Abstract
The experiments were conducted for the estimation of the mitigating effect of the static magnetic field (SMF of 200 mT for 1 h) treatment on soybean under ambient UV-B stress. The SMF treated (MT) and untreated (UT) seeds were grown inside iron cages covered with polyester filters for the purpose to filter UV-A + B (< 400 nm) and UV-B (< 300 nm) radiations, polythene filter control (FC) transparent for UV (280-400 nm), and open controls (OC) were without any filters. Our results indicated that specific leaf weight, efficiency of PS II, activity of carbonic anhydrase (CA) and nitrogenase (NRA), nucleic acid and protein content, nitric oxide (NO) and yield were significantly decreased in plants of untreated seeds under UV-B stress. SMF treatment to the soybean seeds was observed to mitigate the adverse effect of ambient UV-B with a significant enhancement in above-measured parameters in plants when compared with plants of untreated seeds grown under OC/FC conditions. Chlorophyll a fluorescence transition curve (OJIP-curve) from SMF treated and UV excluded plants has shown a higher fluorescence yield especially for I–P phase as compared to the plants grown in ambient UV-B stress. Reduction in the level of superoxide anion radicle (\({\text{O}}_{2}^{{ \cdot^{ - } }}\)), hydrogen peroxide (H2O2), malondialdehyde (MDA) and proline content with a remarkable increase in DNA, RNA, protein and NO content, increased photosynthetic efficiency and nitrogen fixation in the leaves of soybean suggested the ameliorating effect of SMF pre-treatment against ambient UV-B induced damage. Consequently, SMF-pretreatment increased the tolerance of soybean seedlings to ambient UV-B stress as compared to the untreated seeds. The increase in carbon and nitrogen fixation ability due to SMF pre-treatment and the omission of solar UV radiation impact can be a direction for the purpose to improve the crop yield. Evaluation of the consequences of SMF treated seeds under ambient UV-B stress, and the plants from untreated seeds under solar UV exclusion indicated parallelism among the two effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the last century, anthropogenic activities have led to a rapid deterioration in the environment. Among different impacts, ozone depletion is a serious problem, which leads to the penetration of ultraviolet radiation (UV-B, 280–320 nm) to the Earth’s surface. UV-B constitute a small fraction of total solar radiation which reaches the Earth’s surface (Kataria et al. 2014a, b). The problem has been given attention and since the mid-1990s, the UV-B alleviation is monitored through the Montreal Protocol. However, it is very complicated to reach the level of the ozone layer to a pre-1980 stage and may require numerous decades (McKenzie et al. 2011; Bais et al. 2019). UV-B contributes to a tiny fraction of the solar spectrum; however, its high energy level leads to substantial damage to the plants (Santos et al. 1993; Kataria et al. 2014a; Robson et al. 2015). It has been previously reported that exposure to UV-B radiations may cause a change in several structural molecules such nucleic acids, proteins, and lipids. Several reports have also shown an enhancement in the production of reactive oxygen species (ROS), with the observation related to a decrease in several physiological processes such as photosynthetic capacity and N2 fixation in plants (Kataria et al. 2014a; Kurth et al. 2015). It is established that UV-B radiation damage the DNA by creating several dimers (such as cyclobutane pyrimidine dimers, and pyrimidine-pyrimidinone (6-4) photoproducts) (Santos et al. 2012). DNA damage resulting due to UV-B leads to the impairment in DNA replication, resulting in failure of transcription and ultimate cell death (Santos et al. 2012; Chudobova et al. 2015).
There are several studies where specific filters were applied to filter out solar UV, these studies were generally focused on the impacts of ambient UV on plant growth parameters, yield, and photosynthesis (Kataria et al. 2014a, b). The UV exclusion studies have reported that the exposure of plants to UV-B radiations caused an adverse effect on the plant growth parameters, biomass accumulation, photosynthesis, nitrogen fixation and yield of several plant species like wheat, barley, sorghum, maize, cucumber, soybean, cotton, amaranthus, mung bean and pea (Pal et al. 2006; Kataria et al. 2013; Kataria and Guruprasad 2014, 2015).
Recently, methods such as radiofrequency energies, electron beam, gamma rays, microwaves, laser, and magnetic fields were used for the purpose to stimulate seed germination. With the application of different physical methods, seeds were observed to increased its vigor and improved the development of plants, and ultimately an increase in crop yield (Stange et al. 2002; Aladjadjiyan 2007; Akshatha et al. 2013; Kumar et al. 2014; Kataria and Jain 2018).
In agriculture, static magnetic field (SMF) pre-treatments was used as a novel environmentally friendly physical method for seed priming for the purpose to improve seed germination, growth, photosynthesis, and crop yield under non-stress as well as abiotic stress conditions (Martinez et al. 2000; Kataria et al. 2015, 2017a, b; Kataria and Jain 2018; Kataria et al. 2019). Although several works have been performed previously, where SMF was shown to have a positive impact on seed germination, plant growth, protein biosynthesis, seedling elongation and root development (Yinan et al. 2005; Kataria et al. 2019, 2020). Earlier studies also suggested the changes in structural molecules such as nucleic acids, and protein synthesis gets influenced by the changes in strengths of MF in G1 phase of the cell division, where the cell division rate was observed to be increased in cells exposed to MF (Atak et al. 2003). Previously, it has been found that the pre-treatment of SMF (200 mT for 1 h) to the soybean seeds leads to the improvement of plant growth, and photosynthetic parameters as compared to untreated ones in presence of salt, drought and ambient UV-B stress (Kataria et al. 2017a, b; Baghel et al. 2016, 2018, 2019). To best of our knowledge, the effects of SMF along with ambient UV-B stress on Chl a fluorescence induction curve (OJIP), leaf phenological models, along with the activity of CA and NRA, and metabolites like DNA, RNA and protein, proline and MDA content in soybean have not been reported previously in soybean. Soybean (Glycine max) is among the most important leguminous crop, which is very sensitive to UV-B stress (Baroniya et al. 2011; Kataria et al. 2017b). Therefore, through this experimental work, we evaluated the changes in physiological and biochemical parameters of soybean in response to SMF pretreatment and ambient UV-B. In the present work, we evaluated the impact of magnetopriming with SMF (200 mT for 1 h) particularly on leaf growth, chlorophyll a fluorescence parameters, activities of CA in the leaves and NRA in the root nodules, nucleic acids and protein, ROS, NO, proline, and MDA content in leaves of the soybean plants under the ambient UV (280 - 400 nm) stress and under solar UV exclusion filters.
Materials and methods
Plant material and experimental setup
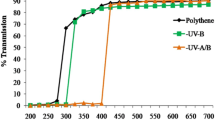
The soybean (Glycine max) var. JS-335 seeds were obtained from ICAR-Indian Institute of Soybean Research, Khandwa Road, Indore, India. The experiments were conducted under the natural light condition from October 2018 to January 2019 at an open terrace of the School of Biochemistry, Devi Ahilya Vishwavidhalaya, Indore (22° 44′ N), India. The uniformed size soybean seeds were pre-treated with SMF of 200 mT for 1 h before its plantation. The moistened unprimed (UT-untreated) and magnetoprimed (SMF-treated) seeds of soybean were treated with fungicides like Diathane M (Sagan Agro Industries, Meerut, India) and Bevistin (Ankur Agro company, Etawah, India) at 2 g kg−1 seeds, and then, mixed with powder of Rhizobium culture 3 g kg−1 seed. The treated (MT) and untreated (UT) seeds were cultivated in nursery bags of the size 34 × 34 cm. The nursery bags were filled with a mixture of soil, sand, and farm-yard manure (in the ratio of 2:2:1). The plastic nursery bags were kept under a designed iron mesh cages, The cages were wrapped with a UV cut-off Polyester filters (Garware polyester Ltd., Mumbai) with a cut-off range for UV-A + B < 400 nm and UV-B < 315 nm. In this study, we planned two types of controls; plants were grown either in the cages covered with transparent polythene that transmits ambient UV (filter control, FC) or without any polythene in open field, exposed to ambient solar radiation (open control, OC). The Shimadzu Spectrophotometer (UV-1800) was used to measure the transmission characters of the used filters (Fig. 1a).
Magnetic field generation and treatment
For the generation of the electromagnetic field, “AETec” (Academy of Embedded Technology, Delhi, India) was used (as shown in Fig. 1b), which generate variable magnetic field strength (50–500 mT). For the experiments, electromagnetic field of 200 mT was generated with a gap of 5 cm between pole pieces. The pole pieces of the electromagnet were cylindrical in shape with 9 cm diameter and 16 cm length. The total number of turns of copper coil per pole piece was 3000 and resistance of the coil was 16 Ω. A DC power supply (0–85 V/10 A) with a continuously variable output current was used for the electromagnet. The digital gauss meter (AEtech model DGM-102) operating on the principle of Hall Effect monitored the field strength produced in the pole gap. The magnetic field of 200 mT was applied to soybean seeds for an hour in a sample holder (transparent plastic) of 42 cm3 capacity (2.7 L × 2.6 B × 7.3 H) at 25 °C. The seeds not exposed to magnetic field (kept away from the magnetic field) were considered as untreated seeds. The local geomagnetic field was observed to be < 10 mT. The experiments were conducted for SMF-primed and unprimed seeds simultaneously under similar conditions.
Measurement of growth, chlorophyll (Chl) content and chlorophyll fluorescence parameters
The experiments were conducted in triplicates (n = 3); different samples from five plants of each replica were analyzed for each treatment at 45 days after the emergence of seedlings (DAE).
For the assessment of morphological variation, specific leaf weight (SLW) and leaf weight ratio (LWR) of the third trifoliate leaves was measured as described by Hunt (1982). The leaves were oven-dried at 105 °C for 24 h for constant weight. The dry mass of the third trifoliate leaves was measured. A portable laser leaf area meter CID-202 scanning planimeter (CID Inc., USA) was used to measure leaf area. The concentration of chlorophyll (Chl) a and Chl b, were measured as per Hiscox and Israelstam (1979).
Chlorophyll a fluorescence (Chl F) of dark-adapted third trifoliate leaves of soybean at 45 DAE was measured by a Handy PEA fluorimeter (Plant Efficiency Analyzer, Hansatech Instruments Ltd., King’slynn Norfolk) as previously described in Kalaji et al. 2018; Rastogi et al. 2019a, b). The light intensity reaching the leaf was 3200 µE m−2 s−1. The plants were dark-adapted for 30 min. Data were recorded for 1 s with 12-bit resolution; the data acquirement was for every 10 µs for the first 2 ms and subsequently for every 1 ms (Strasser et al. 1995). Different chlorophyll fluorescence characteristics such as specific quantum yield of electron transport (PHIE0), the density of active photosynthetic reaction centers (RC/CSm) and leaf phenomenological models were calculated by using the software Biolyzer HP 3 (the chlorophyll fluorescence analyzing program by Bioenergetics Laboratory, University of Geneva, Switzerland) (Strasser et al. 1995).
Measurement of carbonic anhydrase activity (CA)
CA activity was measured in the third trifoliate leaves at 45 DAE by using the methods described in Kataria and Guruprasad (2014). Enzyme activity was defined as 1 unit = 10 (To − T)/T, in which T and To represent the time (s) needed for a pH decrease from 8.25 to 6.45, respectively with and without enzyme.
Measurement of nitrogenase activity (NRA)
Nitrogenase (EC 1.18.6.1) activity was determined in root nodules of soybean by the acetylene reduction assay (ARA) (Hardy et al. 1973). Standard curve for ethylene was plotted for this analysis. Nitrogenase activity was calculated for the linear phase of reaction and expressed as n mole C2H4 produced g−1 fresh weight of root nodules h−1.
Biochemical analysis
DNA, RNA, protein, superoxide radical, proline and MDA content were measured in third trifoliate leaves of the soybean seedlings emerged from SMF treated and untreated soybean seeds after 45 DAE.
DNA, RNA and protein
The total DNA content was estimated by diphenylamine (DPA) reagent as described in Gendimenico et al. (1988). The standard curve of DNA was prepared by 40–400 µg of horse sperm DNA and was expressed in µg g−1 fresh weight of leaves.
The total RNA content was estimated using orcinol reagent by the method of Webb et al. (1958). The standard curve for RNA was prepared using 40–200 µg of yeast RNA and it was expressed as µg g−1 fresh weight of leaves.
Total soluble protein was estimated as per Lowry (1951) using bovine serum albumin as the standard and expressed in mg g−1 fresh weight of leaves.
Superoxide radical (\({\text{O}}_{2}^{{ \cdot^{ - } }}\)) and nitric oxide (NO)
Superoxide anion radical (\({\text{O}}_{2}^{{ \cdot^{ - } }}\)) was quantified by its capacity to reduce nitroblue tetrazolium chloride (NBT) in soybean leaves following the method of Chaitanya and Naithani (1994). For \({\text{O}}_{2}^{{ \cdot^{ - } }}\) estimation, 0.1 g radicles were homogenized with cold (4 °C) sodium phosphate buffer (0.2 M, pH 7.2) containing diethyldithiocarbamate (10−3 M) to inhibit active superoxide dismutase, and this homogenate was centrifuged (Remi Centrifuge C-24 Plus) at 13800g for 10 min at 4 °C. The resultant supernatant was used for estimation of \({\text{O}}_{2}^{{ \cdot^{ - } }}\). The optical density of the end product was measured at 540 nm using a UV–Vis spectrophotometer (UV-1800). It was calculated using a molar absorption coefficient of 12.8 mM−1 cm−1 and expressed as µmoles g−1 fresh weight of leaves.
Nitric oxide (NO) content was measured according to the method of Zhou et al. (2005) by Greiss reagent (1% sulphanilamide and 0.1% N-1-napthylethylenediaminedihydrochloride in 5% H2PO4 solution). The NO content was measured at 540 nm with a Shimadzu Spectrophotometer (UV-1800) and it was calculated using a standard curve prepared with NaNO2 and expressed in n moles g−1 fresh weight of leaves.
Malondialdehyde (MDA) and Proline
MDA content was measured by estimating thiobarbutaric acid reactive substances (TBARS) using the method described by Heath and Packer (1968). The amount of MDA was calculated using the extinction coefficient (e = 155 mM cm) and expressed in mM mg−1 fresh weight of leaves.
The proline content was estimated as per Bates et al. (1973). The proline content was expressed as µg proline g−1 fresh weight of the leaves.
Yield
After the harvest at 120 DAE, soybean biological yield and seed yield were measured. For the calculation of seed yield, seed weight per plant, whereas, the biological yield was measured by weighing above-ground parts. The % Harvest index was calculated using:
Statistical analysis
The data are presented as the mean ± SE of triplicates (n = 3) and analyzed by the analysis of variance (ANOVA) followed by post hoc Newman–Keuls Multiple Comparison Test by using Prism 4 software for Windows, GrafPad Software, La Jolla, California. ###p < 0.001; ##p < 0.01; #p < 0.05 indicates the significant difference between OC with FC and UV exclusion conditions in soybean plants emerged from untreated seeds; ***p < 0.001; **p < 0.01; *p < 0.05 indicates significant difference between SMF pre-treated (MT) and untreated (UT) seedlings grown in different treatments like OC/FC as well as in UV exclusion conditions.
Results
Growth, chlorophyll (Chl) content and chlorophyll fluorescence parameters
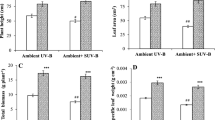
In the present study, we have observed that solar UV exclusion has promoting effects on leaf growth, specific leaf weight, leaf weight ratio in soybean plants, which emerged from UT seeds (Fig. 2a, b). Solar UV-B exclusion caused 44% increase whereas, exclusion of UV-A + B caused 37% increase in SLW in UT plants when compared with plants from UT seeds under ambient UV (OC) conditions. On the other hand, in presence of ambient UV stress conditions (OC and FC), the plants that emerged from SMF treated seeds also caused significant increase in SLW and LWR as compared to the plants emerged from untreated seeds (Fig. 2a, b). The maximum enhancement of 32–26% in LWR was observed respectively under OC and FC conditions after SMF pre-treatment when compared with untreated ones (Fig. 2a). While SMF caused a 20% increase in SLW under OC and 35% increase was found under FC conditions (Fig. 2a). When the SMF pre-treated plants were grown under UV exclusion filters then SMF treatment does not cause any significant changes in UV exclusion induced enhancement in the studied parameters.
Effect of SMF pre-treatment (200 mT for 1 h) on specific leaf weight (a), and leaf weight ratio (b) of third trifoliate leaves at 45 days after emergence of soybean seedlings grown under different treatments. The vertical bar indicates ± SE for mean. OC = ambient control, FC = filter control, –UV-B = exclusion of solar UV-B and –UV-A + B = exclusion of solar UV-A and B both
The Chl a/b ratio was observed to be higher in leaves of soybean plants from UT seed in comparison to SMF pretreatment and samples from solar UV exclusion (Fig. 3). The reduction in Chl a/b by SMF and solar UV exclusion may be due to an increase in Chl b then Chl a (data not given).
Effect of SMF pre-treatment (200 mT for 1 h) on Chla/b ratio of third trifoliate leaves at 45 days after emergence of soybean seedlings grown under different treatments. The vertical bar indicates ± SE for mean. OC = ambient control, FC = filter control, –UV-B = exclusion of solar UV-B and –UV-A + B = exclusion of solar UV-A and B both
Assessment of ChlF parameters indicated that the ambient UV-B exposure results in a significant decrease in maximum fluorescence (Fm) when compared with ChlF from plants of solar UV exclusion or after SMF pretreatment. The transient fluorescence curve (O-J-I-P) from SMF treated and UV excluded plants (OC/FC) is shown in Fig. 4. For better presentation, the O–J–I–P transients were normalized at Fo. A significant difference in O–J–I–P curve between UT and MT were observed in OC and FC conditions. The O–J–I–P curve showed an increase in I–P phase due to the exclusion of solar UV and also after SMF treatment as compared to the leaves of plants emerged from UT seeds in the presence of UV stress (OC/FC) (Fig. 4).
Effect of SMF pre-treatment on chlorophyll fluorescence emission transients in third trifoliate leaves at 45 days after emergence of the soybean seedlings grown under different treatments. OC = ambient control, FC = filter control, –UV-B = exclusion of solar UV-B and –UV-A + B = exclusion of solar UV-A and B both. O–J–I–P steps, where O is the minimal fluorescence (Fo), P is the peak at about 500 ms (Fp), and J and I are inflections
The photosynthetic parameters such as yield of electron transport (φEo = ETo/ABS), was observed to be increased by 11% and 10% after the exclusion of UV-B and UV-A + B, respectively as compared to UT ones grown in OC conditions (Fig. 5a). SMF treatment caused slight increase in ETo/ABS as compared to their UT ones under the presence of UV-B stress. The density of active photosynthetic reaction centers (RC/CSm) was slightly improved by SMF treatment and solar UV exclusion in comparison to untreated plants grown under ambient UV conditions (OC/FC) (Fig. 5b).
Effect of SMF pre-treatment on specific quantum yield of electron transport (PHIE0) (a), and the density of active photosynthetic reaction centers (RC/CSm) (b) in the third trifoliate leaves at 45 days after emergence of the soybean seedlings grown under different treatments. The vertical bar indicates ± SE for mean. OC = ambient control, FC = filter control, –UV-B = exclusion of solar UV-B and –UV-A + B = exclusion of solar UV-A and B both
In leaves of soybean plants that emerged from SMF treated seeds and untreated seeds grown in presence and absence of UV stress, the phenomenological leaf model was obtained through Biolyzer HP 3 software. The model showed that SMF treatment and solar UV exclusion enhanced electron transport (ETo/CSm) as compared to the UT ones grown under ambient UV stress (Fig. 6). In model, the open circles showed the active reaction center; the plants after SMF treatment grown under UV-B and UV-A/B exclusion filters had higher number of active reaction centers combined with higher efficiency of ETo/CSm indicated by broader width of the arrow in the leaf models as compared to their UT ones (Fig. 6).
Effect of SMF pre-treatment on leaf phenomological models in the third trifoliate leaves at 45 days after emergence of the soybean seedlings grown under different treatments. OC = ambient control, FC = filter control, –UV-B = exclusion of solar UV-B and –UV-A + B = exclusion of solar UV-A and B both. The arrows showed fluxes for light absorbance (ABS), excitation energy trapping (TRo), energy dissipation (DIo) and electron transport (ETo) beyond QA-. ABS/CSm = absorption flux CS approximately by Fm; TRo/CSm = trapped energy per CS, ETo/CSm = electron transport flux per CS; DIo/CSm = dissipated energy per CS. By the size of the proper parameters (arrow), each relative value is stands for the thickness of each arrow indicates the relative size of the fluxes or the antenna, empty circles stands for reducing QA active reaction centers, full black circles represents for non-reducing QA inactive or silent reaction center
Carbonic anhydrase (CA) and nitrogenase (NRA) activity
SMF treatment significantly increased the activity of CA in third trifoliate leaves of soybean by 33% in OC and 46% in FC conditions as compared to their UT ones (Fig. 7a). It was enhanced by 37% by UV-B exclusion and 22% by UV-A + B exclusion as compared to UT ones under OC conditions (Fig. 7a).
Effect of SMF pre-treatment on activity of carbonic anhydrase in the third trifoliate leaves (a), and nitrogenase in root nodules (b) at 45 days after emergence of the soybean seedlings grown under different treatments. The vertical bar indicates ± SE for mean. OC = ambient control, FC = filter control, –UV-B = exclusion of solar UV-B and –UV-A + B = exclusion of solar UV-A and B both
Similarly, SMF treatment caused a remarkable increase in activity of nitrogen-fixing enzyme NRA by 151% and 138% respectively under OC and FC conditions (Fig. 7b). The significant increase of 183% and 100% respectively was observed in NRA activity after solar UV-B and UV-A + B exclusion in comparison to UT ones grown under OC conditions (Fig. 7b).
SMF treatment does not cause any significant changes in the activity of CA and NRA under solar UV exclusion filters (Fig. 7b).
DNA, RNA, and protein
Exclusion of solar UV-B and UV-A + B suggested that ambient UV-B caused a decrease in the content of DNA, RNA and protein while SMF treatment and exclusion of solar UV increase these bio-molecules (Fig. 8a–c).
Effect of SMF pre-treatment on DNA (a), RNA (b) and protein (c) content in the third trifoliate leaves at 45 days after emergence of the soybean seedlings grown under different treatments. The vertical bar indicates ± SE for mean. OC = ambient control, FC = filter control, –UV-B = exclusion of solar UV-B and –UV-A + B = exclusion of solar UV-A and B both
SMF pretreatment caused 38% increase in DNA content in OC conditions (+UV-B) and in FC conditions (+UV-B), SMF caused 32% increase as compared to their UT ones (Fig. 8a). Exclusion of solar UV-B caused 33% and exclusion of solar UV-A + B caused 28% increase in DNA content of leaves of plants that emerged from UT seeds as compared to the plants grown under ambient UV stress (OC) (Fig. 8a).
Similarly, RNA and protein content was also increased by SMF treatment and solar UV exclusion but when SMF treated seeds grown under UV exclusion filters then SMF does cause any significant change (Fig. 8b,c). The plants emerged from UT seeds under exclusion of solar UV-B and UV-A + B caused 17% and 27% increase respectively in the RNA content as compared to the plants of UT seeds grown in presence of UV-B stress (OC) (Fig. 8b).
The protein content was also dramatically increased by SMF treatment even under the presence of UV stress (OC and FC conditions) as compared to their UT ones (Fig. 8c). It caused 92% and 80% promotion in protein content respectively in OC and FC conditions as compared to their UT ones (Fig. 8c). Similarly, exclusion of solar UV-B caused 29% and solar UV-A + B caused 35% increase in protein content in the leaves of plants from UT seeds as compared to the plants grown under ambient UV conditions (OC) (Fig. 8c).
Superoxide anion radical and nitric oxide content
Superoxide anion radical (\({\text{O}}_{2}^{{ \cdot^{ - } }}\)) content was observed to be higher in the leaves of plants that emerged from UT seeds (OC and FC) when compared with plants from SMF-pre treated seeds. Whereas under ambient UV stress, the \({\text{O}}_{2}^{{ \cdot^{ - } }}\) content was observed to be low in the plants emerged from SMF-pre-treated seeds and in the plants which emerged from untreated seeds under the exclusion of solar UV components (Fig. 9a).
Effect of SMF pre-treatment on superoxide radical (a) and nitric oxide (b) content in the third trifoliate leaves at 45 days after emergence of the soybean seedlings grown under different treatments. The vertical bar indicates ± SE for mean. OC = ambient control, FC = filter control, –UV-B = exclusion of solar UV-B and –UV-A + B = exclusion of solar UV-A and B both
Nitric oxide (NO) content was remarkably increased by SMF treatment and solar UV exclusion alone and in a combination of SMF and UV exclusion filters (Fig. 9b). SMF treatment significantly increases the NO content by 89% and 86% respectively in OC and FC conditions as compared to their UT ones (Fig. 9b). Whereas the plants of UT seeds under exclusion of UV-B caused 70% and exclusion of solar UV-A + B caused 57% increase in NO content in comparison to the plants of UT seeds grown under OC conditions (Fig. 9b).
The SMF treated plants under UV-B exclusion filters showed 24% increase and UV-A + B exclusion filters showed 48% increase in NO content as compared to their UT ones (Fig. 9b).
MDA and Proline
The MDA and proline contents were observed to be decreased due to solar UV exclusion and SMF treatment, but the extent of reduction was more in proline content by solar UV exclusion and SMF treatment (Fig. 10a, b). The proline content was observed to be decreased by 54% and 56% by SMF treatment respectively under OC and FC conditions (Fig. 10b). And the exclusion of solar UV-B caused 24% and UV-A + B exclusion caused 19% reduction in proline content as compared to their UT ones in OC conditions (Fig. 10b).
Effect of SMF pre-treatment on MDA (a) and proline (b) content in the third trifoliate leaves at 45 days after emergence of the soybean seedlings grown under different treatments. The vertical bar indicates ± SE for mean. OC = ambient control, FC = filter control, –UV-B = exclusion of solar UV-B and –UV-A + B = exclusion of solar UV-A and B both
Yield attributes
In the present study, a significant difference in yield parameters like total biomass accumulation, number of pods and weight of seeds per plant was observed at maturity on 120 DAE in the plants grown under UV exclusion filters and plants emerged from SMF treated seeds under ambient UV-B radiation (Fig. 11a, b). We have found negative effect of ambient UV-B stress on the yield of soybean, but the plants emerged from SMF treated seeds showed 25% increase in harvest index as compared to UT ones under the presence of ambient UV stress (Fig. 11b). In the same way, -UV-B caused 42% increase and -UV-A + B caused 21% enhancement in the harvest index of UT plants as compared to the plants emerged from UT seeds under ambient UV stress (OC) conditions (Fig. 11b).
Effect of SMF pre-treatment on yield attributes (a) and harvest index (b) of soybean seedlings grown under different treatments at maturity (120 DAE). The vertical bar indicates ± SE for mean. OC = ambient control, FC = filter control, –UV-B = exclusion of solar UV-B and –UV-A + B = exclusion of solar UV-A and B both
Discussion
In the present study, the exclusion of solar UV-B radiations indicated that ambient UV-B radiation severely affected the different growth and physiological parameters of soybean as indicated by the changes in leaf growth, photosynthetic parameters, and metabolites. On the other hand, SMF pre-treatment and exclusion of solar UV enhanced the growth, PSII efficiency, carbon and nitrogen fixation and yield in soybean plants as compared to their UT ones grown under ambient UV-B stress. Similarly, earlier individual studies on SMF treatment and solar UV exclusion suggested the enhancement in the plant growth, leaf area, biomass accumulation, and photosynthetic efficiency; and stimulated the activities of CA, NRA and Rubisco in several crops wheat, maize, cotton, soybean (Kataria et al. 2013, 2017b, 2019; Kataria and Guruprasad 2012, 2014, 2015).
This is the first report showing that SMF pretreatment and solar UV-B and UV-A + B exclusion significantly increased the JIP test parameters like rise in I–P phase, Quantum yield of electron transport, RC/CSm; active reaction centers and electron transport per cross-section; activity of CA and NRA and biomolecules like DNA, RNA and proteins and NO content as compared to the plants emerged from UT seeds grown under ambient UV-B stress (OC or FC) conditions. Thus, our data indicate that ambient UV-B stress suppressed the fixation of carbon and nitrogen in the soybean plants emerged from UT seeds under OC and FC.
A significant increase in LWR and SLW as a response to solar UV exclusion and SMF pre-treatment influenced the plant’s higher biomass and an increase in leaf thickness. Previous studies have shown a similar response of UV exclusion in sorghum and amaranthus (Kataria and Guruprasad 2012, 2014). The plants grown in ambient UV conditions (OC/FC) showed lesser SLW indicating that UV-B radiation decreases leaf thickness, which results in a reduction in dry weight and rate of photosynthesis as reported in other studies (Musil and Wand 1994; Correia et al. 1999; Singh et al. 2009).
Feng et al. (2001) found that enhanced UV-B radiation altered the time of flowering in soybean cultivars and reduce the chlorophyll a and b contents, total leaf area and total leaf number. Whereas, solar UV exclusion caused an increase in the Chl a and Chl b content and a decrease in the ratio of Chl a/b in C3 and C4 plants (Kataria et al. 2013). We have also found higher Chl a/b in plants emerged from UT seeds under ambient UV stress (OC and FC) and lower Chl a/b under exclusion of UV and SMF treatment in plants grown under ambient UV stress as well as under UV exclusion filters. MF increased the chlorophyll and protein content in onion (Novitsky et al. 2001).
Previous studies have identified photosynthetic mechanism as an important target for supplemental and ambient UV-B stress (Keiller et al. 2003; Shine and Guruprasad 2012; Kataria et al. 2013, 2017b). Under UV-B stress, photosystem II is the most susceptible site in chloroplast which affects crop productivity (Kataria et al. 2014a). The changes in chlorophyll fluorescence have been widely used to detect the stress response in the photosynthetic apparatus of the plants (Kalaji et al. 2016; Rastogi et al. 2019a, b). The changes in chlorophyll fluorescence were considered as a vulnerable common biomarker to UV stress (Cordi et al. 1997). The changes in transient fluorescence curve (OJIP) (Fig. 4) reflected damage to photosynthetic apparatus and its kinetics in the leaves of plants emerged from SMF treated and untreated seeds under ambient UV stress as well as UV exclusion filter. At I–P phases, the fluorescence yield was significantly higher in the SMF treated plants compared to untreated plants under OC/FC conditions. On the other hand, solar UV exclusion also increased the I–P phase. The O–J phase contains information on antenna size and connectivity between PSII reaction centers. The changes in J–I phase indicated the reduction of the secondary electron acceptor QB, plastoquinone (PQ), cytochrome (Cyt b6f), and plastocyanin (PC). The increase in chlorophyll fluorescence specifically in the I–P phase of the OJIP curve is characteristically recognized to the reduction of electron transporters (ferredoxin, intermediary acceptors, and NADP) of the PS I acceptor side (Kalaji et al. 2016). In the present study, under ambient UV-B stress (OC/FC) conditions, the reduction in maximum chlorophyll fluorescence (Fm) was observed in the leaves of soybean plants emerged from UT seeds indicating a decrease in the amount PS II reaction centers which are able to reduce QA. Our results indicated that quantum yield of electron transport and RC/CSm were increased by the exclusion of ambient UV irradiation and SMF treatment as compared to the plants emerged from UT seeds under OC/FC. Previously, UV-B exclusion has been shown to positively impact different fluorescence parameters in cotton, wheat, soybean, maize, amaranthus, and sorghum (Shine and Guruprasad 2012; Kataria et al. 2013; Kataria and Guruprasad 2014, 2015) and also after magnetopriming in soybean plants (Shine et al. 2011).
A phenomenological leaf model was generated through Biolyzer HP 3 software (Fig. 6). The model represents more active reaction centers per unit area of the leaf after the treatment of seeds with SMF under exclusion of UV-B and UV-A + B. SMF (200 mT for 1 h) treated plants under ambient UV stress (OC/FC) and UV-B and UV-A + B excluded plants of UT seeds showed higher efficiency of electron transport (ETo/Csm), indicated by a broader thickness of the arrow in the leaf model (Fig. 6). The obtained results are in agreement with previous studies of the phenomenological leaf models after solar UV exclusion studies on wheat, cotton, amaranthus, and sorghum (Kataria et al. 2013; Kataria and Guruprasad 2015) and also after magnetopriming in soybean plants (Shine et al. 2011; Kataria and Jain 2018).
In this study, the activities of enzymes related to carbon and nitrogen metabolism such as CA in the leaves and NRA in root nodules were significantly enhanced by SMF treatment under ambient UV stress conditions (OC/FC) as compared to their UT ones (Fig. 7). The increase in the NRA activity by SMF pretreatment in the root nodules of soybean was observed under non-stress as well as in salt stress conditions (Kataria et al. 2019). Solar UV exclusion also caused enhancement in NRA activity in root nodules of soybean and trigonella (Singh 2012; Sharma and Guruprasad 2012) and CA activity in amaranthus (Kataria and Guruprasad 2014). Enhanced UV-B and solar UV-B caused reduction in the activities of enzymes involved in CO2 assimilation like carbonic anhydrase and Rubisco and nitrogen fixation by NRA in several plant species (Takeuchi et al. 2002; Xu et al. 2008; Sharma and Guruprasad 2012; Kataria et al. 2013; Kataria and Guruprasad 2014, 2015). The data of the present study suggested that the linear flow of electron beyond PS II may be increased by SMF pre-treatment and exclusion of UV radiation as it is apparent by the Chl a fluorescence data, which leads to greater proportion of electrons available for the Calvin cycle.
The impact of UV-B radiations on plants includes an alteration in structural molecules (such as DNA, RNA, proteins), increase in ROS generation, inhibition of physiological processes as N2 fixation, and photosynthesis (Santos et al. 1993; Rastogi and Pospisil 2013; Chudobova et al. 2015; Kurth et al. 2015). UV-B can induce the formation of oxidative compounds that is highly reactive and can cause DNA damage indirectly. UV-A has no direct effect on DNA because it is not absorbed by DNA. Reactive oxygen species accumulation like in present study \({\text{O}}_{2}^{{ \cdot^{ - } }}\) radical content was more in OC/FC conditions which may cause damage to biomolecules like DNA, RNA, and protein which altered the metabolic processes in the soybean plants. UV-B inactivates different proteins and enzymes due to the photolysis of aromatic amino acids (Peykarestan et al. 2012). Also, UV-B can cause protein damage by impairing RNA. But the total soluble protein content in the leaves of several plant species increased in UV exclusion studies (Kataria et al. 2014a, b; Chen et al. 2019). These studies indicated that solar UV exclusion enhanced different growth and physiological parameters (Kataria et al. 2013; Chen et al. 2019). In contrast to UV-B, MFs have been stimulating effect on DNA, RNA, and protein synthesis (Goodman et al. 1995; Kataria and Jain 2018). An increase in DNA synthesis by MFs intensities of 1700G and 3600G has been found to increase in cells in Tunica zone of roots (Majd and Farzpourmachiani 2013). The metabolic changes after MF pre-treatment involved the protein biosynthesis from mRNA, gene transcription and cellular repairs (Racuciu et al. 2008; Shabrangi and Majd 2009). Piacentini et al. (2001) observed that the protein values were higher in MF-exposed cucumber seedlings as compared with the controls. In accordance with these studies, in the present study we have also found that SMF pre-treatment enhanced the DNA, RNA and protein content and reduced the amount of superoxide radical in leaves of soybean even in the presence of ambient UV-B stress (OC/FC) conditions.
Nitric oxide (NO) is known to enhance the plant’s growth and development under several abiotic stress conditions such as UV-B radiation. Several of the previous studies has established NO as a crucial supporting factor for plants under UV-B stress. NO may act in two different ways first by its interaction with ROS and transition metals and also secondary through the generation of reactive nitrogen species (RNS) (Tossi et al. 2011). As a signaling molecule, NO is known to exhibit an important role in a plant developmental process. The protective role of NO in plants is based on lowering the levels of ROS and in such way regulating its toxicity (Hu et al. 2016). Interestingly, in our experiments with soybean a significant increase in NO was observed after SMF pre-treatment under UV-B stress (OC/FC) conditions as compared to their untreated ones. Previously it has been reported that an external application of SNP defends the Larix gmelinii seedlings and increased their tolerance to ultraviolet-B radiation (Hu et al. 2016). In the present study, first time, we have reported the increase in the DNA, RNA and NO content along with decrease in the \({\text{O}}_{2}^{{ \cdot^{ - } }}\), proline and MDA content in soybean seedlings grown after SMF pre-treatment in the presence of ambient UV-B stress.
An elevated level of MDA contents is a typical sign for lipid peroxidation and it has been reported that thylakoid membranes with abundant unsaturated fatty acids can be peroxidized by stress-related ROS (Pospisil 2016). High doses of UV-B can cause membrane peroxidation through the development of reactive oxygen species (ROS) in plants (Köhler et al. 2017). During environmental stresses, plants modify their metabolism in various ways including the production of osmoregulatory compounds, such as proline (Ma et al. 2016). The accumulation of proline is one of the most important non-enzymatic defense systems, in plants, which protects the plant against ROS. We have also found a higher level of proline and MDA under ambient UV-B stress (OC/FC) whereas these metabolites were lower after solar UV exclusion and SMF pre-treatment. Solar UV exclusion was previously observed to enhance the soluble protein content but decrease the proline and MDA content in Prunella vulgaris (Chen et al. 2019). Under cadmium stress, MF (600 mT) decreased the malondialdehyde, H2O2, and \({\text{O}}_{2}^{{ \cdot^{ - } }}\) content and the conductivity of electrolyte leakage, though the NO content and activity of nitric oxide synthase were increased in mung bean seedlings (Chen et al. 2011). These authors suggested that MF mitigated the toxicological effects of cadmium and are related to NO signal (Chen et al. 2011). Similarly, Azimian and Roshandel (2015) also found that MF treatment (200 mT for 20 min) increased the dry weight, radical-scavenging activity and decreased the lipid peroxidation (MDA content) in Artemisia sieberi under salt stress.
The accumulation of proline in a plant is a typical response to environmental stresses (Anjum 2008). It has been reported that higher proline content may be due to increased proteolysis or a decrease in protein synthesis. Overall, our results indicated that the plants grown from SMF pre-treated seeds and under solar UV exclusion filters are better protected against oxidative damage caused by ambient UV-B stress. Mahdavian et al. (2008) found that increased proline synthesis reduced the UV-B and UV-C induced damage by the elimination of excess H+ and promoted the peroxidation processes. The data of our results of SMF pre-treatment under ambient UV-B stress and solar UV exclusion indicated the elimination of oxidative damage caused by ambient UV-B stress and altered metabolism of plants which results in the improved harvested yield of soybean. Similarly, the yield of soybean was enhanced by SMF pre-treatment under salt, drought and ambient UV stress (Kataria et al. 2014b, 2017a, 2019; Baghel et al. 2018) and by solar UV exclusion also (Baroniya et al. 2011).
Thus, SMF pre-sowing treatment altered the metabolism of soybean plants and improved the tolerance towards the ambient UV stress. Similarly, the absence of solar UV components removes UV stress and reduces the defense which eventually enhances the growth, photosynthetic efficiency, and yield of soybean. The enhancement in the crop yield by SMF and solar UV exclusion was due to the better leaf growth, leaf biomass and efficiency of PS II (J-I-P parameters), carbon and nitrogen fixation, higher DNA, RNA and protein content in the soybean plants in comparison to untreated ones grown under ambient UV-B stress (OC/FC) conditions. Our results revealed that SMF-pretreatment enhanced the tolerance of soybean seedlings to ambient UV-B stress (OC/FC) as compared to the untreated ones. Using SMF-treatment could be a promising technique for agricultural improvements under abiotic stresses, although extensive research is required at the field level.
References
Akshatha CKR, Somashekarappa HM, Souframanien J (2013) Effect of gamma irradiation on germination, growth, and biochemical parameters of Terminalia arjuna Roxb. Radiat Prot Environ 36:38–44
Aladjadjiyan A (2007) The use of physical methods for plant growing stimulation in Bulgaria. J Cent Eur Agric 8:369–380
Anjum MA (2008) Effect of NaCl concentrations in irrigation water on growth and polyamine metabolism in two citrus rootstocks with different levels of salinity tolerance. Acta Physiol Plant 30:43–52
Atak Ç, Emiroğlu Ö, Alikamanoğlu S, Rzakoulieva A (2003) Stimulation of regeneration by magnetic field in soybean (Glycine max L. Merrill) tissue cultures. J Cell Mol Biol 2:113–119
Azimian F, Roshandel P (2015) Magnetic field effects on total phenolic content and antioxidant activity in Artemisia sieberi under salinity. Ind J Plant Physiol 20:264–270
Baghel L, Kataria S, Guruprasad KN (2016) Static magnetic field treatment of seeds improves carbon and nitrogen metabolism under salinity stress in soybean. Bioelectromagnetics 37:455–470
Baghel L, Kataria S, Guruprasad KN (2018) Effect of SMF pretreatment on growth, photosynthetic performance and yield of soybean under water stress. Photosynthetica 56:718–730
Baghel L, Kataria S, Jain M (2019) Mitigation of adverse effects of salt stress on germination, growth, photosynthetic efficiency and yield in maize (Zea mays L.) through magnetopriming. Acta Agrobot 72:1757
Bais AF, Bernhard G, McKenzie RL, Aucamp PJ, Young PJ, Ilyas M, Jöckel P, Deushi M (2019) Ozone-climate interactions and effects on solar ultraviolet radiation. Photochem Photobiol Sci 18:602–640
Baroniya SS, Kataria S, Pandey GP, Guruprasad KN (2011) Intraspecific variation in sensitivity to ambient ultraviolet-B radiation in growth and yield characteristics of eight soybean cultivars grown under field conditions. Braz J Plant Physiol 23:197–202
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Chaitanya KS, Naithani SC (1994) Role of superoxide, lipid peroxidation and superoxide dismutase in membrane perturbation during loss of viability in seeds of Shorea robusta Gaertn.f. New Phytol 126:623–627
Chen YP, Li R, He JM (2011) Magnetic field can alleviate toxicological effect induced by cadmium in mungbean seedlings. Ecotoxic 20:760–769
Chen Y, Zhang X, Guo Q, Cao L, Qin Q, Li C, Zhao M, Wang W (2019) Plant morphology, physiological characteristics, accumulation of secondary metabolites and antioxidant activities of Prunella vulgaris L. under UV solar exclusion. Biol Res 52:17. https://doi.org/10.1186/s40659-019-0225-8
Chudobova D, Cihalova K, Jelinkova P, Zitka J, NejdlL Guran R et al (2015) Effects of stratospheric conditions on the viability, metabolism and proteome of prokaryotic cells. Atmosphere 6:1290–1306
Cordi B, Depledge MH, Price DN, Salter LF, Donkin ME (1997) Evaluation of chlorophyll fluorescence, in vivo spectrophotometric pigment absorption and ion leakage as bio-markers of UV-B exposure in marine macroalgae. Mar Biol 130:41–49
Correia CM, Areal ELV, Torres-Pereira MS, Torres-Pereira JMG (1999) Intraspecific variation in sensitivity to ultraviolet-B radiation in maize grown under field conditions. II. Physiological and biochemical aspects. Field Crops Res 62:97–105
Feng HY, An LZ, Xu SJ, Qiang WY, Chen T, Wang XL (2001) Effect of enhanced ultraviolet-B radiation on growth, development, pigments and yield of soybean (Glycine max (L.) Merr.). Acta Agron Sin 27:319–323
Gendimenico GJ, Bouquin PL, Tramposch KM (1988) Diphenylamine-colormetric method for DNA assay: a shortened incubating sample at 50 °C. Anal Biochem 173(1):45–48
Goodman EM, Greenebaum B, Marron MT (1995) Effects of electromagnetic fields on molecules and cells. Int Rev Cytol 158:279–338
Hardy R, Burns RC, Holsten RD (1973) Applications of the acetylene-ethylene assay for measurement of nitrogen fixation. Soil Biol Biochem 5(1):47–81
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. l Kinetics and stoichiometry of fatty acid per oxidation. Biochem Biophys 125:189–198
Hiscox JD, Israelstam GF (1979) A method for the extraction of chlorophyll from leaf tissue without maceration. Can J Bot 57:1332–1334
Hu H, Zhou Z, Sun X, Zhang Z, Meng Q (2016) Protective effect of nitric oxide (NO) against oxidative damage in larix gmelinii seedlings under ultraviolet-B irradiation. Forests 7:251. https://doi.org/10.3390/f7110251
Hunt R (1982) Plant growth analysis. University Press, Baltimore
Kalaji MH, Jajoo A, Oukarroum A, Brestic M, Zivcak M, Samborska AI, Cetner DM, Lukasik I, Goltsev V, Ladle JR (2016) Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol Plant 38:102
Kalaji HM, Rastogi A, Živčák M, Brestic M, Daszkowska-Golec A, Sitko K, Alsharafa KY, Lotfi R, Stypiński P, Samborska IA, Cetner MD (2018) Prompt chlorophyll fluorescence as a tool for crop phenotyping: an example of barley landraces exposed to various abiotic stress factors. Photosynthetica 56:353–361
Kataria S, Guruprasad KN (2012) Solar UV-B and UV-A/B exclusion effects on intraspecific variations in crop growth and yield of wheat genotypes. Field Crops Res 125:8–13
Kataria S, Guruprasad KN (2014) Exclusion of solar UV components improves growth and performance of Amaranthus tricolor varieties. Sci Hortic 174:36–45
Kataria S, Guruprasad KN (2015) Exclusion of solar UV radiation improves photosynthetic performance and yield of wheat varieties. Plant Physiol Biochem 97:400–411
Kataria S, Jain M (2018) Magnetopriming alleviates adverse effects of abiotic stresses on plants. In: Hasanuzzaman M, Fujita M, Oku H, Tofazzal Islam M (eds) Plant tolerance to environmental stress: role of phytoprotectants, Chap 26, 1st edn. CRC Press, Boca Raton, pp 427–438
Kataria S, Guruprasad KN, Ahuja S, Singh B (2013) Enhancement of growth, photosynthetic performance and yield by exclusion of ambient UV components in C3 and C4 plants. Photochem Photobiol B Biol 127:140–152
Kataria S, Jajoo A, Guruprasad KN (2014a) Impact of increasing ultraviolet-B radiation on photosynthetic processes. J Photochem Photobiol B 137:55–66
Kataria S, Baroniya SS, Baghel L, Kanungo M (2014b) Effect of exclusion of solar UV radiation on plants. Plant Sci Today 1:224–232
Kataria S, Baghel L, Guruprasad KN (2015) Effect of seed pretreatment by magnetic field on the sensitivity of maize seedlings toambient ultraviolet radiation (280–400 nm). Int J Trop Agric 33:1–7
Kataria S, Baghel L, Guruprasad KN (2017a) Pre-treatment of seeds with static magnetic field improves germination and early growth characteristics under salt stress in maize and soybean. Biocatal Agric Biotechnol 10:83–90
Kataria S, Baghel L, Guruprasad KN (2017b) Alleviation of adverse effects of ambient UV stress on growth and some potential physiological attributes in soybean (Glycine max) by seed pre-treatment with static magnetic field. J Plant Growth Regul 36:550–565
Kataria S, Baghel L, Jain M, Guruprasad KN (2019) Magnetopriming regulates antioxidant defense system in soybean against salt stress. Biocatal Agric Biotechnol 18:101090
Kataria S, Jain M, Tripathi DK, Singh VP (2020) Involvement of nitrate reductase-dependent nitric oxide production in magnetopriming-induced salt tolerance in soybean. Physiol Plant 168:422–436
Keiller DR, Mackerness SAH, Holmes MG (2003) The action of a range of sup-plementary ultraviolet (UV) wavelengths on photosynthesis in Brassica napus L. in the natural environment: effects on PS-II, CO2 assimilation and level of chloroplast proteins. Photosynth Res 75:139–150
Köhler H, Contreras RA, Pizarro M, Cortés-Antíquera R, Zúñiga GE (2017) Antioxidant Responses Induced by UVB Radiation in Deschampsia antarctica Desv. Front Plant Sci 8:921
Kumar M, Bhupinder S, Ahuja S, Dahuja A, Anand A (2014) Gamma radiation and magnetic field mediated delay in effect of accelerated ageing of soybean. J Food Sci Technol 52:4785–4796
Kurth D, Belfiore C, Gorriti MF, Cortez N, Farias ME, Albarracín VH (2015) Genomic and proteomic evidences unravel the UV-resistome of the poly-extremophile Acinetobacter sp. Ver3. Front Microbiol 6:328. https://doi.org/10.3389/fmicb.2015.00328
Lowary OH (1951) Protein measurement with the folin phenol reagent. Biol Chem 193(1):265–275
Ma Z, Marsolais F, Bykova NV, Igamberdiev AU (2016) Nitric oxide and reactive oxygen species mediate metabolic changes in barley seed embryo during germination. Front Plant Sci 7:138
Mahdavian K, Ghorbanli M, Kalantari M (2008) The effects of ultraviolet radiation on the contents of chlorophyll, flavonoid, anthocyanin and proline in Capsicum annuum L. Turk J Bot 32:25–33
Majd A, Farzpourmachiani S (2013) Effect of magnetic fields on growth and anatomical structure of Vicia sativa L. Glob J Plant Ecophysiol 3(2):87–95
Martinez E, Carbonell MV, Amaya JMA (2000) Static magnetic field of 125 mT stimulates the initial growth stages of barley (Hordeum vulagare, L.). Electromagn Biol Med 19:271–277
McKenzie RL, Aucamp PJ, Bais AF, Björn LO, Ilyas M, Madronich S (2011) Ozone depletion and climate change: impacts on UV radiation. Photochem Photobiol Sci 10:182–198
Musil CF, Wand SJE (1994) Differential stimulation of an arid-environment winter ephemeral Dimorphotheca pluvialis (L.) Moench by ultraviolet-B radiation under nutrient limitation. Plant Cell Environ 17:245–255
Novitsky YI, Novitskaya GV, Kocheshkova TK, Nechiporenko GA, Dobrovolskii MV (2001) Growth of green onions in a weak permanent magnetic field. Russ J Plant Physiol 48:709–715
Pal M, Zaidi PH, Voleti SR, Raj A (2006) Solar UV-B exclusion effect on growth and photosynthetic characteristics of wheat and pea. J New Seeds 8:19–34
Peykarestan B, Seify M, Shoukat-Fadaei M, Hatim M (2012) UV irradiation effects on seed germination and growth, protein content, peroxidase and protease activity in Portulaca grandiflora and Portula caoleracea. World Appl Sci J 17(7):802–808
Piacentini MP, Fraternale D, Piatti E, Ricci D, Vetrano F, Dacha M, Accorsia A (2001) Senescence delay and change of antioxidant enzyme levels in Cucumis sativus L. etiolated seedlings by ELF magnetic fields. Plant Sci 161:45–53
Pospisil P (2016) Production of reactive oxygen species by photosystem II as a response to light and temperature stress. Front Plant Sci 7:1950
Racuciu M, Creanga D, Horga I (2008) Plant growth under static magnetic field influence. Roman J Phys 53:353–359
Rastogi A, Pospisil P (2013) Ultra-weak photon emission as a non-invasive tool for the measurement of oxidative stress induced by UVA radiation in Arabidopsis thaliana. J Photochem Photobiol B 123:59–64
Rastogi A, Stróżecki M, Kalaji HM, Łuców D, Lamentowicz M, Juszczak R (2019a) Impact of warming and reduced precipitation on photosynthetic and remote sensing properties of peatland vegetation. Environ Exp Bot 160:71–80
Rastogi A, Zivcak M, Tripathi DK, Yadav S, Kalaji HM, Brestic M (2019b) Phytotoxic effect of silver nanoparticles in Triticum aestivum: improper regulation of photosystem I activity as the reason for oxidative damage in the chloroplast. Photosynthetica 57:209–216
Robson TM, Klem K, Urban O, Jansen MAK (2015) Re-interpreting plant morphological responses to UV-B radiation. Plant Cell Environ 38:856–866
Santos I, Almeida JM, Salema R (1993) Plants of Zea mays L. developed under enhanced UV-B radiation. I. Some ultra structural and biochemical aspects. J Plant Physiol 141:450–456
Santos AL, Gomes NCM, Henriques I, Almeida A, Correia A, Cunha  (2012) Contribution of reactive oxygen species to UV-B-induced damage in bacteria. J Photochem Photobiol B 117:40–46
Shabrangi A, Majd A (2009) Comparing effects of electromagnetic fields (60 hz) on seed germination and seedling development in monocotyledon and dicotyledons. In: Progress in electromagnetic research symposium proceedings. Moscow, Russia, 18–21 Aug 2009
Sharma S, Guruprasad KN (2012) Enhancement of root growth and nitrogen fixation in Trigonella by UV-exclusion from solar radiation. Plant Physiol Biochem 61:97–102
Shine M, Guruprasad KN (2012) Impact of pre-sowing magnetic field exposure of seeds to stationary magnetic field on growth, reactive oxygen species and photosynthesis of maize under field conditions. Acta Physiol Plant 34:255–265
Shine MB, Guruprasad KN, Anand A (2011) Enhancement of germination, growth and photosynthesis in soybean by pretreatment of seeds with magnetic field. Bioelectromagnetics 32:474–484
Singh P (2012) Impact of exclusion of solar UV components on photosynthesis, nitrogen fixation and growth of soybean. Ph.D thesis, School of Life Sciences, Devi Ahilya University, Indore, India
Singh S, Kumari R, Agrawal M, Agrawal SB (2009) Modification of growth and yield responses of Amaranthus tricolor L. to sUV-B under varying mineral nutrient supply. Sci Hortic 120:173–180
Stange BC, Rowland RE, Rapley BI, Podd JV (2002) ELF magnetic fields increase amino acid uptake into Vicia faba L. roots and alter ion movement across the plasma membrane. Bioelectromagnetics 23:347–354
Strasser RJ, Srivastava A, Govindjee (1995) Polyphasic chlorophyll a fluorescence transients in plants and cyanobacteria. Photochem Photobiol 61:32–42
Takeuchi A, Yamaguchi T, Hidema J, Strid A, Kumagai T (2002) Changes in synthesis and degradation of Rubisco and LHC II with leaf age in rice (Oryza sativa L.) growing under supplementary UV-B radiation. Plant Cell Environ 25:695–706
Tossi V, Amenta MN, Lamattina L, Cassia R (2011) Nitric oxide enhances plant ultraviolet-B protection up-regulating gene expression of the phenylpropanoid biosynthetic pathway. Plant Cell Environ 34:909–921
Webb JM, Levy HB, Glick D (1958) Methods in biochemical analysis. Interscience, New York
Xu C, Natarajan S, Sullivan JH (2008) Impact of solar ultraviolet-B radiation onthe antioxidant defense system in soybean lines differing in flavonoids content. Environ Exp Bot 63:39–48
Yinan L, Yuan L, Yongquing Y, Chunyang L (2005) Effect of seed pre-treatment by magnetic field on the sensitivity of cucumber (Cucumis sativus) seedlings to ultraviolet-B radiation. Environ Exp Bot 54:286–294
Zhou B, Guo Z, Xing J, Huang B (2005) Nitric oxide is involved in abscisic acid-induced antioxidant activities in Stylosanthes guianensis. J Exp Bot 56:3223–3228
Acknowledgements
This research work was carried out with the financial support of Department of Science and Technology, New Delhi, Women Scientists Scheme-A (DST/SR/WOS-A/LS-17/2017).
Author information
Authors and Affiliations
Contributions
SK conceived, designed and performed all the research experiments, and analyzed the data; AB helped in some experiments; SK wrote the manuscript; SK, AR, and MJ read, corrected and approved the manuscript in its final form.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kataria, S., Rastogi, A., Bele, A. et al. Role of nitric oxide and reactive oxygen species in static magnetic field pre-treatment induced tolerance to ambient UV-B stress in soybean. Physiol Mol Biol Plants 26, 931–945 (2020). https://doi.org/10.1007/s12298-020-00802-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-020-00802-5