Abstract
To increase food production, prevalent agricultural malpractices such as intensive use of fertilizers and pesticides have led to degradation of the ecosystem. In this situation, there is a need to encourage eco-friendly and sustainable methods for improving crop production under ever increasing abiotic stress conditions. One such method can be through use of arbuscular mycorrhizal fungi (AMF or AM fungi). Soil microorganisms such as AMF serve as a link between plants and the soil resources. AMF represent a key functional group of soil microbiota that is fundamental for soil fertility, crop productivity, yield, quality and ecosystem resilience. AMF potentially increases bioavailability of water as well as various micro- and macro- nutrients which enhances production of plant photosynthates. In plants, inoculation with AMF led to increased photochemical efficiency ultimately resulting in enhanced plant growth. In this review we have summarized amelioration of drought or water scarcity, salt stress, increasing temperature or high temperature and heavy metal stresses etc. in crop plants by AMF through its effects on various physiological and biochemical processes including photosynthesis. The review also highlights AMF induced tolerance and adaptive mechanisms which protect crops from stresses. We conclude the review with a discussion of unseen issues and suggestions for future researches.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction: crop production, photosynthesis and arbuscular mycorrhizal fungi
Plants use several protective mechanisms to cope up with undesirable environmental conditions. Stress conditions affect plants in a number of ways, such as disturbance in osmotic and ionic balance, disturbance of stomatal opening, inhibition in photosynthetic activity, reduction and modifications in important proteins and gene expression etc. which results in decreased plant growth rate and overall productivity (Lenoir et al. 2016; Begum et al. 2019). There is an urgent need for eco-friendly and sustainable techniques for improving crop production without disturbing the ecosystem. The interaction between beneficial soil microbes and plants can play a major role in sustainable crop management. To increase crop productivity, adoption of environment friendly technique, such as use of arbuscular mycorrhizal fungi (AMF or AM fungi) may prove to be an excellent alternative to chemical fertilizers. Soil microorganisms like AMF represent an important link between plants and soil mineral nutrients. AMF, often referred to as “agro-ecosystem engineers”, represent an important component of soil micro-biota which are fundamental for soil fertility, crop productivity, yield, quality and ecosystem resilience and thus are very important for agriculture (Smith and Read 2008). AMF play an important role in agriculture as it may reduce the dependency on chemical fertilizers (Srivastava et al. 2017; Ryan and Graham 2018). AMF form symbiotic association with the plant-roots and help in providing important nutrients to host or colonized plants, enhance their growth photosynthesis and overall crop production. It forms hyphal network in roots and improve the access and exposure of roots to a larger soil surface area. AMF also helps in improving soil structure, quality and texture (Thirkell et al. 2017) by accelerating the decomposition in soil organic matter. For these reasons, AMF have been able to generate growing interest as natural fertilizers. It is well known that AMF facilitate fixation of atmospheric CO2 in host plants (Begum et al. 2019). This is mainly done by enhancing “sink effect” and diverting transportation of photo-assimilates from the above ground parts (leaves) to the below ground parts i.e., roots. An increased activity of numerous antioxidant enzymes have been reported in response to many type of climatic stress. Theses antioxidant enzymes facilitate to inhibit and scavenge the ROS production thereby decreasing the negative impact of oxidative stress on different molecules in the cell. Under stress conditions, rapid scavenging of ROS occurs due to increased activity of antioxidant enzymes helping in keeping ROS at low levels when inoculated with AMF (Hashem et al. 2015). AMF have the potential to improve the production of photo-assimilates by increasing photosynthetic rate and indirectly reducing ROS formation (Hashem et al. 2016). As compared to non inoculated plants, AMF colonized plants showed a much reduce amount of H2O2 and malondialdehyde (Talaat and Shawky 2014). This lower content is in corroboration that AMF helped the plants from major ROS, oxidative stress and membrane damage under salinity stress (Talaat and Shawky 2014). Increased proline and glycine betaine activity is considered as one of the important cause for thylakoid protection against ROS (Talaat and Shawky 2014). AMF not only increased proline and glycine betaine activity under stress but also promoted activities of α-tocopherols, ascorbate (AsA), glutathione (GSH), and carotenoids in plants (Evelin and Kapoor 2014). Presence of higher α-tocopherol in AMF plants probably protects auto-oxidation of lipids and thus protects plants against lipid peroxidation (Serbinova and Packer 1994). High α-tocopherol content limits auto-oxidation of lipids, thus preventing damage to AMF plants from lipid peroxidation (Serbinova and Packer 1994). High α-tocopherol content in AMF colonized plants results in improved production of tocopheroxyl radicals, facilitated by the high AsA content (Noctor and Foyer 1998). Higher reduced glutathione (GSH) levels and glutathione reductase (GR) activity result in higher AsA levels in plants. GSH enables plants to directly scavenge more.O2– and H2O2 as well as other ROS (Noctor and Foyer 1998), and recycle more AsA via the AsA glutathione cycle (Foyer and Halliwell 1976). Carotenoids are known to prevent.O2– production (Ramel et al. 2012; Evelin et al. 2019). In plants, AMF reduced the amount of ROS and thus led to amelioration of stress.
Photosynthesis is the fundamental process on earth which help to synthesize organic matter for plant by using solar energy. Photosystems I (PSI) and II (PSII), which are important components of the photosynthetic apparatus, are sensitive to various environmental stresses (Mathur et al. 2014). AMF colonizing the host plant’s root alter the carbon cycle and the process of photosynthesis in plants. Higher the photosynthesis more is the production of photoassimilates in the host plants. The higher photosynthetic rates associated with AMF colonization with host plant roots can result in higher concentration of soluble sugars and photosynthetic by products in the leaf symplasm, which is reflected as an increased osmolarity in cytoplasm in AM plants (Porcel and Ruiz-Lozano 2004). Abiotic stresses inhibit enzymes involved in the process of chlorophyll biosynthesis along with increase in chlorophyllase activity which triggers chlorophyll degradation (Zhu et al. 2017). In crop plants, down-regulation of chlorophyllase activity and up-regulation chlorophyll biosynthetic genes by AMF inoculation results in increased pigment synthesis (Shahabivand et al. 2012). There is increased activity of sucrose phosphate synthase in AMF inoculated plants which probably contribute to the maintenance of sufficient carbon pool for several other metabolic pathways (Al-Arjani et al. 2020). Levels of N and P in leaf are more affected in mycorrhizal plants and have been observed to scale equally with photosynthesis (Grimoldi et al. 2006). This may be due to the reason that efficiency of biochemical processes in chloroplasts (such as electron transport and carboxylation capacity) is limited by tissue concentrations of N and P (Walker et al. 2014). Ribulose bisphosphate carboxylase oxygenase (RuBisCO) in chloroplast is the major pool for leaf N (Evans 1989) while P mainly constitutes ATP and reduction equivalents responsible for photo-phosphorylation (Jacob and Lawlor 1993). Sinks like rhizobia and AMF may help in overcoming end-product limitation of photosynthesis (Kaschuk et al. 2012).
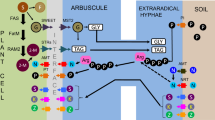
Increase in uptake of several micro and macro nutrients have been observed under AMF colonization. The increase is followed by an enhanced biomass production of the plants (Chen et al. 2017). Not only macronutrients, but AMF association also increases the availability of micronutrients like zinc and copper, nitrogen, potassium, calcium, and phosphorus. This results in increased leaf area and enhanced photosynthetic activity. AMF provides more nutrition and moisture to plants even under adverse conditions inside the root cells (Smith and Read 2008). The surface area (~100 folds) and length of the hyphae is much higher than that of the plant root system. As a result they can facilitate improved nutrient and water uptake by releasing nutrients from soil particles (Rabie 2005). Arbuscules are the main site in AMF where actual nutrient and mineral exchange take place. This exchange protect and provide stability to the host plant (Prasad et al. 2017).
In phosphorus (P) deficient conditions, AMF symbiosis makes availability of P to the host plants. Maize plants inoculated with AMF showed increased inorganic P absorption (Garcés-Ruiz et al. 2017). Amiri et al. (2017) have reported that in drought stressed conditions AMF increased N, P, and Fe concentrations in Pelargonium graveolens L. (Amiri et al. 2017). In the same way, Euonymus japonica inoculated with AMF showed enhanced P, Ca, and K concentrations under salinity stress (Gomez-Bellot et al. 2015). AMF also improved the seedling weight by improving water content and intercellular CO2, P, and N contents in Leymus chinensis (Lin et al. 2017) which ultimately improved photosynthesis.
AMF play a pivotal role in the N cycle (Hodge and Fitter 2010). The extra-radical hyphae of AMF can absorb and assimilate inorganic N. Around 20–75% of the total uptake of N by the AM plants can be transferred to their hosts (Govindarajulu et al. 2005). AMF inoculated plants are rich in N content which ultimately result in increased chlorophyll content (since chlorophyll molecules can effectively trap N) in the host plants (De Andrade et al. 2015).
AMF facilitates nutrient accumulation in tissues, provide moisture, stimulate sink metabolism, leading to higher photosynthesis rates. Symbiotic AMF use carbon resource of plant, but compensates it by higher photosynthetic rates. It happens because a strong C sink is created due to fungal metabolism which prevents accumulation of photosynthates and down regulation of photosynthetic process. Gavito et al. (2019) provided first direct experimental evidence for the carbon sink strength effects exerted by AMF on plant photosynthesis. They reported that there is a strong relationship among photosynthesis and nitrogen availability since more than half of N in leaves is engaged in the photosynthetic machinery (Rubisco). Plants come across a number of stresses that inhibit their growth and photosynthetic activity. However AMF could overcome inhibitory effects of these stresses to a significant extent. A summary of protective effects of AMF in different crops and under various abiotic stresses is presented to emphasize the usefulness of AMF to improve crop production through its effect on various metabolic processes (Table 1). As is evident from Table 1, AMF promotes root colonization and facilitates nutrient uptake, sugar transport, photosynthetic rate, water content, CO2 assimilation etc. Apart from this AMF also changes soil characteristics making it more porous, improves surface absorbing capacity of host roots.
Thus, because of these unique properties, AMF have proven to be an important measure for sustainable agricultural practices by helping the plant to cope up with stress conditions. We have compiled and presented data and information from our own recently published work on AMF and from hundreds of other published research papers. The present review provides comprehensive and up-to-date information on role of AMF in crop improvement, their mechanism of action and their beneficial effects on crop photosynthesis and productivity under various abiotic stresses.
AMF and protection of crops under various abiotic stresses
AMF is beneficial for crops facing abiotic stresses. Table 2 shows crop plants studied for protection provided by AMF under various types of abiotic stresses. Studies have been done on various crops like soybean, maize, wheat, sorghum, barley, garlic, peach etc. colonized with AMF. The various species of AMF were effective in ameliorating several abiotic stresses like high temperature, drought, salinity, environmental stress etc. (Table 2). More specific effects of AMF on crops to ameliorate particular stress are discussed below.
Water stress
Drought stress
Drought (water scarcity) has a negative impact on growth, development, and productivity of plants by affecting enzyme activity, ion uptake, and nutrient assimilation. Wilting of leaves, reduction in the rate of net photosynthesis, stomatal conductance, water use efficiency, are the major symptoms of drought stress (Abbaspour et al. 2012). Drought condition leads to closing of stomata, resulting in decreased CO2 uptake and impaired photosynthesis (Ganugi et al. 2019; Mathur et al. 2019). AMF has proven to ameliorate drought stress (Fig. 1) in many crops such as wheat (Mathur et al. 2019), barley, maize, soybean, strawberry, and onion (Ruiz-Lozano et al. 2015; Yooyongwech et al. 2016). AMF not only protects the plant under drought stress but also plays an important role in improvising leaf area, root efficacy in terms of its size and biomass (Begum et al. 2019).
Improved leaf water potential, gas exchange, photosynthetic activities are some of the known positive effects of AM symbiosis in response to drought stress (Lee et al. 2012). This may be because of more water uptake by the extraradical hyphae in mycorrhizal roots. This leads to higher water content and upregulation of photosynthetic rate by increasing root hydraulic conductivity or by altering root architecture. AMF is also reported to alter water regulation in the host plant by regulating hormonal signalling or by stimulating osmolytes (such as higher concentration of photosynthetic by-products and soluble sugars in leaf symplasm) (Fan and Liu 2011). Better photosynthetic performance in AMF inoculated and drought exposed plants was correlated with the accumulation of the antioxidant compounds such as glutathione, which in turn lowered cellular H2O2 and decreased membrane lipids (Ruiz-Sanchez et al. 2010). A decline in Mg uptake (an important component of chlorophyll) was observed under drought conditions while AMF colonized plants under similar conditions not only reinstate Mg uptake but also made it accessible for chlorophyll synthesis. Cheng et al. (2021) in their study on trifoliate orange observed higher photosynthetic activity, stomatal conductance, intercellular CO2 concentration, and transpiration rate. They reported that AMF inoculated plants had improved root volume and diameter as compared to non—AMF plants under drought stress.
Shoot and root weights, leaf area, total chlorophyll and flavonoid content increased significantly in mycorrhizal plants under drought conditions in Pistachio plants (Abbaspour et al. 2012). AM seedlings consistently had a higher content of soluble sugars in leaves than in non-AM under water stress conditions which indicate a higher photosynthetic activity. This increase in plant growth has been attributed to the improvement of water uptake resulting in the enhancement of P nutrition. External hyphae help in water absorption and transport (Augé et al. 2003; Abbaspour et al. 2012). AMF uses one more strategy for stress tolerance. Chaperon production is one of those strategies that forbid aggregation or missfolding of proteins under stress conditions. In R. irregularis under drought and salinity, genes encoding for 14–3-3 protein and the luminal binding protein (BiP) have been reported. Specific genes encoding for a 14–3-3 protein and the luminal binding protein (BiP) have been identified under drought and saline stress (Estrada et al. 2012) (Fig. 1). Trehalose, a nonreducing disaccharide, plays an important physiological role as drought stress protectant. In fungi, trehalose is a universal storage carbohydrate involved in defence mechanisms. Under drought conditions, cell structure is stabilised and proteins maintain their native structure by trehalose accumulation (Lenoir et al. 2016). A remarkable feature of trehalose is that once the stress conditions are over, trehalose returns to original concentration. Thus, trehalose can have a potential protective role in AMF colonized host plants under drought, high temperature, saline and chemical exposure stress conditions. (Lenoir et al. 2016). Many signalling molecules have the potential to act as phytohormones under specific conditions. Example for these include ethylene, ABA, cytokinins, salicylic acid (SA), jasmonic acid (JA), and auxin which are triggered during the process of AMF symbiosis against drought stress by improvement in plant water status by increasing hydraulic conductivity. In Solanum lycopersicum, AMF enhanced the plant drought tolerance by regulating the 14–3-3 genes (TFT1-TFT12) in the ABA signaling pathway and improved the plant water relations (Bahadur et al. 2019). ABA is one of the non-nutritional mechanisms by which AM symbiosis regulate stomatal conductance and other physiological traits in response to drought stress (Bahadur et al. 2019; Doubková et al. 2013).
Drought stress (DS) was further studied in detail in wheat plants with and without AMF by Mathur et al. (2019). Authors observed following changes in response to AMF colonization of the wheat plant’s root. Improved relative water content (RWC) for leaf and soil was observed for AMF enriched plants, advocating that AMF hyphae has the potential to penetrate profoundly in the soil and provide increased moisture to the host plants. Plants colonized with AMF had more total chlorophyll content as compared to control wheat plants (grown in soil without AMF inoculation). Due to drought stress, very less water was available and as a result of which Chl content decreased drastically in control plants. However, AMF + DS plants showed higher Chl concentration as they could get water through hyphae. AMF inoculation presumably enhanced Mg absorption via hyphae to plants, which is considered as probable reason to improve total chlorophyll content in AMF and AMF + DS wheat plants. AMF enrichment simultaneously enhanced photosynthates and their transportation. Chlorophyll (Chl) a fluorescence induction curves were measured in wheat plants with and without AMF inoculated in drought conditions. Based on these biophysical measurements, it was demonstrated that, AMF inoculated wheat plants showed better photochemical efficiency and photosynthetic capacity in comparison to control plants. In DS wheat plants absorbance, electron transport, and energy trapping were declined while dissipation increased followed by closure of active reaction centers whereas all these parameters recovered in DS + AMF wheat plants. The number of active reaction centers also increased in AMF + DS plants. Along with this, total chlorophyll content of the drought stressed leaves reduced which was recovered in AMF + DS plants. The reason could be an adequate supply of N and other nutrients in AMF plants which protected them under stress condition. Rate of primary photochemistry decreased in DS plants, while AMF plants showed maximum photochemistry. Further, quantum efficiency of PSII (YII) in AMF plants was found to be higher as compared to control plants. This indicated that AMF plants had photochemically active PS II reaction centres which could use the absorbed photons to carry our actual photochemistry. Quantum yield of PSII decreased drastically in DS plants while Y(II) enhanced in AMF + DS plants. A decreased PSI quantum efficiency was observed in drought stressed wheat plants, while it showed recovery for AMF + DS wheat plants. Drought stress downregulated electron transport rates for PSI and PSII but upregulated quantum yield of non-photochemical quenching Y(NPQ) (Mathur et al. 2019). Thus AMF enrichment effectively reduced the negative impact of drought stress for PSII and PSI in wheat plants (Mathur et al. 2019).
Water logging
Soil water logging has strong and negative effect on plant growth and production (Ren et al. 2014). During water logging conditions, diffusion of oxygen into the soil is limited resulting in an anaerobic condition which inhibits several physiological activities of the plant (Malik et al. 2002). AMF potentially enhances the tolerance of the host plants to stress due to water logging (Fig. 1). Wu et al. (2013), studied water logging for 37 days in citrus plants using AMF species Diversispora spruca. They observed that during soil water logging situations, an increase in the number of AMF entry points and vesicles were observed (Wu et al. 2013). Tuo et al. (2015) described that F. mosseae conferreda contributed positively to peach plant grown in waterlogged soil by increasing proline accumulation and biosynthesis of chlorophyll. Proline is regarded as an anti-stress organic molecule solute in some plants (Kandowangko et al. 2009). Tuo et al. 2015 observed more proline accumulation in AMF (F. mosseae) inoculated seedlings under water logging conditions. This was possibly dependent on upregulation of P5CS(Δ1-pyrroline-5-carboxylate synthase),activity for the glutamate pathway and the P5CS1 gene could be regulated by abiotic stresses. It is hypothesized that alterations in proline content under AMF colonization may prove to be an indicator for abiotic stress tolerance Augé and Moore (2005). AMF inoculated seedlings showed improved Chl a, b and total chlorophyll content as compared to non-inoculated ones. A reduction in pigment content in non-AMF seedlings was explained by the fact that water-logging resulted in root cell senescence and even death due to the increase in CO2 and ethylene, thereby restricting absorption of nutrients by roots from soils, especially N, which is responsible for chlorophyll decrease in stressed plants (Tuo et al. 2015).
Salinity stress
Salinity (or high salt) stress is known to reduce productivity by affecting the vegetative development and overall growth. Particularly, in the process of photosynthesis, salt stress reduces photosynthetic efficacy by decreasing the number of PSII active reaction centers and inhibiting the electron transport from PSII to PSI (Mehta et al. 2010). Augmentation in photosynthetic efficiency was observed in AMF host plants under salinity stress (Klinsukon et al. 2021). AMF inoculation has proven to improve N content in crop plants. Antirrhinum majus plants showed improved growth rate, leaf water potential (El-Nashar 2017). AMF symbiosis has proven to be beneficial where it enhances photosynthetic rate, stomatal conductance, and leaf water relations in saline conditions (Fig. 1). A declined Na transport while an improved N, Mg absorption and chlorophyll content was observed in AMF inoculated plants under salinity conditions (Talaat and Shawky 2014). Hameed et al. (2014) reported an enhanced cytokinin accumulation and translocation of photosynthetase assimilates in AMF host plants in salinity stress. An improved PSI and PSII efficacy, increased carbonic anhydrase and total chlorophyll content were also observed. AMF inoculation showed better proline and glycine betaine content which shielded the thylakoid membranes against the ROS and (Fig. 1) contributed to the osmotic adjustments and improved photosynthesis under salinity. Aggregation of sugar under improved photosynthesis is one of the tactics of AMF plants in response to salinity condition. Besides, surplus malic acid could enhance sugar synthesis by making possibility of CO2 delivery to Calvin cycle (Kapoor et al. 2013). It is reported that AM rice plants exhibited a better light utilization capacity, higher photochemical efficiency for CO2 fixation which improved salt tolerance in them. This suggested that AM inoculated rice plants had better and improved photosynthetic rate, rubisco activities and greater biomass under salinity stress condition.
As compared to C3 plants, AMF inoculated C4 plant species depicted better salinity stress amelioration for photosynthetic rate (Pn), quantum yield of photochemical energy conversion for PSII (ΦPSII), and electron transport rates (Wang et al. 2019a). AMF inoculated woody plants had better electron transport rate, primary photochemistry followed by lesser energy dissipation. AMF inoculation also showed better photosynthetic rates in monocotyledons as compared to that of dicotyledonous plants. Moreover, AMF could mitigate moderate salinity stress in plants more strongly as compared to high salinity. Enhanced net photosynthesis was found in AMF colonized plants under salinity. The ratio Fv/Fm reflects the extent of photo-inhibition of PSII by measuring the maximum quantum yield of primary acceptor (QA) reduction (Wang et al. 2019b). Under salt stress, AMF inoculation led to a significant increase in Fv/Fm ratio indicating that AMF inoculation ameliorated the photo-damage to PSII caused by stress. The ΦPSII value which reflects the utilization of photons absorbed by PSII antennae and is estimated from the quantum efficiency of photochemical energy dissipation (Ding et al. 2016) enhanced in AMF-inoculated plants under salt stress. Photochemical quenching (qp) tells about susceptibility of PSII to photo-inhibition and demonstrates the state of the primary acceptor (QA) (Chen et al. 2017). Enhanced qp and ΦPSII values in AMF-inoculated plants under salt stress indicate that AMF colonization could improve the utilization of photons and improved PSII tolerance to photo-inhibition. Higher NPQ was observed in salinity stressed plants inoculated with Funneliformis mosseae.
Salt (NaCl) stress reduced Chl a and Chl b content in tomato leaves, which accelerated the degradation of chlorophyll, especially Chl b. Inoculation with AMF could significantly increase the content of photosynthetic pigments, and slowed down the degradation of Chl under salt stress, and increased the photophosphorylase activity. The plants inoculated with AMF had an advantage in the capture of light energy, especially at higher concentrations (0.6%, 1%) and longer time of NaCl stress (Xie et al. 2019). AMF symbiosis led to higher water absorption and retention facilitated via extra radical hyphae. Not only this AMF also improvised soil texture and volume. AMF colonization also facilitates Na+ exudation or extrusion, following the reduction of toxic Na+ build-up in the cell along with the selective absorption of K+ (Chandrasekaran et al. 2016). Aroca et al. (2013) reported that in lettuce plants, it is the higher level of strigolactone in AMF plants that are one of the causes of alleviation of salinity stress in these plants (Aroca et al. 2013).
Phytohormones regulate plant responses under changing environment and are effective even at very low concentrations. AMF inoculated plants had higher content of jasmonic acid (JA), salicylic acid (SA), and various other important inorganic nutrients and acids. Phytohormones such as, abscisic acid (ABA), auxin, brassinosteroids (BR), cytokinin, gibberellic acid;GA, JA, SA, stigolactones, nitric oxide, and triazoles play major role in plants under salinity stress (Fahad et al. 2015). Exogenous applications of GA and SA enhances tolerance against salt stress in Solanum lycopersicum and Cicer arietinum (Khalloufi et al. 2017). Solanum lycopersicum colonized with AMF, GA3 foliar spray showed improved nutrient uptake and increase salinity tolerance (Khalloufi et al. 2017). Improved growth and yield was observed when seeds were primed with SA in Cicer arietinum colonized with AMF. The plants could tolerate salinity stress because of improved ionic homeostasis, modulated carbohydrate metabolism. (Evelin et al. 2019).
Under salinity stress, Cucumis sativus plants with AMF showed improved concentrations for P, Ca2+, N, Mg2+, and K+ as compared non-inoculated ones (Hashem et al. 2018). Wheat plants under saline conditions colonized with AMF had enhanced membrane stability, photochemistry and RWC. They also possessed better carbohydrates, proteins, chlorophyll content, N and K+ (Ganugi et al. 2019). Allium sativum plants growing in high salt were inoculated with AMF and they showed better growth traits such as improved leaf area index, fresh/ dry biomass (Fileccia et al. 2017). Significant increase in fresh/dry weights and nitrogen concentration in roots and shoots was observed due to AM inoculation under saline conditions (Wang et al. 2018a) Under salinity stress, AMF colonization could decrease oxidative stress by inhibiting lipid membrane peroxidation (Talaat and Shawky 2014). D1 and D2 proteins are major protein component of PSII reaction centers. D1 and D2 proteins are structural components of the PSII and play essential roles in electron transport. The efficiency of D1 and D2 proteins was downregulated under salinity condition. AM symbiosis up regulated the genes encoding D1 (RppsbA) and D2 (RppsbD) proteins for PSII under salinity conditions thereby empowering host plant to have more efficient PSII repair system (Chen et al. 2017). One probable reason of up regulation could be higherconcentration of polyamines and glycinebetaine in AM plants (Talaat and Shawky 2014). Glycinebetaine stabilizes PSII pigment-protein (Talaat and Shawky 2014). AM inoculation improved photosynthetic efficacy of plants as compared to non inoculated plants, by decreasing non photochemical quenching (NPQ) and improving photochemical efficiency of plants by converting light harvesting energy to chemical energy. Hashem et al. (2015) observed that salinity stress decrease the activity of Rubisco and NADP-dependent malic enzyme (NADP-ME), while AMF colonization induced activity of this enzyme which led to improved stress tolerance. Role of NADP malic enzymes have been studied in several plant defence pathways (Casati et al. 1999). In C4 plants, increased stress tolerance is associated with the increased activity of NADP-ME due to presence of AMF in roots. This is further associated with an increased C metabolism regulated by pyruvate orthophosphate dikinase (PPDK), phosphoenolpyruvate carboxylase (PEPC) and malate dehydrogenase (MDH). Hashem et al. (2015) observed increased activities of PPDK, PEPC and MDH in salt-stressed plants. Thus, higher NADP-ME in mycorrhizal plants can contribute in an increased carbon metabolism and contribute to improved stress tolerance in plants (Evelin et al. 2019). Under continuous cropping system, Wang et al. (2021) observed an improved endogenous IAA level in AMF inoculated tomato plant which further improved. The NADP-ME activity in tomato plant. This could be a promising solution for the recycle and reuse of continuous cropping substrates with AMF amendment for consecutive years.
Temperature stress
High temperature
High temperature (HT) has negative impact on plant growth, metabolism and productivity (Mathur et al. 2018). Heat stress significantly cause inhibition in seed germination, growth rate, biomass, and photosynthesis. HT leads to leafwilting, smouldering reproductive organs causing cell death (Wahid et al. 2007). Under high-temperature photosynthesis is also considerably affected. In photosynthesis, photosystem II (PSII) is one of the most heat labile component. It is known that the water oxidizing complex (WOC), PSII reaction center and the light harvesting complexes are initially affected with HT (Mathur et al. 2014). Mycorrhizal symbiosis may alter plant physiological activity to enable it to cope with adverse stress conditions. Under high temperature stress, one of protective strategies that AMF adapt to survive is trehalose production. Triacylglycerols (TAGs) are the most common (up to 95%) form of C aggregates in AMF. Translocation of C to the extraradical mycelium (distal part of fungal colony) takes place mainly as glycogen and TAGs. Trehalose metabolizing enzymes (transcription and/or post-transcription regulation) are activated in response to heat shock leading to trehalose accumulation (Van Dijck et al. 2002). Enzymes associated with trehalose synthase complex and neutral trehalase are activated in response to HT and many other types of stresses (Lenoir et al. 2016). Trehalose prevent cells by maintaining cellular structure and native confirmation of proteins under HT stress (Singer and Lindquist 1998). Zhu et al. (2011), have reported that mycorrhizal inoculation led to high net photosynthetic rate (Pn), stomatal conductance (gs), transpiration rate and better PSII photochemistry (as evident from higher Fv/Fm ratio) as compared to non-mycorrhizal maize plants. This indicated that AM symbiosis decreased stomatal resistances and increased CO2 assimilation and transpiration fluxes (Zhu et al. 2011). Kytöviita and Ruotsalainen 2007 observed that HT can lead to increased carbon allocation, phosphorus uptake and improved AMF root (Kytöviita and Ruotsalainen 2007) thereby improvising photosynthesis by production of more photosynthates in leaf.
AMF colonization maintains the structural and functional integrity of photosynthetic apparatus by reducing damage to PSII reaction center. Under heat stress, AMF inoculation protected the plants by enhancing light harvesting capacity, increasing chlorophyll content and improving photosynthetic activity of the plants (Zhu et al. 2017) (Fig. 2). AM colonization helps in lipid phase stabilization of the thylakoid membrane, photoprotection of cellular structure thus protecting photosynthetic apparatus against stress (Zhu et al. 2017). AM symbiosis could improve water conservation leading to prevention of dehydration and metabolic disturbances (Zhu et al. 2017).
Mathur et al. (2018) have studied the effects of high temperature (HT) exposure on photosynthesis in maize plants grown in soil enriched with AMF and without AMF. Various phenological characters such as leaf width, plant height etc. showed improvement under AMF enrichment as compared to non–AMF and high temperature exposed plants. Total chlorophyll content was enhanced in AMF enriched plants. Around 40% increase in leaf width and 20% in leaf length was observed for AMF enriched plants. An increase in leaf area supported more C assimilation and provided more solar absorption for the enriched plants. Thus it was observed that AMF enriched plants had better growth as compared to non AMF ones. The hyphae helped the roots to acquire more Mg which indirectly increased the total chlorophyll in the plants. This in turn result in increased photosynthetic assimilates and increased biomass. Thus quantum efficiency of PSII (Fv/Fm), primary photochemistry was reduced in high temperature exposed maize plants. AMF plants showed an increase in performance index. This suggests that the presence of AMF has supported the plants to improve light reaction and absorbance per reaction center and net photosynthesis under temperature stress. Efficiency of primary photochemical reaction increased in AMF plants as compared to HT plants. Under high temperature stress it was observed that AMF not only protected water splitting complex but also enhanced primary photochemistry of PSII. Recently, Mathur and Jajoo (2020) studied effect of photosystem II heterogeneity on maize plants and concluded that AMF increased active alpha centers in AMF + HT exposed maize plants while less active and inactive beta and gamma centers were increased in control plants. AMF plants showed maximum reducing centers while most of the non-reducing centres which were unable to transfer electrons from QA to QB were increased in high temperature exposed control plants. This suggested that when maize plants are grown in soil inoculated with AMF, they are largely protected by high temperature stress by manipulating photosystem II heterogeneity.
Low temperature
Low temperature causes several detrimental effects including poor seed germination, retarded seedlings, chlorosis, decreased photosynthesis, lessened leaf enlargement and growth, wilting, withering, low tillering, poor plant reproduction ultimately dropping the grain yield (Suzuki et al. 2008; Ganugi et al. 2019). It is well established that AMF plants have better growth and development as compared to non AMF ones (Liu et al. 2013). AMF mediated improvement in cold tolerance is probably due to its role in improving plant photosynthesis which provides the energy for plant growth. AMF host plants show better osmotic balance, gas exchange, PSII photochemistry and nutrient uptake. This is in consensus that AMF hyphae improve water absorption in host plants which may have the above implications (Zhu et al. 2010) (Fig. 2). Involvement of AMF in chlorophyll synthesis and enhanced chlorophyll content was observed in cold stressed plants (Zhu et al. 2010). Hence, higher Chl content could have contributed to the enhanced net photosynthesis of the mycorrhizal plants grown at low temperature (Ma et al. 2019) thereby protecting the crops. AM maize plants had higher maximum fluorescence (Fm), variable fluorescence (Fv), maximum photochemical efficiency (Fv/Fm), and potential photochemical efficiency (Fv/Fo), and lower primary fluorescence (Fo), compared with non-AMF maize plants. The photosynthetic rate and transpiration rate (Tr) of maize inoculated with G. etunicatum increased markedly under low temperature. Under low temperature stress, higher stomatal conductance (gs) and lower intercellular CO2 concentration (Ci) was observed in AM maize plants as compared to non-AM maize plants. It is reported that AM fungi could protect maize plants from low temperature induced damage by improving Chl synthesis and net photosynthesis (Zhu et al. 2010). This is in corroboration with earlier studies that AMF hyphae can penetrate deep in the soil and can absorb more N which is a major component of chlorophyll. AMF retains moisture in the host plant (Zhu et al. 2010). AMF colonization helps in increasing the secondary metabolites of the plants and thus support in improving the defense system of host plant thereby protecting the plants against low temperature stress. Hajiboland et al. (2019) studied the effects of freezing temperature and non-freezing temperature in barley with and without AMF. They reported an enhancement in growth, photosynthesis, osmotic and water homeostasis, and potassium uptake for AMF inoculated plants under low temperature. Under low temperature stress, AM maize plants exhibited higher root proline and sugar content as compared to non-AM plants (Chen et al. 2014; Charest et al. 1993). Zhu et al. 2017, mentioned that soluble sugars can play major role as osmoprotectant under low temperature stress in plants.
Soil pollution
Heavy metal stress
Heavy metals (HM) are naturally present as trace elements in the environment and their presence in soil and water initiate several responses in plants affecting the overall crop yield (Mathur et al. 2016). For example, a high level of Lead (Pb) in the chloroplasts inhibits enzyme related to chlorophyll biosynthesis, CO2 fixation, and aggregation of pigment-protein complexes. PSI is found to be more tolerant to HM pollution as compared to PSII (Yang et al. 2015). AMF could strengthen plant defence system and thereby support plant survival in soil contaminated with heavy metals (Ali et al. 2015). Pea plants inoculated with AMF exposed to arsenic As(V) showed high relative water content (RWC), chlorophyll content, and Mg uptake establishing the role of AM colonization in maintaining high turgor, Chl bio-synthesis and lower leaf chlorosis (Garg and Singla 2012; Latef et al. 2016).
Other studies revealed that AMF inoculation enhanced pigment content in Bassica indica decreased due to Cadmium (Cd) stress (Hashem et al. 2019). AMF hyphae due to extensive surface area can penetrate deep in the soil and can trap deep buried toxic metals. They offer a good adsorption site for the accumulation of cations and prevent their entry to the plants (Lenoir et al. 2016; Ganugi et al. 2019). Aguilera et al. (2014) studied enhanced nutrient absorption for wheat plants inoculated with mycorrhizal under Aluminium (Al) deficiency. AMF repeatedly binds Cd and Zn in the cell wall of mantle hyphae and cortical cells, thereby restricting their uptake which results in improved growth, yield, photosynthetic activity, and nutrient status (Garg and Chandel 2012). Lanfranco et al. (2002) reported that Cu exposure upregulated the gene expression entirely in the symbiotic mycelium. In the germinating spores of Gigaspora margarita, BEG34, could provide tolerance against Cd and Cu (Kapoor et al. 2013). Up regulation of AMF gene namely GintABC1 participates actively in Cu and Zn detoxification was observed (Gonzalez-Guerrero et al. 2007; Latef et al. 2016). AMF increases the nutrient uptake of the hosts by obtaining phosphate, micronutrients and water (Latef et al. 2016). In the same way, metals are taken up by fungal hyphae and transported to the plants. In some cases, mycorrhrizal plants exhibited improved HM uptake and root to shoot transport (phytoextraction) while in some other cases AM fungi reduce the HM mobilization in the soil (phytostabilization). Phytoextraction includes alleviation via increased translocation of metals in roots and shoots and alliance with metal as hyper accumulator. The mechanism of phytostabilization involves metal accumulation or scavenging via hyphae, production of glomalin, formation of chelates, accumulation of metal in spores etc. (Latef et al. 2016). Tolerance of plants to metal stress was found to be alleviated by AMF inoculation. Underlying possible mechanisms could be: i) restriction of metals by fungal exudates, ii) precipitation of metals in polyphosphate granules in the soil, iii) adsorption of metals by chitin, iv) chelation of metals inside the fungus, v) change in rhizosphere pH, vi) regulation of gene expression (Malekzadeh et al. 2011; Latef et al. 2016) (Fig. 2).Furthermore, sometimes these metals are adsorbed in chitin in the cell wall of fungi and chelation of metals inside the fungus takes place. Due to presence of AM fungi the pH of soil rhizosphere is changed, and expression and regulation of some genes occurs in stress conditions (Malekzadeh et al. 2011). Four AM fungal genes (GrosMT1, GinZnT1, GmarMT1 and GintABC1) are responsible for maintaining cellular homeostasis against metals (Azcon et al. 2013). GmarMT1 regulates the redox potential in fungi and protect it against oxidative stress (Gonzalez-Guerrero et al. 2007). AMF produce a glycoprotein glomalin that is predominantly present in spore and hyphal wall (80%) and produced by hyphal wall itself (Treseder and Turner 2007). It has the potential to immobilize high amount of metals (Latef et al. 2016). The glomalin protein sequence found in R. irregularis encodes a putative heat shock protein (Gadkar and Rillig 2006). A positive correlation was observed between glomalin and Cu content in the soil. This indicated that AMF responds to high soil Cu levels by over expressing glomalin and glomalin protects against Cu stress (Lenoir et al. 2016). Etcheverria (2009) showed that glomalin related protein (GRSP) could bind around 4.2–7.5% of Al in acidic soils. The production of GRSP depends on the soil conditions, particularly low pH. Exudation of organic acids (e.g. citrate, malate, and acetate) by AM fungal hyphae can improve plant tolerance to Al toxicity (Muthukumar et al. 2014). Therefore, AMF colonized roots are protected from the deleterious effects of the metals through extensive hyphal network, root exudates, and by modulating detoxifying mechanisms (Muthukumar et al. 2014; Latef et al. 2016).
Extensive and diverse use of Nanoparticles (NPs) has led to their increasing existence in the environment and consequent environmental risks (Nowack 2009). NPs can deform and damage fungal hyphae and bacterial cells leading to inhibited functions and reduced diversity of soil microbiota (Tian et al. 2019). It is reported that AMF inoculation have improved and ameliorated phytotoxicity of ZnONP and AgNPs thereby reducing the metal accrual in plants followed by decreased bioavailability of metals and enhanced glomalin secretion i.e., glomalin-related soil protein (GRSP) (due to stress) (Feng et al. 2013; Siani et al. 2017; Wang et al. 2018b). Inoculation with AMF exerts positive effects against nanotoxicity. ZnO NPs toxicity in plants can be greatly reduced through AMF inoculation. AMF reduces the toxicity by decreasing the availability of Zn from the soil, reduce Zn translocation from roots to shoots, and thus increases nutrition and mineral uptake and antioxidant activity of the host plants (Liu et al. 2015). In non-inoculated plants, the nutrient uptake activity of roots are reduced due to presence of ZnO NPs. In contrast AMF colonization improves root activity and help to acquire nutrients, even if present distantly. An improved soil bacterial community with diversity and better microbial consortium was observed in AM inoculated soil. Thus, AMF could decrease NPs toxicity by increasing root and shoot biomass including increased root nodule number (Tian et al. 2019).
In the same way, AMF protected plants from toxic effects of AgNPs. AMF inoculation in plants enhances glomalin secretion and reduces metal uptake in the plants (Siani et al. 2017). AM fungi may indirectly inhibit the bioavailability of the metal by affecting soil pH. In the same way, inoculation of AM fungi decreased Ag bioavailability and thereby Ag accumulation in plants, by down-regulating genes potentially involved in Ag transport and up-regulating genes involved in P transport (Cao et al. 2020).
Conclusion and future prospectives
-
AMF helps the host plant to grow healthy by improving photosynthetic rate, gas exchange and other related traits thereby ameliorating the impact of abiotic stresses.
-
AMF is able to protect the plants under stress conditions due to its involvement in the mechanisms related to trehalose production (heat stress), water and nutrient uptake (salinity, drought, heavy metal stress), glomalin production (heavy metal stress), proline accumulation (water logging), phytohormones (salinity), chaperone protein production (all abiotic stresses).
-
AMF act as an underground network connectors for soil and roots whose major role is nutrient and mineral transport to plants.
-
Higher the photosynthesis more is the photosynthate accumulation in the host leaves.
-
AMF also has role in C and N cycles thereby promoting photosynthesis even under stress conditions.
AMF should not only be used as pioneer species for land fertility and recycle but also play a major role as soil quality indicators The crosstalk between plants and AM fungi can be a promising field for research for physiological and molecular approaches. Further research should focus on gene expression studies of the involved proteins.
Abbreviations
- AgNPs:
-
Silver Nano particles
- AMF:
-
Arbuscular Mycorrhizal Fungi
- DS:
-
Drought stress
- Fv/Fm:
-
Quantum efficiency of PSII
- HM:
-
Heavy metal
- NaCl:
-
Sodium chloride
- NM:
-
Non mycorrhizal
- NP:
-
Nano particles
- qp:
-
Photochemical quenching
- ΦPSII:
-
Quantum yield of photochemical energy conversion in PSII
- Y(II):
-
Quantum yield of PSII
- Y(NPQ):
-
Yield of regulated energy dissipation
- ZnOP:
-
Zinc nano particle
References
Abbaspour H, Saeidi-Sar S, Afshari H, Abdel-Wahhab MA (2012) Tolerance of Mycorrhiza infected Pistachio (Pistacia vera L.) seedling to drought stress under glasshouse conditions. J Plant Physiol 169:704–709
Aguilera P, Pablo C, Fernando B, Fritz O (2014) Diversity of arbuscular mycorrhizal fungi associated with Triticum aestivum L. plants growing in an andosol with high aluminium level. Agri Eco Environ 186:178–184
Al-Arjani ABF, Hashem A, Abd-Allah EF (2020) Arbuscular mycorrhizal fungi modulates dynamics tolerance expression to mitigate drought stress in Ephedra foliata Boiss. Saudi J Biol Sci 27:380–394
Ali N, Masood S, Mukhtar T, Kamran MA, Rafique M, Munis MFH, Chaudhary HJ (2015) Differential effects of cadmium and chromium on growth, photosynthetic activity, and metal uptake of Linum usitatissimum in association with Glomus intraradices. Environ Monitor Assess 187:311
Amiri R, Ali N, Nematollah E, Mohammad RS (2017) Nutritional status, essential oil changes and water-use efficiency of rose geranium in response to arbuscular mycorrhizal fungi and water deficiency stress. Symbiosis 73:15–25
Aroca R, Ruiz-Lozano JM, Zamarreño AM, Paz JA, García-Mina JM, Pozo MJ, López-Ráez JA (2013) Arbuscular mycorrhizal symbiosis influences strigolactone production under salinity and alleviates salt stress in lettuce plants. J Plant Physiol 170:47–55
Augé RM, Moore JL (2005) Arbuscular mycorrhizal symbiosis and plant drought resistance. In: Mehrotra VS (ed) Mycorrhiza: role and applications. Allied Publishers, Nagpur, pp 136–162
Augé RM, Moore JL, Stutz JC, Sylvia DM, Al-Agely AK, Saxton AM (2003) Relation foliar dehydration tolerance of mycorrhizal Phaselus vulgaris to soil and root colonization by hyphae. J Plant Physiol 160:1147–1156
Azcon R, Medina A, Aroca R, Ruiz-Lozano JM (2013) Abiotic stress remediation by the arbuscular mycorrhizal symbiosis and rhizosphere bacteria/yeast interactions. In: Frans de Bruijn J (ed) Molecular Microbial Ecology of the Rhizosphere, 1st edn. John Wiley & Sons Inc., USA, pp 991–1002
Bahadur A, Batool A, Nasir F, Jiang S, Mingsen Q, Zhang Q, Pan J, Liu Y, Feng H (2019) Mechanistic insights into arbuscular mycorrhizal fungi-mediated drought stress tolerance in plants. Int J Mol Sci 20:4199
Begum N, Qin C, Ahanger MA, Raza S, Khan MI, Ashraf M, Ahmed N, Zhang L (2019) Role of arbuscular mycorrhizal fungi in plant growth regulation: implications in abiotic stress tolerance. Front Plant Sci 10:1068
Cao J, Feng Y, Lin X, Wang J (2020) A beneficial role of arbuscular mycorrhizal fungi in influencing the effects of silver nanoparticles on plant-microbe systems in a soil matrix. Environ Sci Pollut Res 27:11782–11796
Casati P, Drincovich MF, Edwards GE, Andreo CS (1999) Malate metabolism by NADP-malic enzyme in plant defense. Photosynth Res 61:99–105
Chandrasekaran M, Kim K, Krishnamoorthy R, Walitang D, Sundaram S, Joe MM, Selvakumar G, Hu S, Oh SH, Sa T (2016) Mycorrhizal symbiotic efficiency on C3 and C4 plants under salinity stress - a meta-analysis. Front Microbiol 7:1246
Charest C, Dalpé Y, Brown A (1993) The effect of vesicular arbuscular mycorrhizae and chilling on two hybrids of Zea mays L. Mycorrhiza 4:89–92
Chen XY, Song FB, Liu FL, Tian C, Liu S, Xu H, Zhu X (2014) Effect of different arbuscular mycorrhizal fungi on growth and physiology of maize at ambient and low temperature regimes. Sci World J 2014:956141
Chen J, Zhang H, Zhang X, Tang M (2017) Arbuscular mycorrhizal symbiosis alleviates salt stress in black locust through improved photosynthesis, water status, and K+/Na+ homeostasis. Front Plant Sci 8:1739
Cheng H-Q, Zou Y-N, Wu Q-S, Kuca K (2021) Arbuscular mycorrhizal fungi alleviate drought stress in trifoliate orange by regulating H+-ATPase activity and gene expression. Front Plant Sci 12:659694
De Andrade SAL, Domingues AP, Mazzafera P (2015) Photosynthesis is induced in rice plants that associate with arbuscular mycorrhizal fungi and are grown under arsenate and arsenite stress. Chemosphere 134:141–149
Ding XT, Jiang YP, Hao T, Jin HJ, Zhang HM, He LZ (2016) Effects of heat shock on photosynthetic properties, antioxidant enzyme activity, and downy mildew of cucumber (Cucumis sativus L). PLoS ONE 11:e0152429
Doubková P, Vlasáková E, Sudová R (2013) Arbuscular mycorrhizal symbiosis alleviates drought stress imposed on Knautia arvensis plants in serpentine soil. Plant Soil 370:149–161
El-Nashar YI (2017) Response of snapdragon Antirrhinum majus L. to blended water irrigation and arbuscular mycorrhizal fungi inoculation: uptake of minerals and leaf water relations. Photosynthetica 5:201–209
Estrada B, Barea JM, Aroca R, Ruiz-Lozano J (2012) A native Glomus irregularis strain from a mediterranean saline area exhibits salt tolerance and enhanced symbiotic efficiency with maize plants under salt stress conditions. Plant Soil 366:333–349
Etcheverria P (2009) Glomalin in evergreen forest associations, deciduous forest and a plantation of Pseudotsuga menziesii in the X Region, Chile. Ph.D Dissertation, Universidad de La Frontera
Evans JR (1989) Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 78:9–19
Evelin H, Kapoor R (2014) Arbuscular mycorrhizal symbiosis modulates antioxidant response in salt-stressed Trigonella foenum-graecum plants. Mycorrhiza 24:197–208
Evelin H, Devi TS, Gupta S, Kapoor R (2019) Mitigation of salinity stress in plants by arbuscular mycorrhizal symbiosis: current understanding and new challenges. Front Plant Sci 10:470
Fahad S, Nie L, Chen Y, Wu C, Xiong D, Saud S, Hongyan L, Cui K, Huang J (2015) Crop plant hormones and environmental stress. In: Lichtfouse E (ed) Sustainable agriculture reviews. Springer, Cham, pp 371–400
Fan QJ, Liu JH (2011) Colonization with arbuscular mycorrhizal fungus affects growth, drought tolerance and expression of stress-responsive genes in Poncirus trifoliata. Acta Physiol Plant 33:1533–1542
Feng Y, Cui XS, He S, Dong G, Chen M, Wang J, Lin X (2013) The role of metal nanoparticles in influencing arbuscular mycorrhizal fungi effects on plant growth. Environ Sci Technol 47:9496–9504
Fileccia V, Ruisi P, Ingraffia R, Giambalvo D, Frenda AS, Martinelli F (2017) Arbuscular mycorrhizal symbiosis mitigates the negative effects of salinity on durum wheat. PLoS ONE 12(9):e0184158
Foyer CH, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133:21–25
Gadkar V, Rillig MC (2006) The arbuscular mycorrhizal fungal protein glomalin is a putative homolog of heat shock protein 60. FEMS Microbiol Lett 263:93–101
Ganugi P, Masoni A, Pietramellara G, Benedettelli S (2019) A review of studies from the last twenty years on plant–arbuscular mycorrhizal fungi associations and their uses for wheat crops. Agronomy 9:840
Garcés-Ruiz M, Calonne-Salmon M, Plouznikoff K, Misson C, Navarrete-Mier M, Cranenbrouck S, Declerck S (2017) Dynamics of short-term phosphorus uptake by intact mycorrhizal and non-mycorrhizal maize plants grown in a circulatory semi-hydroponic cultivation system. Front Plant Sci 8:1471
Garg N, Chandel S (2012) Role of arbuscular mycorrhizal (AM) fungi on growth, cadmium uptake, osmolyte, and phytochelatin synthesis in Cajanus cajan (L.) Millsp. under NaCl and Cd stresses. J Plant Growth Regul 31:292–308
Garg N, Singla P (2012) The role of Glomus mosseae on key physiological and biochemical parameters of pea plants grown in arsenic contaminated soil. Sci Hortic 143:92–101
Gavito Mayra E, Jakobsen I, Mikkelsen TN, Mora F (2019) Direct evidence for modulation of photosynthesis by an arbuscular mycorrhiza-induced carbon sink strength. New Phytol 223:896–907
Gomez-Bellot MJ, Ortuño MF, Nortes PA, Vicente-Sánchez J, Bañón S, Sánchez Blanco MJ (2015) Mycorrhizal euonymus plants and reclaimed water: biomass, water status and nutritional responses. Sci Hort 186:61–69
Gonzalez-Guerrero M, Cano C, Azcon-Aguilar C, Ferrol N (2007) GintMTl encodes a functional metallothionein in Glomus intraradices that responds to oxidative stress. Mycorrhiza 17:327
Govindarajulu M, Pfeffer PE, Jin HR, Abubaker J, Douds DD, Allen JW, Bücking H, Lammers PJ, Shachar-Hill Y (2005) Nitrogen transfer in the arbuscular mycorrhizal symbiosis. Nature 435:819–823
Grimoldi AA, Kavanová M, Lattanzi FA, Schäufele R, Schnyder H (2006) Arbuscular mycorrhizal colonization on carbon economy in perennial ryegrass: quantification by 13CO2/12CO2 steady–state labelling and gas exchange. New Phytol 172:544–553
Hajiboland R, Joudmand A, Aliasgharzad N, Tolrá R, Poschenrieder C (2019) Arbuscular mycorrhizal fungi alleviate low-temperature stress and increase freezing resistance as a substitute for acclimation treatment in barley. Crop Pasture Sci 70:218–233
Hameed A, Egamberdieva D, Abd-Allah EF, Hashem A, Kumar A, Ahmad P (2014) Salinity stress and arbuscular mycorrhizal symbiosis in plants. In: Miransari M (ed) Use of microbes for the alleviation of soil stresses. Springer Science Business Media, NY, pp 139–159
Hashem A, Abd-Allah EF, Alqarawi AA, Aldubise A, Egamberdieva D (2015) Arbuscular mycorrhizal fungi enhances salinity tolerance of Panicum turgidum Forssk by altering photosynthetic and antioxidant pathways. J Plant Interact 10:230–242
Hashem A, Abd-Allah EF, Alqarawi AA, Al-Huqail AA, Egamberdieva A, Wirth D (2016) Alleviation of cadmium stress in Solanum lycopersicum L. by Arbuscular mycorrhizal fungi via induction of acquired systemic tolerance. Saudi J Biol Sci 23:272–281
Hashem A, Alqarawi AA, Radhakrishnan R, Al-Arjani AF, Aldehaish HA, Egamberdieva D, Abd-Allah EF (2018) Arbuscular mycorrhizal fungi regulate the oxidative system, hormones and ionic equilibrium to trigger salt stress tolerance in Cucumis sativus L. Saudi J Biol Sci 25:1102–1114
Hashem A, Abd-Allah EF, Alqarawi AA, Malik JA, Wirth S, Egamberdieva D (2019) Role of calcium in AMF-mediated alleviation of the adverse impacts of cadmium stress in Bassia indica [Wight] AJ Scott. Saudi J Biol Sci 26:828–838
Hodge A, Fitter H (2010) Substantial nitrogen acquisition by arbuscular mycorrhizal fungi from organic material has implications for N cycling. Proc Natl Acad Sci 107:13754–13759
Jacob J, Lawlor DW (1993) In vivo photosynthetic electron transport does not limit photosynthetic capacity in phosphate-deficient sunflower and maize leaves. Plant Cell Environ 16:785–795
Kandowangko NY, Suryatmana G, Nurlaeny N, Simanungkalit RDM (2009) Proline and abscisic acid content in droughted corn plant inoculated with Azospirillum sp. and arbuscular mycorrhizae fungi. HAYATI J Biosci 16:15–20
Kapoor R, Evelin H, Mathur P, Giri B (2013) Arbuscular mycorrhiza: approaches for abiotic stress tolerance in crop plants for sustainable agriculture. In: Tuteja N, Gill SS (eds) Plant acclimation to environmental stress. LLC, Springer, Science+Business Media, pp 359–401
Kaschuk G, Yin X, Hungria M, Leffelaar PA, Giller KE, Kuyper TW (2012) Photosynthetic adaptation of soybean due to varying effectiveness of N2 fixation by two distinct Bradyrhizobium japonicum strains. Environ Exp Bot 76:1–6
Khalloufi M, Martínez-Andújar C, Lachaâl M, Karray-Bouraoui N, Pérez-Alfocea F, Albacete A (2017) The interaction between foliar GA3 application and arbuscular mycorrhizal fungi inoculation improves growth in salinized tomato (Solanum lycopersicum L.) plants by modifying the hormonal balance. J Plant Physiol 214:134–144
Klinsukon C, Lumyong S, Kuyper TW, Boonlue S (2021) Colonization by Arbuscular mycorrhizal fungi improves salinity tolerance of eucalyptus (Eucalyptus camaldulensis) seedlings. Sci Rep 11:4362
Kytöviita M, Ruotsalainen AL (2007) Mycorrhizal benefit in two low arctic herbs increases with increasing temperature. Am J Bot 94:1309–1315
Lanfranco L, Bolchi A, Ros EC, Ottonello S, Bonfante P (2002) Differential expression of a metallothionein gene during the presymbiotic versus the symbiotic phase of an arbuscular mycorrhizal fungus. Plant Physiol 130:58–67
Latef AAHA, Hashem A, Rasool S, Abd-Allah EF, Alqarawi AA, Egamberdieva D, Jan S, Anjum NA, Ahmad P (2016) Arbuscular mycorrhizal symbiosis and abiotic stress in plants: A Review. J Plant Biol 59:407–426
Lee BR, Muneer S, Avice JC, Jung WJ, Kim TH (2012) Mycorrhizal colonisation and P-supplement effects on N uptake and N assimilation in perennial ryegrass under well-watered and drought-stressed conditions. Mycorrhiza 22:525–534
Lenoir I, Fontaine J, Lounès-Hadj Sahraoui A (2016) Arbuscular mycorrhizal fungal responses to abiotic stresses: A review. Phytochemistry 123:4–15
Lin J, Wang Y, Sun S, Mu C, Yan X (2017) Effects of arbuscular mycorrhizal fungi on the growth, photosynthesis and photosynthetic pigments of Leymus chinensis seedlings under salt-alkali stress and nitrogen deposition. Sci Total Environ 576:234–241
Liu ZL, Li YJ, Hou HY, Zhu XC, Rai V, He XY, Tian CJ (2013) Differences in the arbuscular mycorrhizal fungi improved rice resistance to low temperature at two N levels: Aspects of N and C metabolism on the plant side. Plant Physiol Biochem 71:87–95
Liu X, Wang F, Shi Z, Tong R, Shi X (2015) Bioavailability of Zn in ZnO nanoparticles-spiked soil and the implications to maize plants. J Nanopart Res 17:175
Ma J, Janoušková M, Ye L, Bai LQ, Dong RR, Yan Y, Yu XC, Zou ZR, Li YS, He CX (2019) Role of arbuscular mycorrhiza in alleviating the effect of cold on the photosynthesis of cucumber seedlings. Photosynthetica 57:86–95
Malekzadeh E, Alikhani AH, Savaghebi-Fioozabadi RG, Zarei M (2011) Influence of arbuscular mycorrhizal fungi and an improving growth bacterium on Cd uptake and maize growth in Cd polluted soils. Spanish J Agric Res 9:1213–1223
Malik AI, Colmer TD, Lambers H, Setter TL, Schortemeyer M (2002) Short-term waterlogging has long-term effects on the growth and physiology of wheat. New Phytol 153:225–236
Mathur S, Jajoo A (2014) Alterations in photochemical efficiency of photosystem II in wheat plant on hot summer day. Physiol Mol Biol Plants 20:527–531
Mathur S, Jajoo A (2020) Arbuscular mycorrhizal fungi protects maize plants from high temperature stress by regulating photosystem II heterogeneity. Ind Crop Prod 143:111934
Mathur S, Kalaji HM, Jajoo A (2016) Investigation of deleterious effects of chromium phytotoxicity and photosynthesis in wheat plant. Photosynthetica 54(2):185–192
Mathur S, Sharma MP, Jajoo A (2018) Improved photosynthetic efficacy of maize (Zea mays) plants with Arbuscular mycorrhizal fungi (AMF) under high temperature stress. J Photochem Photobiol B Biol 180:149–154
Mathur S, Tomar RS, Jajoo A (2019) Arbuscular mycorrhizal fungi (AMF) protects photosynthetic apparatus of wheat under drought stress. Photosynth Res 139:227–238
Mehta P, Jajoo A, Mathur S, Bharti S (2010) Chlorophyll a fluorescence study revealing effects of high salt stress on Photosystem II. Plant Physiol Biochem 48:16–20
Muthukumar T, Priyadharsini P, Uma E, Jaison S, Pandey RR (2014) Role of arbuscular mycorrhizal fungi in alleviation of acidity stress on plant growth. In: Miransari M (ed) Use of microbes for the alleviation of soil stresses, vol 1. Springer Science+Business Media, NY, p 43
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Biol 49:249–279
Nowack B (2009) The behavior and effects of nanoparticles in the environment. Environ Pollut 157:1063–1064
Porcel R, Ruiz-Lozano JM (2004) Arbuscular mycorrhizal influence on leaf water potential, solute accumulation, and oxidative stress in soybean plants subject to drought stress. J Exp Bot 55:1743–1750
Prasad R, Bhola D, Akdi K, Cruz C, Sairam KVSS, Tuteja N, Varma A (2017) Introduction to mycorrhiza: historical development, in Mycorrhiza. In: Prasad R, Tuteja N (eds) Varma A. Cham, Springer, pp 1–7
Rabie GH (2005) Role of arbuscular mycorrhizal fungi in phytoremediation of soil rhizosphere spiked with poly aromatic hydrocarbons. Mycobiology 33:41–50
Ramel F, Birtic S, Ginies C, Soubigou-Taconnat L, Triantaphylidès C, Havaux M (2012) Carotenoid oxidation products are stress signals that mediate gene responses to singlet oxygen in plants. Proc Natl Acad Sci USA 109:5535–5540
Ren BZ, Zhang JW, Li X, Fan X, Dong ST, Liu P, Zhao B (2014) Effects of water logging on the yield and growth of summer maize under field conditions. Can J Plant Sci 94:23–31
Ruiz-Lozano JM, Aroca R, Zamarreño ÁM, Molina S, Andreo-Jiménez B, Porcel R, García-Mina JM, Ruyter-Spira C, López-Ráez JA (2015) Arbuscular mycorrhizal symbiosis induces strigolactone biosynthesis under drought and improves drought tolerance in lettuce and tomato. Plant Cell Environ 39:441–452
Ruiz-Sánchez M, Aroca R, Muñoz Y, Polón R, Ruiz-Lozano JM (2010) The arbuscular mycorrhizal symbiosis enhances the photosynthetic efficiency and the antioxidative response of rice plants subjected to drought stress. J Plant Physiol 167:862–869
Ryan MH, Graham JH (2018) Little evidence that farmers should consider abundance or diversity of Arbuscular mycorrhizal fungi when managing crops. New Phytol 220:1092–1107
Serbinova EA, Packer L (1994) Antioxidant properties of α-tocopherol and α -tocotrienol. Methods Enzymol 234:354–366
Shahabivand S, Maivan HZ, Goltapeh EM, Sharfi M, Ali S, Aliloo A (2012) The effects of root endophyte and arbuscular mycorrhizal fungi on growth and cadmium accumulation in wheat under cadmium toxicity. Plant Physiol Biochem 60:53–58
Siani NG, Fallah S, Pokhrel LR, Rostamnejadi A (2017) Natural amelioration of Zinc oxide nanoparticle toxicity in fenugreek (Trigonella foenum-gracum) by arbuscular mycorrhizal (Glomus intraradices) secretion of glomalin. Plant Physiol Biochem 112:227–238
Singer MA, Lindquist S (1998) Multiple effects of trehalose on protein folding in vitro and in vivo. Mol Cell 1:639–648
Smith S, Read D (2008) Mycorrhiza symbiosis. Academic Press, San Diego, CA
Srivastava P, Saxena B, Giri B (2017) Arbuscular mycorrhizal fungi: green approach/technology for sustainable agriculture and environment. In: Varma A, Prasad R, Tuteja N (eds) Mycorrhiza – nutrient uptake, biocontrol, ecorestoration. Springer International Publishing, Cham, Switzerland, pp 355–386
Suzuki K, Nagasuga K, Okada M (2008) The chilling injury induced by high root temperature in the leaves of rice seedlings. Plant Cell Physiol 49:433–442
Talaat NB, Shawky BT (2014) Protective effects of arbuscular mycorrhizal fungi on wheat (Triticum aestivum L.) plants exposed to salinity. Environ Exp Bot 98:20–31
Thirkell TJ, Charters MD, Elliott AJ, Sait SM, Field KJ (2017) Are mycorrhizal fungi our sustainable saviours considerations for achieving food security. J Ecol 105:921–929
Thomas CE, McLean LR, Parker RA, Ohlweiler DF (1992) Ascorbate and phenolic antioxidant interactions in prevention of liposomal oxidation. Lipids 27:543–550
Tian H, Kah M, Kariman K (2019) Are nanoparticles a threat to mycorrhizal and rhizobial symbioses? A Critical Review Front Microbiol 10:1660
Treseder KK, Turner KM (2007) Glomalin in ecosystems. Soil Soc Am J 71:1257
Tuo XQ, Li S, Wu QS, Zou YN (2015) Alleviation of waterlogged stress in peach seedlings inoculated with Funneliformis mosseae: Changes in chlorophyll and proline metabolism. Sci Hortic 197:130–134
Van Dijck P, De Rop L, Szlufcik K, Van Ael E, Thevelein JM (2002) Disruption of the Candida albicans TPS2 gene encoding trehalose-6-phosphate phosphatise decreases infectivity without affecting hypha formation. Infect Immun 147:1772–1782
Wahid A, Gelani S, Ashraf M, Foolad MR (2007) Heat tolerance in plants: an overview. Environ Exp Bot 6:199–223
Walker AP, Beckerman AP, Gu L, Kattge J, Cernusak LA, Domingues TF, Scales JC, Wohlfahrt G, Wullschleger SD, Woodward FI (2014) The relationship of leaf photosynthetic traits: Vcmax and Jmax to leaf nitrogen, leaf phosphorus, and specific leaf area: a meta-analysis and modeling study. Ecol Evol 4:3218–3235
Wang Y, Wang M, Li Y, Wu A, Huang J (2018a) Effects of arbuscular mycorrhizal fungi on growth and nitrogen uptake of Chrysanthemum morifolium under salt stress. PLoS ONE 13:e0196408
Wang F, Jing X, Adams CA, Shi Z, Sun Y (2018b) Decreased ZnO nanoparticle phytotoxicity to maize by arbuscular mycorrhizal fungus and organic phosphorus. Environ Sci Pollut Res 25:23736–23747
Wang Y, Wang J, Yan X, Sun S, Lin J (2019a) The effect of arbuscular mycorrhizal fungi on PhotosystemII of the host plant under salt stress: a meta-analysis. Agronomy 9:806
Wang YN, Jie WG, Peng XY, Hua XY, Yan XF, Zhou ZQ, Lin JX (2019b) Physiological adaptive strategies of oil seed crop Ricinus communis early seedlings (cotyledon vs true Leaf) under salt and alkali stresses: From the growth, photosynthesis and chlorophyll fluorescence. Front Plant Sci 9:1939
Wang Y, Zhang W, Liu W, Ahammed GJ, Wen W, Guo S, Shu S, Sun J (2021) Auxin is involved in arbuscular mycorrhizal fungi-promoted tomato growth and NADP-malic enzymes expression in continuous cropping substrates. BMC Plant Biol 21:48
Wu QS, Zou YN, Huang YM (2013) The arbuscular mycorrhizal fungus Diversispora spurca ameliorates effects of water logging on growth, root system, architecture and antioxidant enzymes activities of citrus seedlings. Fungal Ecol 6:37–43
Xie Y, Yang L, He Z (2019) Effects of AMF infection on photosynthetic characteristics of tomato under salt stress. IOP Conf Series, Earth and Environmental Science, 295
Yang Y, Han X, Liang Y, Ghosh A, Chen J, Tang M (2015) The combined effects of arbuscular mycorrhizal fungi (AMF) and lead (Pb) stress on Pb accumulation, plant growth parameters, photosynthesis, and antioxidant enzymes in Robinia pseudoacacia L. PLoS ONE 10(12):e0145726
Yooyongwech S, Samphumphuang T, Tisarum R, Theerawitaya C, Chaum S (2016) Arbuscular mycorrhizal fungi (AMF) improved water deficit tolerance in two different sweet potato genotypes involves osmotic adjustments via soluble sugar and free proline. Sci Hort 198:107–117
Zhu XC, Song FB, Xu HW (2010) Effects of arbuscular mycorrhizal fungi on photosynthetic characteristics of maize under low temperature stress. Chinese 21:470–475
Zhu XC, Song FB, Liu SQ, Liu TD (2011) Effects of arbuscular mycorrhizal fungus on photosynthesis and water status of maize under high temperature stress. Plant Soil 346:189–199
Zhu X, Song F, Liu F (2017) Arbuscular mycorrhizal fungi and tolerance of temperature stress in plants. In: Wu QS (ed) Arbuscular mycorrhizas and stress tolerance of plants. Springer Nature, Singapore. https://doi.org/10.1007/978-981-10-4115-0_8
Funding
This work was supported by University Grants Commission, [UGC], India for awarding Post-Doctoral Fellowship for Women [PDFWM-2014–15-GEMAD-23945] to SM.
Author information
Authors and Affiliations
Contributions
Review was written and edited by SM and AJ.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have No Conflict of Interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jajoo, A., Mathur, S. Role of arbuscular mycorrhizal fungi as an underground saviuor for protecting plants from abiotic stresses. Physiol Mol Biol Plants 27, 2589–2603 (2021). https://doi.org/10.1007/s12298-021-01091-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-021-01091-2