatment of cartilage defects of the knee: a 2-year randomised study. Knee Surg Sports Traumatol Arthrosc 18:519–527
Hangody L, Ráthonyi GK, Duska Z, Vásárhelyi G, Füles P, Módis L (2004) Autologous osteochondral mosaicplasty. Surgical technique. J Bone Joint Surg Am 86-A Suppl 1:65–72
Marcacci M, Kon E, Zaffagnini S, Filardo G, Delcogliano M, Neri MP, Iacono F, Hollander AP (2007) Arthroscopic second generation autologous chondrocyte implantation. Knee Surg Sports Traumatol Arthrosc 15(5):610–619
Kon E, Gobbi A, Filardo G, Delcogliano M, Zaffagnini S, Marcacci M (2009) Arthroscopic second generation autologous chondrocyte implantation compared with microfracture for chondral lesions of the knee: prospective nonrandomized study at 5 years. Am J Sports Med 37(1):33–41
Nehrer S, Domayer S, Dorotka R, Schatz K, Bindreiter U, Kotz R (2006) Three-year clinical outcome after chondrocyte transplantation using a hyaluronan matrix for cartilage repair. Eur J Radiol 57:3–8
Ossendorf C, Kaps C, Kreuz P, Burmester GR, Sittinger M, Erggelet C (2007) Treatment of posttraumatic and focal osteoarthritic cartilage defects of the knee with autologous polymer-based three-dimensional chondrocyte grafts: 2-year clinical results. Arthritis Res Ther. 9(2):R41
Selmi TA, Verdonk P, Chambat P et al (2008) Autologous chondrocyte implantation in a novel alginate-agarose hydrogel: outcome at two years. J Bone Joint Surg Br 90(5):597–604
Giannini S, Buda R, Vannini F, Cavallo M, Grigolo B (2009) One-step bone marrow-derived cell transplantation in talar osteochondral lesions. Clin Orthop Relat Res 467(12):3307–3320
Mainil-Varlet P, Aigner T, Brittberg M, Bullough P, Hollander A, Hunziker E, Kandel R, Nehrer S, Pritzker K, Roberts S, Stauffer E; International Cartilage Repair Society. Histological assessment of cartilage repair: a report by the Histology Endpoint Committee of the International Cartilage Repair Society (ICRS). J Bone Joint Surg Am. 2003;85-A Suppl 2:45–57
Buda R, Vannini F, Grigolo B, Cenacchi A, DiCaprio F, Giannini S (2007) Ingegneria Tissutale: tecnica Chirurgica. J Orthopaed Traumatol 33(Suppl 1):S215–S222
Buda R, Di Caprio F, Cavallo M, Ruffilli A, Giannini S (2009) Artroscopia della caviglia: ankle arthroscopy. J Orthopaed Traumatol 35(Suppl. 1):S221–S225
Irrgang JJ, Anderson AF, Boland AL et al (2001) Development and validation of the International Knee Documentation Committee subjective knee form. Am J Sports Med 29(5):600–613
Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD (1998) Knee Injury and Osteoarthritis Outcome Score (KOOS)—development of a self-administered outcome measure. J Orthop Sports Phys Ther 28(2):88–96
Marlovits S, Singer P, Zeller P, Mandl I, Haller J, Trattnig S (2009) Evaluation and comparison of cartilage repair tissue of the patella and medial femoral condyle by using morphological MRI and biochemical zonal T2 mapping. Eur Radiol 19(5):1253–1262. [Epub 2008 Dec 23]
Brittberg M, Winalski CS (2003) Evaluation of cartilage injuries and repair. J Bone Joint Surg Am 85(Suppl 2):58–69
Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O (1994) Peterson L Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med 331:889–895
Brittberg M, Tallheden T, Sjogren-Jansson B, Lindahl A (2001) Peterson L Autologous chondrocytes used for articular cartilage repair: an update. Clin Orthop Relat Res 391:S337–S348
Peterson L, Brittberg M, Lindahl A (2003) Autologous chondrocyte transplantation of the ankle. Foot Ankle Clin 8:291–303
Peterson L, Minas T, Brittberg M, Nilsson A, Sjogren-Jansson E (2000) Lindahl ATwo- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res 374:212–234
Peterson L, Brittberg M, Kiviranta I, Akerlund EL (2002) Autologous chondrocyte transplantation. Biomechanics and long-term durability. Am J Sports Med 30:2–12
Henderson I, Lavigne P, Valenzuela H (2007) Oakes B Autologous chondrocyte implantation: superior biologic properties of hyaline cartilage repairs. Clin Orthop Relat Res 455:253–261
Lindahl A, Brittberg M, Peterson L (2001) Health economics beneWts following autologous chondrocyte transplantation for patients with focal chondral lesions of the knee. Knee Surg Sports Traumatol Arthrosc 9:358–363
Minas T (1998) Chondrocyte implantation in the repair of chondral lesions of the knee: economics and quality of life. Am J Orthop 27:739–744
Bosnakovski D, Mizuno M, Kim G, Takagi S, Okumura M, Fujinaga T (2006) Chondrogenic differentiation of bovine marrow mesenchymal stem cells (MSCs) in different hydrogels: influence of collagen type II extracellular matrix on MSC chondrogenesis. Biotechnol Bioeng 93:1152–1163
Dominici M, Pritchard C, Garlits JE, Hofmann TJ, Persons DA, Horwitz EM (2004) Hematopoietic cells and osteoblasts are derived from a common marrow progenitor after bone marrow transplantation. PNAS 101:11761–11766
Kacena MA, Gundberg CM, Horowitz MC (2006) A reciprocal regulatory interaction between megacaryocytes, bone cells and hematopoietic stem cells. Bone 39:978–984
Longobardi L, O’Rear L, Aakula S, Johnstone B, Shimer K, Chytil A, Horton WA, Moses HL, Spagnoli A (2006) Effect of IGF-I in the chondrogenesis of bone marrow mesenchymal stem cells in the presence or absence of TGF-beta signalling. J Bone Miner Res 21:626–636
Lucarelli E, Beccheroni A, Donati D, Sangiorgi L, Cenacchi A, Del Vento AM, Meotti C, Bertoja AZ, Giardino R, Fornasari PM, Mercuri M, Picci P (2003) Platelet-derived growth factors enhance proliferation of human stromal stem cells. Biomaterials 24:3095–3100
Olmsted-Davis EA, Gugala Z, Camargo F, Gannon FH, Jackson K, Kienstra KA, Shine HD, Lindsey RW, Hirschi KK, Goodell MA, Brenner MK, Davis AR (2003) Primitive adult hematopoietic stem cells can function as osteoblast precursors. PNAS 100:15877–15882
Taichman RS (2005) Blood and bone: two tissues whose fates are intertwined to create the hematopoietic stem-cell niche. Blood 105:2631–2639
Capone C, Frigerio S, Fumagalli S, Gelati M, Principato MC, Storini C, Montinaro M, Kraftsik R, De Curtis M, Parati E, De Simoni MG (2007) Neurosphere-derived cells exert a neuroprotective action by changing the ischemic microenvironment. PLoS ONE 2:e373
Lepore AC, Han SS, Tiler-Polsz CJ, Cai J, Rao MS, Fischer I (2004) Transplantation into the adult CNS. Neuron Glia Biol. 1:113–126
Kon E, Verdonk P, Condello V, Delcogliano M, Dhollander A, Filardo G, Pignotti E, Marcacci M (2009) Matrix-assisted autologous chondrocyte transplantation for the repair of cartilage defects of the knee: systematic clinical data review and study quality analysis. Am J Sports Med 37 Suppl 1:156–66S. [Epub 2009 Oct 27]
Di Martino A, Liverani L, Rainer A, Salvatore G, Trombetta M, Denaro V (2011) Electrospun scaffolds for bone tissue engineering. Musc Surg 95(2):69–80. doi:10.1007/s12306-011-0097-8
Tigani D, Rimondi E, Trentani P, Ansaloni M, Amendola L, Testi D (2011) Three-dimensional analysis of image-free navigation system for total knee arthroplasty. Musc Surg 95(2):81–87. doi:10.1007/s12306-010-0090-7
Cenni Elisabetta, Savarino Lucia (2010) Francesca Perut. Caterina Fotia and Sofia Avnet, et al Background and rationale of platelet gel in orthopaedic surgery Musc Surg 94(1):1–8
Paderni S, Terzi S, Amendola L (2009) Major defect treatment with an osteoconductive bone substitute. Musc Surg 93(2):89–96
Notarnicola A, Moretti L, Tafuri S, Vacca A, Marella G, Moretti B (2011) Postoperative pain monitor after total knee replacement. Musc Surg 95(1):19–24. doi:10.1007/s12306-011-0102
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Buda, R., Vannini, F., Cavallo, M. et al. One-step arthroscopic technique for the treatment of osteochondral lesions of the knee with bone-marrow-derived cells: three years results. Musculoskelet Surg 97, 145–151 (2013). https://doi.org/10.1007/s12306-013-0242-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12306-013-0242-7