Abstract
Application of beneficial microbes in soil is an important avenue to control plant stresses. In this study, the salinity tolerance of halotolerant bacteria (Bacillus tequilensis) was investigated and the bacterium was inoculated in the soil to mitigate salinity stress. The results revealed the highest floc yield and biofilm formation ability of B. tequilensis at 100 mM NaCl concentration. Fourier transformed infrared spectroscopy depicted the presence of carbohydrates and proteins which binds with sodium ions (Na+) and provide tolerance against salinity. Using PCR, plant growth-promoting bacterial genes viz., 1-aminocyclopropane-1-carboxylate deaminase and pyrroloquinoline quinone were successfully amplified from the genome of B. tequilensis. In the saline soil, B. tequilensis was inoculated and chickpea plants were grown. The bacterial strain improved the physiology, biochemistry, and antioxidant enzyme activities of the chickpea plant under salt stress. Plants inoculated with B. tequilensis exhibited higher relative water content, higher photosynthetic pigments, lower levels of hydrogen peroxide (H2O2) and malondialdehyde, and improved enzymatic activity for the scavenging of reactive oxygen species. The findings of this study suggest the sustainable use of B. tequilensis to mitigate the salinity stress of chickpea and other crops. This bacterium not only helps in the alleviation of the toxic effects of salt but also increases plant growth along with a reduction in crop losses due to salinity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Crop production is seriously hindered by the adverse effects of abiotic and biotic stresses. Plants are immobile and they are exposed to a range of challenges including water stress, heavy rains, alkalinity, temperature extremities, metal stress, nutrient depletion, phytopathogen and insect attacks. Among many environmental pressures, the extreme availability of dissolved salts (soil salinity) is one of the most significant issues, leading to decreased plant growth and compromised crop yield (Gupta and Pandey 2019). Around 7% of the total land and 20% of the cultivated area is influenced by salinity. Plants use a variety of halotolerant mechanisms, such as the synthesis of polyamine and osmolytes, reduction of reactive oxygen species, ion transport, antioxidant defense mechanisms and compartmentalization, etc. (Shukla et al. 2012).
The word “rhizobacteria” was first used to describe the soil bacterial community in 1978 by Kloepper and Schroth. Rhizobacteria competitively penetrate plant roots, stimulate growth, and decrease the incidence of plant infections. These helpful rhizobacteria are referred to as plant growth-promoting rhizobacteria (PGPR) by Kloepper and Schroth (1981). When growing alongside the host plants, PGPR is an essential component of the rhizosphere biota that can promote the growth of the host plant. Due to their high adaptability in a variety of conditions, rapid growth rate, and biochemical plasticity to metabolize a wide range of natural and xenobiotic substances, PGPRs appear as successful rhizobacteria in establishing themselves in the soil ecosystems. According to Cook (2002), PGPR is a crucial part of managing agricultural practices with innate genetic potential. Currently, the idea of PGPR is only applicable to bacterial strains that can meet at least two of the three requirements, i.e., aggressive colonization, plant growth stimulation, and biocontrol (Weller et al. 2002; Vessey 2003).
Many plant growth promoting rhizobacteria (PGPR) enhance the growth of plants by minimizing phytopathogens or by enabling nutrient availability from the surroundings. PGPR can play a protective role in adverse environmental conditions such as waterlogging, alkalinity, toxic metals, and plant pathogens. PGPR also mitigates these stresses by improving plant growth and development (Tank and Saraf 2010). PGPR promotes phosphate solubilization, IAA production, ACC deaminase activity, siderophores production and NH3 production. Bacterial cells release exopolysaccharides (EPS) and reduce ethylene levels through ACC deaminase activities to regulate the growth of the plant in response to severe environmental stresses. A wide range of PGPRs are being used to enhance growth and development of plants under both normal and saline conditions, across the globe (Kumar et al. 2021). Enterobacter sp. has been reported to alleviate the salinity stress of rice (Sarkar et al. 2018), while Serratia marcescens, a multi-faceted PGPR, enhances salt tolerance in wheat (Singh and Jha 2016). Many PGPRs including Pseudomonas putida, Alcaligenes sp., Klebsiella sp., and Pseudomonas cedrina have been described to maintain their typical salt tolerance characteristics under salinity stress. These traits enable plants to tolerate the adverse effects of salinity and grow better (Tirry et al. 2021). Bacillus species are halotolerant and they have been reported to possess growth promoting abilities (Ibarra-Villarreal et al. 2021).
Many scientists are working on the application of PGPR on chickpea (El Esawi et al. 2019; Mazumdar et al. 2020; Laranjeira et al. 2022). Chickpea (Cicer arietinum L.) is a major pulse crop and a high quality protein source in several areas of the world. It is a nitrogen-fixing crop and is frequently used as green manure and fodder. The nutritional profiling of chickpea seeds reveals 20.6% protein content, 2.2% fat content, and 61.2% total carbohydrates (Madurapperumage et al. 2021). It is one of the oldest pulses known and cultivated in both Asia and Europe dating back to ancient times. Gram is thought to have originated in the Himalayas or the Mediterranean region. It is currently grown in Pakistan, Italy, India, Romania, Greece, Russia, North Africa, Egypt, and several other countries around the world (Singh and Sood 2020). Chickpea is sensitive to salinity and saline conditions have a negative impact on chickpea germination, vegetative growth, and in particular, reproductive processes (Kotula et al. 2019).
The current study was designed to first time characterize, identify, and evaluate the effect of a halotolerant B. tequilensis on salinity tolerance and growth promotion of chickpea. This research will aid in the application of PGPR to improve plant tolerance in stressful environments, particularly salinity, and to promote the growth of chickpea plants.
Materials and methods
Selection of PGPR
For the estimation of flocculation yield potential and biofilm formation ability, the available strain of Bacillus tequilensis was selected. A recent study reported the capability of this PGPR in phosphorus solubilization and the production of indole acetic acid, siderophore, HCN, EPS, and ACC-deaminase (Haroon et al. 2021a). It was isolated from the halophyte rhizosphere collected from the Khewra salt mine in Jhelum, Pakistan (Haroon et al. 2021b).
Estimation of bacterial flocculation
To make Tryptic soy broth (TSB) media, 17 g of Tryptone, 3 g of Soy, 5 g of NaCl, 2.5 g of dipotassium phosphate (K2HPO4), and 2.5 g of glucose were dissolved in 1000 mL of distilled water and autoclaved. B. tequilensis was cultured in TSB broth and incubated at 30–35 °C for 4 days. The culture was filtered, using Whatman filter paper No. 1 and the collected flocculation was dried by placing it into an oven for 2 h at 60 °C. The dry weight was recorded and presented as floc yield (Sadasivan and Neyra 1985).
Biofilm formation
The microtiter plate-based protocol was used to estimate biofilm formation. For 24 h, B. tequilensis was cultured in TSB medium, amended with NaCl at 30–35 °C and its optical density (OD) was adjusted to 0.3 (752 N UV–VIS, Beijing, China). The bacterial culture (200 mL) was transferred into the wells of a microtiter plate and incubated at 35–37 °C for 4 days. The growth medium was removed after 4 days and the wells were stained with 0.01% crystal violet for 20–25 min. The stained biofilm, formed on the walls of microtiter plate wells was extracted with 95% ethanol and its OD was recorded at 590 nm (Christensen et al. 1985).
Scanning electron microscopy (SEM) of B. tequilensis under salinity stress
B. tequilensis was cultured in TSB medium, amended with 100 mM NaCl, and placed in an incubator shaker for 4 days at 120 rpm. The culture was centrifuged for 10 min at 5000 rpm and a bacterial pellet was collected. The bacterial cells were treated with 2.5% glutaraldehyde for 5–6 h at 4 °C and centrifugation at 5000 rpm for 6–8 min. The pellet was washed for 10 min with 0.1 M sodium cacodylate buffer. For post fixation, the sample was treated with 1% osmium tetraoxide and dehydrated using 35–100% acetone. The sample was dried for 1–2 h in a critical dryer. Dried sample was mounted on a stub, sputter coated with gold and observed under Scanning Electron Microscope (SEM, JEOLJSM 25910).
Fourier transform infrared spectroscopy (FTIR)
In a previous study, we found that B. tequilensis could produce exopolysaccharides (EPS) under varying levels of salt stress (Haroon et al. 2021a). To characterize EPS in this study, two milligrams of the extracted dried EPS from B. tequilensis was mixed with 200 mg of potassium bromide and subjected to FTIR spectroscopy. Functional groups of EPS were determined in a range of 4000–5000 cm−1 (Model No. FTSW 300 MX, BIO-RAD, California, USA).
Screening of genes conferring PGP traits
Two most important plant growth promoting (PGP) genes, in bacterial genome were detected by their amplification with conventional PCR. DNA of B. tequilensis (PCR template) was isolated (William et al. 2012) and gene specific primers (Table 1) were used to amplify 1-aminocyclopropane-1-carboxylate deaminase (acdS) and pyrroloquinoline quinone (pqqE) genes. acdS gene enhances plant growth by reducing ethylene levels (Naing et al. 2021), while pqqE gene encodes PPQ cofactor for phosphate solubilization (Kim et al. 2003).
Pot experiment
Inoculation and sowing of chickpea seeds
Seeds of Kabuli chickpea variety (Punjab 2008) were obtained from National agricultural research center (NARC), Islamabad. Seeds were washed with running tap water, soaked in 1% sodium hypochlorite (NaOCl) solution for 3 min and rinsed three times with deionized water. Sterilized seeds were dried on filter paper and nicked with a nail clipper. Chickpea seeds were inoculated by soaking in bacterial culture suspension for 1 h and air dried aseptically in the laminar air flow. Control seeds were surface sterilized and immersed in sterilized distilled water only.
Seeds were sown in plastic pots and kept in a controlled environment in the growth chamber at 20–25 °C, 60% relative humidity, and a light/dark cycle of 14/10 h. Seven seeds were planted in each pot, with three replicates for each treatment (Table 2). For three weeks, seedlings were subjected to saline water, every third day. Pots with control plants were irrigated with normal water.
Germination rate
Following the protocol of Manmathan and Lapitan (2013), the germination rate was calculated in percentage after 2 mm emergence of radicals from the seed.
Plant analysis
The chickpea seedlings were collected three weeks after planting and their root and shoot lengths were measured by using a measuring tape. The fresh weight of plants in each treatment was recorded with the help of a weighing balance. According to Lutts et al. (1996) electrical conductivity meter was used to measure electrolyte leakage. Leaf tissue was removed, and cleaned with deionized water. 1 g of fresh leaves were dried with filter paper, then cut into little pieces, placed in 20 mL of deionized water, and incubated at 25 °C and electrical conductivity (EC1) was measured after 24 h. The tissues in these samples were autoclaved at 120 °C for 20 min. After samples had reached a temperature of 25 °C, the ultimate electrical conductivity (EC2) was assessed. The formula used to express the electrolyte leakage (EL) was REL = EC1/EC2100.
RWC was assessed by following the method of Whetherley (1950). Fresh leaf was taken and weighed as fresh weight (FW). This leaf was positioned in petri plate filled with distilled water, overnight, in dark. After 24 h, the leaf turgid weight (TW) was determined by using sensitive weighing balance. The leaf was placed at 72 °C in an oven for overnight and the dry weight (DW) was determined. The seedling leaves were ground in acetone, centrifuged, and absorbance of supernatant was measured at 480, 663 and 645 nm using a spectrophotometer to determine chlorophyll (Porra 2002) and carotenoid contents (Lichtenthaler and Wellburn 1983).
The procedure outlined by Bates et al. (1973) was used to determine the proline content. With the help of 2 mL of 40% methanol, proline was isolated from leaf samples weighing 100 mg FW. 25 mg of ninhydrin and 1 mL of a combination of glacial acetic acid and orthophosphoric acid (6 M) (3: 2, v/v) were added to the extract. The reaction was stopped by placing the tubes in an ice bath after 1 h of incubation at 100 °C, and 5 mL of toluene was then added. At 520 nm, the absorbance of the upper phase was measured spectrophotometrically. An established standard curve was used to estimate the proline content.
The methodology proposed by Hahm et al. (2017) was used to calculate the total soluble sugar content. In a glass tube containing 5 ml of warmed 80% (v/v) ethanol, 0.1 g of leaf tissue was homogenized. The aliquots of the homogenates were transferred to 2 ml centrifuge tubes and incubated at 80 °C for 30 min. The homogenates were then centrifuged for 10 min at 4 °C at 16,200 × g and the supernatants were transferred in 1.5 ml falcon tubes. A standard calibration curve ranging from 0 to 10 mg of carbohydrate sugar was used to quantify the total soluble sugar levels (µg/g FW) and determine the optical density (OD) at a wavelength of 620 nm.
In 5 mL of potassium phosphate buffer that had been thoroughly chilled, 500 mg of fresh leaf was thoroughly ground (50 mM). Following a vortex, the liquid was centrifuged at 12,000 × g for 15 min. The technique suggested by Giannopolitis and Ries (1977) was used to separate the extract and measure SOD activity. SOD activity was evaluated using the inhibition of nitroblue tetrazolium (NBT) photoreduction. The reaction mixture (1 mL) in cuvettes was exposed to light for 15 min. It consisted of 400 mL of distilled water, 250 mL of phosphate buffer (pH 7.8; 50 mM), 100 mL of 13 mM L-methionine, 100 mL of 0.1% v/v triton-X-100, 50 mL of 50 mM NBT, 50 mL of 1.3 mM riboflavin. SOD enzyme activity per unit was calculated using the amount of enzyme that inhibited 50% of NBT photoreduction and the OD of the irradiated aliquot, which was measured at 560 nm.
The methodology developed by Chance and Maehly (1955) was applied to identify POD and CAT activities. 0.1 mL of plant extract, 20 mM guaiacol, 40 mM H2O2, and phosphate buffer were used in the reaction mixture (pH 5.0). At 470 nm, the variations in the reaction mixture's OD were noted every 30 s. One unit of POD activity was determined as a change in OD of the reaction mixture per minute. The reaction mixture (3.0 mL) contains plant extract, phosphate buffer, and H2O2 (0.1 mL). The plant extract was mixed to start the reaction for CAT activity, and changes in the reaction mixture's optical density (OD) were noted at 240 nm every 20 s. The activities of all the three enzymes were measured on the basis of total protein measured as described by Bradford (1976).
Salt tolerance index (STI) of each seedling was calculated as the ratio of the value for the NaCl-treated seedlings to the value of the control seedlings.
The Yasin et al. (2018) methodology was used to assess the malondialdehyde (MDA) activity, and absorbance measurements were made at 532 and 600 nm. By combining 0.2 g of leaf sample with 5 ml of cold 0.1% trichloroacetic acid, the generation of H2O2 was measured (Loreto and Velikova 2001). A wavelength of 390 nm was used to measure the absorbance.
Statistical analysis
With three replicates, the experiments were set up in a completely randomized design (CRD) factorial. The database of parameters and results was created in MS Excel. Statistix 8.1 was used to perform an analysis of variance (ANOVA) on the collected data. The statistical significance of treatment mean values was determined using the HSD value of p < 0.05. Principal component analysis (PCA) correlation was performed using XL-STAT 2021. RStudio was used to create scatter plots.
Results
Bacterial flocculation
Bacterial flocculation is the aggregation of dispersed bacterial cells into flocs or flakes. Floc yield at varying concentrations of NaCl was recorded in mg/L. It was observed that floc yield increased with increasing concentration of NaCl and maximum floc yield was observed at 100 mM concentration of NaCl (Fig. 1A).
Biofilm formation
B. tequilensis was found to be capable of producing biofilm under varying salinity levels. Biofilm formation increased at increasing salinity levels and the greatest biofilm was formed at 100 mM concentration of NaCl (Fig. 1B).
Scanning electron microscopic observation
The scanning electron microscopic photographs under saline and control conditions were compared. B. tequilensis produced EPS under saline conditions. Under control conditions, cells were scattered, while these were formed and aggregated under NaCl stress, by the production of EPS (Fig. 2).
Fourier transform infrared spectroscopic (FTIR) analysis
The main functional groups were successfully identified by FTIR spectroscopy. FTIR spectrum of B. tequilensis-EPS revealed characteristic absorption peaks of polysaccharides. Peak at 3245 cm−1 indicated the presence of hydroxyl groups. As each monosaccharide has more than one hydroxyl, it confirmed the presence of polysaccharides. The band at around 1400 cm−1 showed C–H stretching and angular vibration, indicating the presence of carbohydrates. C–N (aliphatic amines), C–Br (alkyl halide), and C=O stretching of carboxylate and amide groups (amide I band) were represented by the peaks at 664 cm−1, 1085 cm−1, and 1630 cm−1, respectively. The flocculating activity could be attributed to the presence of hydroxyl, carboxylate and amino functional groups (Fig. 3).
Fourier transform infrared (FTIR) spectrum of B. tequilensis-EPS in 400–4000 cm−1. Different functional groups (1–5) belonging to the expected type of chemical compounds that were identified based on characteristic IR bands. Absorbance (%) is given along the y-axis, while the wavelength of infrared radiation is indicated along the x-axis
Screening of genes conferring PGP traits
PCR results revealed the presence of acdS and pqqE genes in the genome of B. tequilensis (Fig. S1). Presence of these genes indicated ACC deaminase and phosphate solubilizing ability of B. tequilensis.
Germination rate
The increasing concentration of NaCl reduced germination rate of chickpea seeds. Treatment of B. tequilensis displayed a positive effect and improved germination percentage of chickpea seeds (Fig. 4A).
Influence of different salt treatments on germination percentage (a) shoot length (b), root length (c), fresh weight (d), relative electrolyte leakage (e) relative water content (f) of chickpea seedlings. Capped bars above means represent ± SE of three replicates. One-way ANOVA was applied to the collected data and the statistical significance of treatment mean values were determined using the HSD value of p < 0.05. Means with different letters varied significantly from each other
Plant analysis
Saline conditions negatively affected the lengths of shoots and roots of chickpea seedlings. Application of B. tequilensis reduced this effect, significantly. Positive influence of bacterial inoculation was obvious at all NaCl concentrations (Fig. 4B, C). Soil inoculation of B. tequilensis helped stressed plants to maintain high fresh weight of seedlings (Fig. 4D). Salinity stress damaged the seedlings of chickpea and resulted in the higher leakage of electrolytes. Inoculation of B. tequilensis positively played a role to decrease this leakage (Fig. 4E). Salinity stress also decreased the relative water content of leaves. Like other physiological parameters, the soil application of B. tequilensis helped chickpea seedlings to maintain higher RWC and grow better (Fig. 4F).
Inoculation of B. tequilensis also helped plants to maintain higher contents of chlorophyll and carotenoids (Fig. 5A, B). Deterioration of these pigments indicates plant damage. Their higher concentration described less damage to chickpea and elaborated the positive influence of B. tequilensis.
Influence of different salt treatments on chlorophyll (a) and carotenoid contents (b) of chickpea seedlings. Capped bars above means represent ± SE of three replicates. One-way ANOVA was applied to the collected data and the statistical significance of treatment mean values were determined using the HSD value of p < 0.05. Means with different letters varied significantly from each other
Total soluble sugar and proline are osmolytes that serve as antioxidants. They are abundantly accumulated in plants under stressful conditions. Proline and TSS contents were estimated in control and treated plants subjected to varying levels of salinity stress. Both of them were observed to be increased under bacterial inoculated treatments as compared to control. The plants treated with PGPR accumulated more osmolytes under saline conditions as compared to the un-inoculated ones (Fig. 6).
Influence of different salt treatments on proline content (a) and total soluble sugars (b) of chickpea seedlings. Capped bars above means represent ± SE of three replicates. One-way ANOVA was applied to the collected data and the statistical significance of treatment mean values was determined using the HSD value of p < 0.05. Means with different letters varied significantly from each other
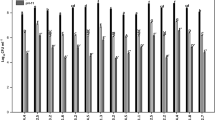
Salt stress conditions triggered the production of all tested antioxidant enzymes (SOD, POD and CAT). Interestingly, the application of B. tequilensis further increased their concentrations at all salinity levels (Fig. 7). The greatest salinity-induced increase in SOD, POD, and CAT levels of chickpea seedlings was observed at 100 mM concentration of NaCl.
Influence of different salt treatments on the production of important antioxidant enzymes including SOD (a), POD (b) and Catalase (c). Capped bars above means represent ± SE of three replicates. One-way ANOVA was applied on the collected data and the statistical significance of treatment mean values was determined using the HSD value of p < 0.05. Means with different letters varied significantly from each other
Production of MDA and H2O2 was increased under salinity stress. Increasing salt concentrations resulted in elevated production of these harmful reactive compounds. Inoculation of soil with B. tequilensis resulted in the decreased accumulation of both MDA and H2O2 at all concentration of NaCl (Fig. 8). In comparison to the non-inoculated control, B. tequilensis inoculation significantly increased the salt tolerance indices of chickpea seedlings (Fig. 9).
Influence of different salt treatments on the production of MDA (a) and H2O2 (b) of chickpea seedlings. Capped bars above means represent ± SE of three replicates. One-way ANOVA was applied on the collected data and the statistical significance of treatment mean values was determined using the HSD value of p < 0.05. Means with different letters varied significantly from each other
Salt tolerance index of chickpea seedlings under control and treated conditions. Salt was provided in three different concentrations including 25 mM, 50 mM and 100 mM. Capped bars above means represent ± SE of three replicates. One-way ANOVA was applied to the collected data and the statistical significance of treatment mean values was determined using the HSD value of p < 0.05. Means with different letters varied significantly from each other
Pearson correlation biplot
Positive influence of B. tequilensis inoculation on chickpea seedlings was confirmed by principal component analysis (PCA). Orange dots showed the correlation among different control and treated plants under normal and saline conditions. Whereas blue dots represented the correlation among different parameters. The variables that were clustered together in the same quadrant had a positive correlation. Morphological parameters like chlorophyll content and RWC showed a positive correlation with each other and had a negative correlation with antioxidant enzymes, osmolytes, and oxidative compounds (Fig. 10).
Scatter plot
Scatter plot depicted the a correlation among the parameters under 100 mM of NaCl stress. While a correlation coefficients described the linkage of two variables. The correlation coefficient, larger than zero, indicated a positive correlation, while a negative correlation was signified by coefficient values less than zero. The zero value signified no correlation. In the correlation analysis, we have tried to disentangle the possible link and relation between different studied parameters. Correlation coefficients were calculated for the values of different parameters. Chlorophyll contents, growth attibutes, RWC, SOD, POD and CAT had a positive correlation among them but they showed a negative correlation with REL, H2O2 and MDA. STI was negatively correlated with REL, MDA and H2O2 (Fig. S2).
Discussion
Plant growth promoting rhizobacteria (PGPR) play a significant role in plant growth, under various stresses (Ullah et al. 2015). In this study, a PGPR (B. tequilensis) was successfully inoculated to mitigate salinity stress of chickpea. B. tequilensis exhibited bacterial flocculation and biofilm formation traits. Bacterial flocculation has been directly related to the production of bacterial exopolysaccharides. It aids bacterial existence in stressed environments and assists plants in stress tolerance (Qureshi and Sabri 2012). Exopolysaccharides are associated with the formation of a bacterial biofilm, which facilitates bacterial adhesion on plant roots (Chen et al. 2013). The mechanism of EPS-mediated amelioration of salt stress in plants has been gradually investigated in recent years (Abbas et al. 2019). According to reports, EPS directly affects how well plants tolerate salt (Liu et al. 2022). Because it may chelate free Na + from the soil, prevent Na+ from entering plants, assist the production of biofilms, and improve soil stability, EPS generated by PGPR under salt stress is essential for plant protection (Liu et al. 2022). High floc yield protects host plants at higher salt concentrations (Hong et al. 2017). These findings about enhanced floc yield at different NaCl concentrations support a prior study that found that increasing the NaCl concentration had an influence on flocculation, which changed how bacterial strains adhered to one another (Asmassie et al. 2022). Furthermore, under salt stress conditions, biofilm serves as a barrier between both bacteria and surroundings and safeguards them, inside the EPS layer. This study revealed maximum biofilm formation at higher salt concentrations. Kasim et al. (2016) previously stated that the increasing concentrations of NaCl enhance the formation of biofilms. An essential trait of Bacillus strains that aids in their resistance to environmental stressors is biofilm development. The development of bacteria in the self-secreted matrix for biofilm formation aids their ability to endure challenging circumstances (Ayaz et al. 2022).
SEM also supported salt-tolerance characteristics of tested bacteria. Bacterial cells can work together with the root system of plants to enhance their water retention and stress tolerance ability. In the prior study, typical cell structural alterations and increased aggregation were seen in the SEM image under stressed conditions, supporting their ability to adapt to challenging environments (Gunasekaran et al. 2022).
FTIR spectroscopy revealed the presence of hydroxyl, amino and carboxyl groups, which bind with Na+ ions and provide tolerance against salinity (Watanabe et al. 2003; Nunkaew et al. 2015; Sultana et al. 2020). In this study, the amplification of 1-aminocyclopropane-1-carboxylate deaminase (acdS) and pyrroloquinoline quinone (pqqE) genes described the growth promoting ability of B. tequilensis. Gene acdS promotes the formation of ammonia and alpha ketobutyrate from ACC, leading to lower ethylene production and promoting plant development and growth (Kang et al. 2019). Gene pqqE is a key element of the PQQE operon and is engaged in phosphate solubilization (Hayat et al. 2010; Alyousif 2022).
Inoculation of B. tequilensis improved the physiological traits of chickpea seedlings, in this study. PGPR has been described to improve the germination of different plants (Nelson 2004; Ureche et al. 2021). During salinity stress, an increase in RWC has also been described earlier (Rakshapal et al. 2013). Under the stress condition, electrolytic discharge such as potassium ions increases by replacing calcium ions present in the plasma membrane. As a result, membrane permeability is compromised, resulting in increased electrolyte efflux within plant cells/tissues (Garg and Manchanda 2009). The results of our study depicted that the plants under the influence of B. tequilensis and salinity stress had low electrolytic leakage in comparison to control treatments. The findings of the current study showed that under the salt stress conditions chlorophyll contents were decreased. This might be because the chlorophyllase which is salinity stimulated, degrades pigment proteins that decreases the synthesis of chlorophyll level in plants (Abd Allah et al. 2018; Petjukevics and Skute 2022). Under salinity stress, carotenoid synthesis was increased in chickpea. Carotenoids act as antioxidants to manage stressful conditions (El Esawi et al. 2019).
For the stress management, plant growth promoting rhizobacteria stimulates the production of ROS scavenging enzymes. In the present investigation, B. tequilensis increased the production of SOD, POD and CAT, under the influence of salinity stress. It has been reported earlier that under the influence of high salt stress, the antioxidant enzyme activities are enhanced to subsequently eliminate harmful free radicals (Abd Allah et al. 2018; Rasool et al. 2013). Over the course of their long evolutionary history, plants have evolved a variety of defence mechanisms to adapt to various stressful situations, one of which is the scavenging of ROS by antioxidant enzyme systems, which is a key strategy to maintain normal plant growth under salt stress (Wang et al. 2022).
Oxidative damage under stress is indicated by lipid peroxidation that is dignified by MDA production in plants. In the current investigation, it was observed that B. tequilensis inoculation decreased the production of MDA, even under varying levels of salinity. The production of H2O2 was high in chickpea seedlings under saline conditions but the inoculation of B. tequilensis stimulated defense mechanism and lowered its production (Gupta et al. 2017). This study has linked the bacterial production of exopolysaccharides with the increased STI of chickpea seedlings. Exopolysaccharides may alter chickpea rhizosphere by forming a biofilm on the root surface, resulting in improved water and nutrient availability (Hussain et al. 2014).
Conclusion
The goal of this study was to investigate the positive effects of locally isolated, salt-tolerant PGPR B. tequilensis on the development of chickpea plants under salinity stress. Hence it is concluded that, B. tequilensis is a halotolerant PGPR and its inoculation in soil can promote the growth and development of chickpea plant. This bacterium helps plants to tolerate salinity stress conditions by enhancing the production of total soluble sugar, proline and antioxidant enzymes. Bacillus tequilensis can be used as an excellent biofertilizer to mitigate salinity stress.
Data availability
Not applicable.
Code availability
Not applicable.
References
Abbas R, Rasul S, Aslam K, Baber M, Shahid M, Mubeen F, Naqqash T (2019) Halotolerant PGPR: a hope for cultivation of saline soils. J King Saud Uni Sci 31(4):1195–1201
AbdAllah EF, Hashem A, Alqarawi AA, Bahkali AH, Alwhibi MS (2015) Enhancing growth performance and systemic acquired resistance of medicinal plant Sesbania sesban (L.) Merr using arbuscular mycorrhizal fungi under salt stress. Saudi J Biol Sci 22(3):274–283
Admassie M, Woldehawariat Y, Alemu T, Gonzalez E, Jimenez JF (2022) The role of plant growth-promoting bacteria in alleviating drought stress on pepper plants. Agric Water Manag 272:107831
Alyousif NA (2022) Review of genetic analysis and mechanisms of phosphate solubilization by phosphate solubilizing bacteria. Marsh Bulletin 17:1
Ayaz M, Ali Q, Jiang Q, Wang R, Wang Z, Mu G, Gu Q (2022) Salt tolerant bacillus strains improve plant growth traits and regulation of phytohormones in wheat under salinity stress. Plants 11(20):2769
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39(1):205–207
Bradford MM (1976) A rapid and sensitive for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Chance M, Maehly AC (1955) Assay of catalases and peroxidases. Methods Enzymol 2:764–817
Chen Y, Yan F, Chai Y, Liu H, Kolter R, Losick R, Guo JH (2013) Biocontrol of tomato wilt disease by Bacillus subtilis isolates from natural environments depends on conserved genes mediating biofilm formation. Environ Microbiol 15(3):848–864
Christensen GD, Simpson WA, Younger JJ, Baddour LM, Barrett FF, Melton DM, Beachey EH (1985) Adherence of coagulase-negative staphylococci to plastictissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J Clin Microbiol 22(6):996–1006
Cook RJ (2002) Advances in plant health management in the twentieth century. Annu Rev Phytopathol 38:95–116
El-Esawi MA, Al-Ghamdi AA, Ali HM, Alayafi AA (2019) Azospirillum lipoferum FK1 confers improved salt tolerance in chickpea (Cicer arietinum L.) by modulating osmolytes, antioxidant machinery and stress-related genes expression. Environ Exp Bot 159:55–65
Fatima T, Arora NK (2021) Pseudomonas entomophila PE3 and its exopolysaccharides as biostimulants for enhancing growth, yield and tolerance responses of sunflower under saline conditions. Microbiol Res 244:126671
Garg N, Manchanda G (2009) Role of arbuscular mycorrhizae in the alleviation of ionic, osmotic and oxidative stresses induced by salinity in Cajanus cajan (L.) Millsp.(pigeonpea). J Agron Crop Sci 195(2):110–123
Giannopolitis CN, Ries SK (1977) Superoxide dismutase. I. Occurrence in higher plants. Plant Physiol 59:309–314
Gunasekaran Y, Thiyageshwari S, Ariyan M, Roy Choudhury A, Park JH, Selvi D, Anandham R (2022) Alleviation of sodic stress in rice by exploring the exopolysaccharide-producing sodic-tolerant bacteria. Agric 12(9):1451
Gupta S, Pandey S (2019) ACC deaminase producing bacteria with multifarious plant growth promoting traits alleviates salinity stress in French bean (Phaseolus vulgaris) plants. Front Microbiol 10:1506
Gupta R, Singh A, Ajayakumar PV, Pandey R (2017) Microbial interference mitigates Meloidogyne incognita mediated oxidative stress and augments bacoside content in Bacopa monnieri L. Microbiol Res 199:67–78
Hahm MS, Son JS, Hwang YJ, Kwon DK, Ghim SY (2017) Alleviation of salt stress in pepper (Capsicum annum L.) plants by plant growth-promoting rhizobacteria. J Microbiol Biotechnol 27(10):1790–1797
Haroon U, Khizar M, Liaquat F, Ali M, Akbar M, Tahir K, Munis MFH (2021a) Halotolerant plant growth-promoting rhizobacteria induce salinity tolerance in wheat by enhancing the expression of SOS genes. J Plant Growth Regul 40(3):1–14
Haroon U, Liaquat F, Khizar M, Akbar M, Saleem H, Arif S, Munis MFH (2021b) Isolation of halotolerant bacteria from rhizosphere of Khewra salt mine halophytes and their application to induce salt tolerance in wheat. Geomicrobiol J 38(9):768–775
Hayat R, Ali S, Amara U, Khalid R, Ahmed I (2010) Soil beneficial bacteria and their role in plant growth promotion: a review. Ann Microbiol 60(4):579–598
Hong BH, Joe MM, Selvakumar G, Kim KY, Choi JH, Sa TM (2017) Influence of salinity variations on exocellular polysaccharide production, biofilm formation and flocculation in halotolerant bacteria. J Environ Biol 38(4):657
Hussain MB, Zahir ZA, Asghar HN, Mahmood S (2014) Scrutinizing rhizobia to rescue maize growth under reduced water conditions. Soil Sci Soc Am J 78(2):538–545
Ibarra-Villarreal AL, Gándara-Ledezma A, Godoy-Flores AD, Herrera-Sepúlveda A, Díaz-Rodríguez AM, Parra-Cota FI, de los Santos-Villalobos S (2021) Salt-tolerant Bacillus species as a promising strategy to mitigate the salinity stress in wheat (Triticum turgidum subsp. durum). J Arid Environ 186:104399
Kang SM, Shahzad R, Bilal S, Khan AL, Park YG, Lee KE, Lee IJ (2019) Indole-3-acetic-acid and ACC deaminase producing Leclercia adecarboxylata MO1 improves Solanum lycopersicum L. growth and salinity stress tolerance by endogenous secondary metabolites regulation. BMC Microbiol 19(1):1–14
Kasim WA, Gaafar RM, Abou-Ali RM, Omar MN, Hewait HM (2016) Effect of biofilm forming plant growth promoting rhizobacteria on salinity tolerance in barley. Ann Agric Sci 61(2):217–227
Kim CH, Han SH, Kim KY, Cho BH, Kim YH, Koo BS, Kim YC (2003) Cloning and expression of pyrroloquinoline quinone (PQQ) genes from a phosphate-solubilizing bacterium Enterobacter intermedium. Current Microbiol 47(6):457–461
Kloepper JW, Schroth MN (1978) Plant growth-promoting rhizobacteria on radishes. In: Proceedings of the 4th international conference on plant pathogenic bacteria. Gilbert-Clarey, Tours, pp 879–882
Kloepper JW, Schroth MN (1981) Relationship of in vitro antibiosis of plant growth promoting rhizobacteria to plant growth and the displacement of root microflora. Phytopathol 71:1020–1024
Kotula L, Clode PL, Jimenez JDLC, Colmer TD (2019) Salinity tolerance in chickpea is associated with the ability to ‘exclude’ Na from leaf mesophyll cells. J Exp Bot 70(18):4991–5002
Kumar A, Singh S, Mukherjee A, Rastogi RP, Verma JP (2021) Salt-tolerant plant growth-promoting Bacillus pumilus strain JPVS11 to enhance plant growth attributes of rice and improve soil health under salinity stress. Microbiol Res 242:126616
Laranjeira S, Reis S, Torcato C, Raimundo F, Ferreira L, Carnide V, Marques G (2022) Use of plant-growth promoting rhizobacteria and mycorrhizal fungi consortium as a strategy to improve chickpea (Cicer arietinum L.) productivity under different irrigation regimes. Agron 12(6):1383
Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents, pp 591–592
Liu X, Chai J, Zhang Y, Zhang C, Lei Y, Li Q, Yao T (2022) Halotolerant rhizobacteria mitigate the effects of salinity stress on maize growth by secreting exopolysaccharides. Environ Exp Bot 204:105098
Loreto F, Velikova V (2001) Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol 127(4):1781–1787
Lutts S, Kinet JM, Bouharmont J (1996) NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. Ann Bot 78(3):389–398
Madurapperumage A, Tang L, Thavarajah P, Bridges W, Shipe E, Vandemark G, Thavarajah D (2021) Chickpea (Cicer arietinum L.) as a source of essential fatty acids—a biofortification approach. Front Plant Sci 12:1–12
Manmathan H, Lapitan NL (2013) Measuring germination percentage in wheat. Bio-Protoc 3(16):e866–e866
Mazumdar D, Saha SP, Ghosh S (2020) Isolation, screening and application of a potent PGPR for enhancing growth of Chickpea as affected by nitrogen level. Int J Veg Sci 26(4):333–350
Naing AH, Jeong HY, Jung SK, Kim CK (2021) Overexpression of 1-aminocyclopropane-1-carboxylic acid deaminase (acds) gene in Petunia hybrida improves tolerance to abiotic stresses. Front Plant Sci 12:737490
Nelson LM (2004) Plant growth promoting rhizobacteria (PGPR): prospects for new inoculants. Crop Manag 3(1):1–7
Nunkaew T, Kantachote D, Nitoda T, Kanzaki H, Ritchie RJ (2015) Characterization of exopolymeric substances from selected Rhodopseudomonas palustris strains and their ability to adsorb sodium ions. Carbohydr Polym 115:334–341
Petjukevics A, Skute N (2022) Chlorophyll fluorescence changes, as plant early state indicator under different water salinity regimes’ on invasive macrophyte Elodea canadensis (Michx., 1803). ARPHA Preprints 3:e82408
Porra RJ (2002) The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth Res 73(1):149–156
Qurashi AW, Sabri AN (2012) Biofilm formation in moderately halophilic bacteria is influenced by varying salinity levels. J Basic Microbiol 52(5):566–572
Rakshapal S, Sumit KS, Rajendra PP, Alok K (2013) For improving essential oil yield of Ocimum basilicum L. (sweet basil) by application of bioinoculant colonized seeds under organic field conditions. Ind Crop Prod 45:335–342
Rasool S, Ahmad A, Siddiqi TO, Ahmad P (2013) Changes in growth, lipid peroxidation and some key antioxidant enzymes in chickpea genotypes under salt stress. Acta Physiol Plant 35(4):1039–1050
Sadasivan LAKSHMI, Neyra CA (1985) Flocculation in Azospirillum brasilense and Azospirillum lipoferum: exopolysaccharides and cyst formation. J Bacteriol 163(2):716–723
Sarkar A, Pramanik K, Mitra S, Soren T, Maiti TK (2018) Enhancement of growth and salt tolerance of rice seedlings by ACC deaminase-producing Burkholderia sp. MTCC 12259. J Plant Physiol 231:434–442
Shukla PS, Agarwal PK, Jha B (2012) Improved salinity tolerance of Arachis hypogaea (L.) by the interaction of halotolerant plant-growth-promoting rhizobacteria. J Plant Growth Regul 31(2):195–206
Shultana R, Kee Zuan AT, Yusop MR, Saud HM (2020) Characterization of salt-tolerant plant growth-promoting rhizobacteria and the effect on growth and yield of saline-affected rice. PLoS ONE 15(9):e0238537
Singh RP, Jha PN (2016) The multifarious PGPR Serratia marcescens CDP-13 augments induced systemic resistance and enhanced salinity tolerance of wheat (Triticum aestivum L.). PLoS ONE 11(6):e0155026
Singh M, Sood S (2020) Millets and pseudo cereals: genetic resources and breeding advancements. Woodhead Publishing
Tank N, Saraf M (2010) Salinity-resistant plant growth promoting rhizobacteria ameliorates sodium chloride stress on tomato plants. J Plant Interact 5(1):51–58
Tirry N, Kouchou A, Laghmari G, Lemjereb M, Hnadi H, Amrani K, El Ghachtouli N (2021) Improved salinity tolerance of Medicago sativa and soil enzyme activities by PGPR. Biocatal Agric Biotechnol 31:101914
Ullah A, Heng S, Munis MF, Fahad S, Yang X (2015) Phytoremediation of heavy metals assisted by plant growth promoting (PGP) bacteria: a review. Environ Exp Bot 117:28–40
Ureche MAL, Pérez-Rodriguez MM, Ortiz R, Monasterio RP, Cohen AC (2021) Rhizobacteria improve the germination and modify the phenolic compound profile of pepper (Capsicum annum L.). Rhizosphere 18:100334
Vessey JK (2003) Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 255:571–586
Wang W, Liu S, Yan M (2022) Synthesis of γ-aminobutyric acid-modified chitooligosaccharide derivative and enhancing salt resistance of wheat seedlings. Molecules 27(10):3068
Watanabe M, Kawahara K, Sasaki K, Noparatnaraporn N (2003) Biosorption of cadmium ions using a photosynthetic bacterium, Rhodobacter sphaeroides S and a marine photosynthetic bacterium, Rhodovulum sp. and their biosorption kinetics. J Biosci Bioeng 95(4):374–378
Weatherley PE (1950) Studies in the water relations of cotton plants. I. The field measurement of water deficit in leaves. New Phytol 49:81–87
Weller DM, Raaijmakers JM, Gardener BB, Thomashow LS (2002) Microbial populations responsible for specific soil suppressiveness to plant pathogens. Annu Rev Phytopathol 40:309–348
William S, Feil H, Copeland A (2012) Bacterial genomic DNA isolation using CTAB. Sigma 50:6876
Yasin NA, Zaheer MM, Khan WU, Ahmad SR, Ahmad A, Ali A, Akram W (2018) The beneficial role of potassium in Cd-induced stress alleviation and growth improvement in Gladiolus grandiflora L. Int J Phytoremediation 20(3):274‒283
Acknowledgements
The authors would like to acknowledge National Agricultural Research Centre (NARC), Islamabad for providing chickpea seeds.
Author information
Authors and Affiliations
Contributions
UH performed the experiments, prepared the manuscript; FL and MK did bacterial characterization; ME did statisical analysis; HJC did formal analysis; MFHM did supervision, analyzed the results and checked manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Haroon, U., Munis, M.F.H., Liaquat, F. et al. Biofilm formation and flocculation potential analysis of halotolerant Bacillus tequilensis and its inoculation in soil to mitigate salinity stress of chickpea. Physiol Mol Biol Plants 29, 277–288 (2023). https://doi.org/10.1007/s12298-023-01280-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-023-01280-1