Abstract
Nitrogen (N) is the basis of plant growth and development and, is considered as one of the priming agents to elevate a range of stresses. Plants use solar radiations through photosynthesis, which amasses the assimilatory components of crop yield to meet the global demand for food. Nitrogen is the main regulator in the allocation of photosynthetic apparatus which changes of the photosynthesis (Pn) and quantum yield (Fv/Fm) of the plant. In the present study, dynamics of the photosynthetic establishment, N-dependent relation with chlorophyll fluorescence attributes and Rubisco efficacy was evaluated in low-N tolerant (cv. CR Dhan 311) and low-N sensitive (cv. Rasi) rice cultivars under low-N and optimum-N conditions. There was a decrease in the stored leaf N under low-N condition, resulting in the decreased Pn and Fv/Fm efficiency of the plants through depletion in the activity and content of Rubisco. The Pn and Fv/Fm followed the parallel trend of leaf N content during low-N condition along with depletion of intercellular CO2 concentration and overall conductance under low-N condition. Photosynthetic saturation curve cleared abrupt decrease of effective quantum yield in the low-N sensitive rice cultivar than the low-N tolerant rice. Also, the rapid light curve highlighted the unacclimated regulation of photochemical and non-photochemical quenching in the low-N condition. The low-N sensitive rice cultivar triumphed non-photochemical quenching, whereas the low-N tolerant rice cultivar rose gradually during the light curve. Our study suggested that the quantum yield is the key limitation for photosynthesis in low-N condition. Regulation of Rubisco, photochemical and non-photochemical quenching may help plants to grow under low-N level.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rice (Oryza sativa L.) is the staple food for more than half of the global population. Nitrogen is the limiting factor for plants growth and development. It functions as an imperative compound to synthesize amino acids, nucleic acids, and proteins and also contributes majorly in photosynthetic pigments such as chlorophylls. Due to unavailability of soil N, plants overall performance decreases because of rapid depletion of N at some stage after application in paddy fields. Insufficient availability of any nutrient element especially low N induces changes in physiological processes including low photosynthetic CO2 assimilation in leaves and decrease in low light saturation photosynthetic rate (Terashima and Evans 1988; Rascher et al. 2000) and leaf N content (Lawlor et al. 1987). The N insufficiencies also affect photosynthetic pigments (Xiaochuang et al. 2017) and proteins like Rubisco, not only its content but also its activity (Kumagaia et al. 2014). Rubisco functions for inorganic carbon utilization by carboxylation affinity and becomes a control key to limiting photosynthesis (Carmo-Silva et al. 2015), meanwhile, the photosynthesis is the only way for food generation in plants.
The commonly functioning attributes with photosynthesis like stomatal conductance (gs) and inter-cellular CO2 concentration (Ci) of the photosynthetic active cells play important role to regulate net photosynthesis of plant during growth and development. The N-starvation causes depletion of the attributes of the photosynthetic process (Jin et al. 2015; Liu et al. 2018), and is considered as N-based photo-pool of this process. Additionally, inadequate N supply strongly impacts the photosynthetic structure and functions, including the PSII (Kalaji et al. 2014). Reduced N level has a detrimental effect on the photosynthetic rate, leading to lower crop yield because of dysfunction of PSII (Zhao et al. 2017) that is possible by quantum yield depletion.
Chlorophyll fluorescence represents a powerful tool for monitoring the photochemical efficiency of leaves and is considered as reliable, non-intrusive and in vivo tool for phenotyping physiological phenomena, interfered with photosynthetic apparatus and its associated metabolism (Humplik et al. 2015; Cen et al. 2017). The maximum PSII efficiency and effective photochemical efficiency justifies the photosynthesis status of the plant under applied conditions. The low-N condition has a negative effect on chlorophyll fluorescence efficiency of the plants (Li et al. 2018; Zhou et al. 2018). Along with this, the electron transport rate is slowed down under N-starvation (Li et al. 2018). Photosynthetic saturation and rapid light curves are helpful to understand the fluorescence performance of light harvesting complexes and the photosynthetic ability of the plants in real-time under the applied environment (Zhao et al. 2017). Photochemical and non-photochemical quenching factors are always in fluctuation under different stress conditions and become estimate characters for photosynthesis efficiency (Ware et al. 2014; Kromdijk et al. 2016). Leaf N content shows a close relationship with the gas exchange and chlorophyll fluorescence efficiencies, and their depletion with a decrease in N application (Bernacchi et al. 2002). On the other hand, Rubisco efficiency has a similar report as that of the former one (Nunes et al. 1993; Jin et al. 2015).
At the agricultural field, N is applied through nitrogenous fertilizers. Efficacy to use applied N by rice in relation to other cereal crops is low (Fan et al. 2011). Plants utilize only 30% of applied N. The unused N results in a deleterious effect on the environment and human health. It not only leads to eutrophication through nitrate leaching but also contributes to the global warming effect. It has been reported that nitrous oxide, which is emitted from the agricultural field, has 298 times more global warming effect than CO2 (Baggs et al. 2000). There is an accepted agreement among plant breeders and ecologist to experience benefits of N application mainly for crop yield against its consequences and to reduce N utilization without loss of yields. Rice cultivars show different genetics characteristics with respect to available N (Sun et al. 2014). The present study was conducted to investigate the limiting factors of the photosynthetic efficiency and its effects on photosynthetic parameters of low-N sensitive and low-N tolerant rice cultivars under low-N conditions. This study clarifies the relationship between photosynthetic regulative and responsive attributes in two contrasting N-responsive rice cultivars under low-N stress. The photosynthetic regulative attributes are leaf N content, chlorophyll content, Rubisco protein content and its expression, which regulates the photosynthetic rate in the plants. The photosynthetic responsive attributes are gas exchange and chlorophyll fluorescence by which photosynthetic efficiency of the plants can be estimated at applied conditions. These findings may be helpful to understand the photosynthetic regulation and further development in the photosynthetic process to sustain rice production under low N application.
Materials and methods
Materials and experimental design
The experiment was conducted in the field of the Faculty of Agricultural Sciences at Aligarh Muslim University (Utter Pradesh, India) using low-N sensitive (cv. Rasi) and low-N tolerant (cv. CR Dhan 311) rice cultivars. These two cultivars were selected after screening ten rice cultivars on the basis of their responses to varied levels of N supply. The response was measured in terms of various physio-morphological, biochemical and N-utilization efficiency parameters (Supplementary Table 1). For screening, seeds of the ten rice cultivars were procured from the Division of Agronomy, Indian Agricultural Research Institute, New Delhi (India) and grown hydroponically in 1/4th strength of Yoshida nutrient solution of three N-concentrations (1 mM, 4 mM and 10 mM) in growth chamber under controlled environmental conditions (14:10 h light/dark, relative humidity of 60%, 22/28 °C and a photosynthetic photon flux density of 430 μ mol m−2 s−1). Twenty-five-day-old plantlets were used for screening. Two contrasting cultivars were selected, in which one has shown tolerance towards low-N whereas the other one was sensitive towards low-N level. Rice cultivars, CR Dhan 311 and Rasi were identified as low-N tolerant and low-N sensitive, respectively.
These two rice cultivars were grown in the experimental field to investigate the dynamics of the photosynthetic establishment, N-dependent relationship with chlorophyll fluorescence attributes under low-N and optimum-N conditions. The experimental field (latitude 27.9135° N, 78.0782° E) had sandy loam soil, pH 6.78, 14/10 h light/night, 70% ± 3 relative humidity, average temperature of 30/23 °C ± 2 °C (day/night) and photosynthetically active radiation (PAR) of 450–1000 μ mol m−2 s−1. Before sowing, the seeds were treated with 0.1% mercuric chloride, followed by 75% alcohol for surface sterilization. The seeds were then thoroughly rinsed with mineral free water 4–5 times. Sterilized seeds were pre-soaked for overnight in distilled water and then transferred at next morning into seedbeds of the field which was already prepared. The seeds were uniformly dispersed underneath of 5 cm irrigated water. 25-day-old germinated seedlings were transplanted in the main field. The recommended dose of N for these cultivars was 120 kg h−1. Two levels of N (60 kg h−1 and 120 kg h−1) were applied in the form of urea. The N was supplied in two splits, 50% as basal dose and rest 50% at growing tiller stage. The field was flood-irrigated during the vegetative phase. The gas exchange and chlorophyll fluorescence analysis were done at 45-day-old plants in five replications.
Chlorophyll measurement
Chlorophyll content of the plants was measured by Soil and Plant Analyzer Development (SPAD) chlorophyll meter (SPAD 502 DL PLUS, Spectrum Technologies, USA) in the early morning hours and represented in SPAD values.
Measurement of photosynthetic gas exchange
Fully expanded third leaf of each treatment was selected for this analysis by using InfraRed Gas Analyzer (CID-340, Photosynthesis system, Bio-Science, USA). Net photosynthetic rate (Pn), inter-cellular CO2 concentration (Ci), stomatal conductance (gs) and transpiration rate (Tr) were measured in the sunny day between 10:00 a.m. and 11:00 a.m. at clear saturating light intensity. The conditions of the field at the time of measurement: light intensity ≈ 800 μ mol m−2 s−1; relative humidity ≈ 70%; air temperature ≈ 28 °C and CO2 concentration ≈ 380 μ mol mol−1.
Chlorophyll fluorescence measurements
In vivo PSII activity was measured by pulse amplitude modulation (Mini-PAM) chlorophyll fluorometer (Heinz Walz, Effeltrich, Germany) and using WinControl-3.29 software. Saturation pulse (SP) mode was used for chlorophyll fluorescence measurement. PSII reaction centers were allowed to complete oxidation by giving approximately 30 min of the dark period before all measurements of samples using dark-adaptation leaf clips. Transmutable light (440 nm) were induced to dark-adapted samples just to prior to the SP to determine the minimum fluorescence yield (Fo) and after first SP the maximum fluorescence yield (Fm) were induced. The fluorescence parameters–F [momentary fluorescence level], Fm’ [illuminated maximum fluorescence level], Y(II) [effective photochemical quantum yield], Fo’ [illuminated minimum fluorescence level], ETR-F [relative electron transfer rate-factor], qN [coefficient of non-photochemical fluorescence quenching], qP and qL [coefficient of photochemical fluorescence quenching], NPQ [non-photochemical fluorescence quenching], Y(NO) [quantum yield of non-regulated fluorescence emission], Y(NPQ) [quantum yield non-photochemical fluorescence quenching] and Fv/Fm [maximum photochemical quantum yield] were automatic generated with the help of WinControl-3 through saturation pulse in dark-acclimated and light-exposed state of the sample.
Rapid light curve measurements
The SP mode analyses were used for measurement of rapid light curve (RLC) starting with the light intensity below that of the natural light. Each interval of RLC was about 20 s and Fo and Fm were determined in eight intervals without a dark-acclimation period. The RLC was completed in 2.67 min and regulate at 440 nm wavelength during the curve. The generated data were used to assess the acclimation state of photosynthesis.
Leaf nitrogen content
The leaf N content (LNC) percentage was measured by CHNS analyzer (EuroVector 3000, Italy) based on GC system simultaneous determination. The instrument was calibrated and prepared standard curve by using certified standards. The dry samples were fine powdered and 0.5 to 1.5 mg were used for the analyzing reaction and completed within 10 min after loading to combustion reactor (Jimenez and Ladha, 1993).
Rubisco activity and protein content in leaves
Using the enzyme extract assay (Usuda 1985) to determine Rubisco enzyme activity with the help of a microplate reader (Biotek Instruments Inc., USA) at 340 nm wavelength. Fresh leaf tissue (1 g) were homogenized with extraction Tris–HCl buffer (0.25 M and pH 7.8) contained 2% DTT, 0.0025 M EDTA and 0.05 M MgCl2. Homogenate was centrifuged at 10,000 × g at 4 °C for 10 min. the supernatant was subjected with Tris–HCl (100 M and pH 8.0) reaction mixture of 40 mM NaHCO3, 10 mM MgCl2, 5 mM DTT, 0.2 mM EDTA, 4 mM ATP, 0.2 mM NADH, 0.2 mM ribulose 1,5-bisphosphate, 1 U of 3-phosphoglycerate kinase and 1 U of glyceraldehyde 3-phosphodehydrogenase.
Carvalho et al. (2005) method was adopted for Rubisco protein extraction by homogenizing fresh leaf in potassium phosphate extraction buffer (0.2 M, pH 8.0) containing: 50% (w/w) polyvinylpyrrolidone, 5 mM DTT, 5 mM EDTA and 0.2 mM PMSF. The homogenate was centrifuged at 27,000 × g at 4 °C for 10 min and the supernatant was desalted with the help of Sephadex PD10 columns Amersham Biosciences. The desalted soluble proteins (10 μg) were loaded uniformly onto 12.5% SDS-PAGE of all the samples. The density of the protein bands was compared by ImageJ software with control bands on the gel. The LSU was marked in SDS-PAGE on the basis of molecular weight. Using previous studies the molecular weight of the LSU band (large sub-unit of Rubisco) is mentioned (Wang and Kolattukudy 1996; Bharti et al. 2014).
Data processing
The statistical analyses were done by SPSS (17.0) software and excel (2010). For comparing the difference among different data groups and one-way analysis of variance (one-way ANOVA) and Least significant difference (LSD) tests were conducted, and significance among differences was considered if P ≥ 0.05. The graphs and correlations (Pearson’s correlation method) were employed by SigmaPlot 12.0 and MINITAB 14.0.
Results
Plant growth response
Plant growth decreased more in Rasi than CR Dhan 311 in low-N stress condition and also the leaf colour changed from green to yellowish, as shown in Fig. 1.
Effect of low N-stress on chlorophyll content and LNC
The SPAD values depicted high deviation in the chlorophyll concentration under low-N conditions in both the cultivars. There was 30.36% and 21.34% decrease in the SPAD value of the low-N sensitive cultivar (cv. Rasi) and low-N tolerant cultivar (cv. CR Dhan 311) when compared with optimum-N condition (Fig. 2a). The same trend was attained by LNC under low N-stress conditions. The result showed that there was 45.24% decrease in LNC of cv. Rasi and 41.67% decrease in LNC of cv. CR Dhan 311 at the low-N level, compared with optimum-N level (Fig. 2b).
Effect of low N-stress on gas exchange parameters
The gas exchange parameters construe the photosynthetic response of the plant. The Pn decreased significantly under low-N conditions in both the cultivars, when compared with the optimum-N condition. However, the decrease in Pn was lesser in cv. CR Dhan 311 (12.91%) than cv. Rasi (18.64%) at low-N level. The gs vary from 0.285% to 0.332% among treatments and cultivars, but there was a higher decrease in low N-sensitive cultivar (cv. Rasi) with 11.77% loss of function efficiency under low-N level than optimum-N level. The regular and depletive trend has been attained by Ci parameter of rice cultivars under low-N conditions. The Ci decreased by 9.88% in cv. CR Dhan 311 and 10.68% in cv. Rasi under low-N conditions. Transpiration rate (Tr) was increased under low N-stress in both cultivars, but higher negative deviation was found in low N-sensitive cultivar with 23.53% uplift in low-N treatment when compared with optimum-N treated plants (Fig. 3).
Gas exchange parameters performance of 45 days old plants including under N-optimum and stress conditions. The mean values (n = 5) and standard deviations, with statistically significant differences indicated by students T-test at *P < 0.05, **P < 0.01 and ***P < 0.001 (Pn: net photosynthesis; gs: stomatal conductance; Ci: inter-cellular CO2 concentration and Tr: transpiration rate)
Chlorophyll fluorescence response under low N-stress
The measurement of photosystem-II chlorophyll fluorescence of rice cultivars after N treatments (optimum-N and low-N) are given in Table 1. The Fv/Fm and Y (II) was decreased significantly in both low N-tolerant and low N-sensitive cultivars. The Fv/Fm decreased from 3.86% to 5.39% and Y (II) from 5.69% to 7.31% by low-N treatment, compared to optimum-N treatment. The inversed qP and NPQ results were also obtained. The qP was decreased by 5.99% and 18.63%, whereas the NPQ was increased by 13.63% and 27.13% in cv. CR Dhan 311 and cv. Rasi respectively under low-N condition when compared with the optimum-N condition. The dependent parameter of chlorophyll fluorescence i.e., ETR was also decreased from 201.3 to 165.4 µ mol m−2 s−1 in cv. CR Dhan 311 and 189.6 to 134.7 µ mol m−2 s−1 in cv. Rasi under low-N condition, when compared to the optimum-N condition.
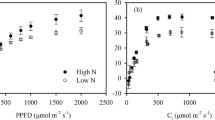
The photosynthesis saturation was measured under optimum-N and low-N conditions of all treatments. The photosynthesis saturation was leisurely deprived at each interval under optimum-N conditions in both the cultivars. At low-N conditions, the tolerant cultivar (cv. CR Dhan311) maintained the photosynthetic saturation curve towards the last flash of the light. Whereas the sensitive cultivar (cv. Rasi) showed abrupt photosynthetic saturation after the first flash of the light and severely decreased at each light interval. Accordingly, the Y (II) was also decreased in all treatments at each interval of light but, cv. Rasi deprived highly under low-N conditions during the experiment.
Rapid light curve response under low N-stress
The rapid light curve (RLC) helps in understanding the acclimation state of photosynthesis in rice plant (Fig. 4). In our experiment, the momentary fluorescence level (Fm’) gradually decreased during the light curve in all treatments. But there was a rapid decrease of Fm’ under low-N conditions in N-sensitive cultivar (cv. Rasi) than tolerant one (cv. CR Dhan 311) when compared with optimum-N conditions. There was a little deviation of qP curve at low-N conditions in cv. CR Dhan 311 when compared with qP curve obtained at optimum-N conditions but, qP curve decreased rapidly in cv. Rasi under low-N conditions when compared with qP curve obtained at optimum-N conditions. Moreover, the NPQ increased slowly at both optimum and low-N conditions in the tolerant cultivar (cv. CR Dhan 311) whereas there was an abrupt increase in NPQ curve under low N-stress conditions in cv. Rasi when compared with NPQ curve obtained at optimum-N conditions during RLC. The qP have shown direct proportionality with quantum yield efficiency whereas the NPQ is inversely proportioned to the quantum yield efficiency. The LRC data clearly showed that there is a short photosynthetic acclimation state in cv. Rasi than cv. CR Dhan 311 under low-N conditions.
Rapid light curves of rice cultivars under Low N-stress and optimum-N conditions, represented as (a) cv. CR Dhan 311 at optimum-N; (b) cv. CR Dhan at low-N; (c) cv. Rasi at optimum-N and (d) cv. Rasi at low-N. The small graph plots of each treatment represent the associated momentary fluorescence characters of corresponding rapid light curves
Effect of low N-stress on Rubisco activity
The Rubisco activity was decreased severely under low-N conditions in both rice cultivars, heralds overall photosynthetic parameters. The low-N sensitive cultivar depicted the higher degree of Rubisco activity function loss (30.78%) than the low-N tolerant cultivar (25.28%) under low-N conditions when compared with optimum-N condition (Fig. 5).
The SDS-PAGE of leaf protein depicted that the Rubisco protein content was also decreased with low-N conditions in both the rice cultivars (Fig. 6). The intensity/density of the protein bands showed depletion in the large subunit of Rubisco protein strength. The cv. Rasi (low N-sensitive cultivar) showed 1.5 folds decrease, whereas cv. CR Dhan 311 (low N-tolerant cultivar) showed a 0.5 fold decrease of protein band strength. The small subunit of Rubisco portrayed slightly greater (2-folds) decrease under low-N conditions in cv. Rasi when compared with optimum-N conditions.
Discussion
Growth, development, and regulation are directly dependent on macro- and micro-nutrients for proper photosynthetic and physiological maintenance of the plants (Zhao et al. 2017; Hou et al. 2018). Overall plant growth was decreased under N-stress and the effect was more prominent in Rasi cultivar than CR Dhan 311 cultivar. As, we have found in experimental results that the low-N conditions caused negative impact on photosynthetic and physiological performances and impact was more in Rasi rice cultivar. The main reason was decreased LNC in both cultivars under stress conditions due to unavailability of N in soil. The N limitation became the key factor for dysfunctional growth, as it is the main component of structural and functional substrates of the plants. Leaf organs show yellow-symptom due to its low mobility under low-N conditions. The plant chlorophyll pigment content decreased under low-N cultivation (Xiaochuang et al. 2017; Zhao et al. 2017). Similar results were depicted by SPAD values in our experiment. The working principle of photosynthetic reaction centers is totally dependent on these pigments for light harvesting (Kursar and Alberte 1983). The photosynthetic efficiency is also related to chlorophyll concentration (Yokoya et al. 2007). Depletion of photosynthetic activity may be due to disapprove of pigment contents under low-N conditions in plants.
Photosynthetic and leaf growth proteins are synthesized with the help of stored leaf N buffer pool (Liu et al. 2018). Almost 80% of the stored N is used in photosynthetic responses and out of which Rubisco protein contains 27% of LNC (Makino et al. 2003). Due to the high investment of N in Rubisco, plants may limit in photosynthetic efficiency with the decrease of Rubisco content under low-N conditions, as results found in our experiment. Not only Rubisco content but Rubisco activase content has also been decreased, which may decline the photosynthetic rate (Yousuf et al. 2017). Our results also depict the decline of Rubisco activity during low-N conditions and, its confirmation was already done by other researchers in their findings (Kumagaia et al. 2014; Yousuf et al. 2017).
Gas exchange parameters declined under low-N conditions in both cultivars of rice. The net photosynthesis rate was more affected in low N-sensitive cultivar than tolerant one because of low-N response. Previous studies have also suggested that low photosynthetic rate in N-starvation cultivated rice, oilseed rape and corn (Jin et al. 2015; Hou et al. 2018; Liu et al. 2018). In past, researchers have found decreased carbon stimulation and partially increased respiration in low LNC (Sakai et al. 2006; Hou et al. 2018), which may be the possible reason for declination of Ci and raise in Tr, as depicted in our results. The stomatal conductance ebbed highly in cv. CR Dhan 311 than cv. Rasi under low-N conditions decreased the availability of internal photosynthetic CO2 and would become one of the reasons for depleted Pn in plants. The same situation was also found with other stress conditions studied previously, N (Kumagaia et al. 2014); phosphate (Li et al. 2018) and water stress (Cao et al. 2018).
Chlorophyll fluorescence measurement is a powerful tool to analyze the photosynthetic response of the plants under abiotic stress. The activity of reaction centers and the photochemical efficiency of PSII was decreased and in reverse, there is raise in photo-inhibition of PSII, under low-N conditions (Jin et al. 2015; Zhao et al. 2017). Our result emphasizes the de-escalation of Fv/Fm and Y (II) under low N-application, but the Y (II) was highly affected in cv. Rasi. At the same point, qP and NPQ showed dramatic behaviour under low-N conditions, as the qP decreased with low-N treatment but on another hand, there was an alarm of NPQ raise in both cultivars when compared with optimum-N treatment, but the deviation was more significant in cv. Rasi than cv. CR Dhan 311. The decreased qP dissipate low excessive light energy and released in the form of heat (Maxwell and Johnson 2000), and may lack photosynthetic inhibition efficiency. Electron transport slow-down with the increase of NPQ of PSII also caused a negative effect on photorespiration and carbon fixation (Zhao et al. 2017). In our results, ETR was greatly decreased with low-N conditions in both cultivars, but the low N-sensitive cultivar showed a higher negative deviation at low-N condition than optimum-N application. This decline of photochemical efficiency was observed in other plants under stress conditions by other researchers also (Li et al. 2018; Zhong et al. 2018; Zhou et al. 2018).
The photosynthesis saturation curve (PSC) represented the gradual decrease of Y (II) in low N-tolerant cultivar whereas, there was abrupt depletion in low N-sensitive cultivar and, this may be due to low—chlorophyll content and Rubisco efficacy under low-N conditions. The inimical height of NPQ under low-N conditions may be another reason for depleted Y (II) and curve explained the loss of photosynthetic efficiency in relation with leaf LNC, qP and NPQ criterion in rice plants. Previous investigations have also highlighted the declination of Y (II) under abiotic stress conditions in other plants, low-N conditions in wheat (Živcak et al. 2014); high temperature in tomato (Pan et al. 2018); salt stress in maize (Chen et al. 2018). Our results also displayed short period of photosynthetic saturation in cv. Rasi under low-N conditions than cv. CR Dhan 311 (Fig. 7), which demystify that there was short photo-dilation of PSII in low N-sensitive cultivar.
The results of rapid light curve (RLC) demonstrated that Fm’ lowered steeply with time under optimum-N but, fall straight and fast under low-N conditions in cv. Rasi gives an explanation of low photon efficiency of PSII photochemistry under low-N conditions. Previous studies demonstrated that importance of photon efficiency of PSII for quantum yield efficiency (Tremblay et al. 2012) and loss of this efficiency is deleterious for plant photosynthetic rate. Our result exhibited slow provoke in light-dependent criterion like qP, qN, and qL, which hints towards the low photo-inhibition efficiency of the plant under low-N conditions but, on the other, the NPQ increased highly only after the first flash of light and attained high peak towards the end of the RLC (Fig. 6). NPQ processes mostly dissipated unadjusted light energy which demystifies the in-efficiency of PSII reaction centers (Juneau et al. 2005). It was suggested in previous findings through RLCs that PSII light responses were deeply swayed by low-N conditions and indicating high susceptibility of N-starved cells to photoinhibition (Zhao et al. 2017).
The correlation interactions between LNC, Rubisco activity, and gas exchange measurements were all significant (P < 0.05), but the interaction between LNC and Tr was negatively (r = − 0.9777) significant (Table 2). There was a highly significant relationship between LNC and Ci (r = 0.8931), which corresponds to parallel depletion of both attributes in low-N conditions cultivation. But when LNC correlated with Rubisco activity, Pn, gs, and Ci all showed a strong positive correlation (r ranges from 0.991 to 0.993), which show their level of reliability during N-application for growth (Bernacchi et al. 2002; Liu et al. 2018).
The correlation interactions between LNC, Rubisco activity and chlorophyll fluorescence measurements (Table 3) demonstrated a strong relationship between LNC and Fv/Fm (r = 0.991); LNC and Y (II) (r = 0.992) than LNC and ETR (r = 0.9667), which elucidate the coordinative behavior of LNC and quantum yield to hold physiology of plant under N-applications (Nunes et al. 1993; Jin et al. 2015; Yang et al. 2016). The qP and NPQ were not significantly correlated either with LNC nor Rubisco activity, could be used for the better estimate of fluorescence efficiency under N-regimes to elucidate the quantum efficiency of plants.
Conclusions
The results of this study showed that the net photosynthesis of the plant is dependent on the LNC, which is the main reason for depletion of photosynthetic pigments (SPAD values) and Rubisco efficiency, either Rubisco protein content or its activity. All the photosynthetic differentia loss their ability in low-N condition crop fields. Also, the PSII have negatively affected by low-N conditions due decrease in quantum yield which evaluates low electron transport flow during light reaction of photosynthesis. The escalated NPQ is the indirect confirmation of low photosynthetic active radiation consumption during light harvesting reaction of photosynthesis under low-N conditions. The significant correlation coefficients existed between LNC and Pn was 0.981 and, between Rubisco activity and Fv/Fm was 0.995, which enables us to localize the impact of low-N conditions on photosynthesis of the rice plant. These findings will help in understanding and development of photosynthetic regulation under low-N applications.
Abbreviations
- cv.:
-
Cultivar
- Ci :
-
Inter-cellular concentration of CO2
- ETR:
-
Electron transfer rate
- Fv/Fm :
-
Maximum photochemical quantum yield
- LNC:
-
Leaf nitrogen content
- NPQ:
-
Non-photochemical fluorescence quenching
- PAR:
-
Photosynthetically active radiation
- Pn :
-
Net photosynthesis
- PSC:
-
Photosynthesis saturation curve
- PSII:
-
Photosystem II
- qP:
-
Photochemical fluorescence quenching
- RLC:
-
Rapid light curve
- Rubisco:
-
Ribulose bisphosphate carboxylase/oxygenase
- SP:
-
Saturation pulse
- Y(II):
-
Effective photochemical quantum yield
References
Baggs E, Rees R, Smith K, Vinten A (2000) Nitrous oxide emission from soils after incorporating crop residues. Soil Use Manag 16:82–87. https://doi.org/10.1111/j.1475-2743.2000.tb00179.x
Bernacchi CJ, Portis AR, Nakano H, von Caemmerer S, Long SP (2002) Temperature response of mesophyll conductance. Implications for the determination of Rubisco enzyme kinetics and for limitations to photosynthesis in vivo. Plant Physiol 130:1992–1998. https://doi.org/10.1104/pp.008250
Bharti RK, Srivastava S, Thakur IS (2014) Proteomic analysis of carbon concentrating chemolithotrophic bacteria Serratia sp. for sequestration of carbon dioxide. PloS One 9(3):e91300. https://doi.org/10.1371/journal.pone.0091300
Cao X, Zhu C, Zhong C, Hussain S, Zhu L, Wu L, Jin Q (2018) Mixed-nitrogen nutrition-mediated enhancement of drought tolerance of rice seedlings associated with photosynthesis, hormone balance and carbohydrate partitioning. Plant Growth Regul 84:451–465. https://doi.org/10.1007/s10725-017-0352-6
Carmo-Silva E, Scales JC, Madgwick PJ, Parry MA (2015) Optimizing Rubisco and its regulation for greater resource use efficiency. Plant Cell Environ 38(9):1817–1832
Carvalho L, Esquível M, Martins I, Ricardo CP, Amâncio S (2005) Monitoring the stability of Rubisco in micro propagated grapevine (Vitis vinifera L.) by two-dimensional electrophoresis. J Plant Physiol 162:365–374. https://doi.org/10.1016/j.jplph.2004.09.013
Cen H, Weng H, Yao J, He M, Lv J, Hua S, Li H, He Y (2017) Chlorophyll fluorescence imaging uncovers photosynthetic fingerprint of Citrus huanglongbing. Front Plant Sci 8:1509. https://doi.org/10.3389/fpls.2017.01509
Chen YE, Mao JJ, Sun LQ, Huang B, Ding CB, Gu Y, Liao JQ, Hu C, Zhang ZW, Yuan S (2018) Exogenous melatonin enhances salt stress tolerance in maize seedlings by improving antioxidant and photosynthetic capacity. Physiol Plant 164:349–363. https://doi.org/10.1111/ppl.12737
Fan M, Shen J, Yuan L, Jiang R, Chen X, Davies WJ, Zhang F (2011) Improving crop productivity and resource use efficiency to ensure food security and environmental quality in China. J Exp Bot 63:13–24. https://doi.org/10.1093/jxb/err248
Hou W, Yan J, Jákli B, Lu J, Ren T, Cong R, Li X (2018) Synergistic effects of nitrogen and potassium on quantitative limitations to photosynthesis in rice (Oryza sativa L.). J Agric Food Chem 66:5125–5132. https://doi.org/10.1021/acs.jafc.8b01135
Humplík JF, Lazár D, Fürst T, Husičková A, Hýbl M, Spíchal L (2015) Automated integrative high-throughput phenotyping of plant shoots: a case study of the cold-tolerance of pea (Pisum sativum L.). Plant Methods 11:20. https://doi.org/10.1186/s13007-015-0063-9
Jimenez RR, Ladha JK (1993) Automated elemental analysis: a rapid and reliable but expensive measurement of total carbon and nitrogen in plant and soil samples. Commun Soil Sci Plant Anal 24:1897–1924. https://doi.org/10.1080/00103629309368926
Jin X, Yang G, Tan C, Zhao C (2015) Effects of nitrogen stress on the photosynthetic CO2 assimilation, chlorophyll fluorescence, and sugar-nitrogen ratio in corn. Sci Rep 5:9311. https://doi.org/10.1038/srep09311
Juneau P, Green B, Harrison P (2005) Simulation of Pulse-Amplitude-Modulated (PAM) fluorescence: limitations of some PAM-parameters in studying environmental stress effects. Photosynthetica 43:75–83. https://doi.org/10.1007/s11099-005-5083-7
Kalaji HM, Oukarroum A, Alexandrov V, Kouzmanova M, Brestic M, Zivcak M, Samborska IA, Cetner MD, Allakhverdiev SI, Goltsev V (2014) Identification of nutrient deficiency in maize and tomato plants by in vivo chlorophyll a fluorescence measurements. Plant Physiol Biochem 81:16–25. https://doi.org/10.1016/j.plaphy.2014.03.029
Kromdijk J, Głowacka K, Leonelli L, Gabilly ST, Iwai M, Niyogi KK, Long SP (2016) Improving photosynthesis and crop productivity by accelerating recovery from photoprotection. Science 354(6314):857–861. https://doi.org/10.1126/science.aai8878
Kumagai E, Hamaoka N, Araki T, Ueno O (2014) Dorsoventral asymmetry of photosynthesis and photoinhibition in flag leaves of two rice cultivars that differ in nitrogen response and leaf angle. Physiol Plant 151:533–543. https://doi.org/10.1111/ppl.12145
Kursar TA, Alberte RS (1983) Photosynthetic unit organization in a red alga: relationships between light-harvesting pigments and reaction centers. Plant Physiol 72:409–414. https://doi.org/10.1104/pp.72.2.409
Lawlor D, Boyle F, Young A, Keys A, Kendall A (1987) Nitrate nutrition and temperature effects on wheat: photosynthesis and photorespiration of leaves. J Exp Bot 38:393–408. https://doi.org/10.1093/jxb/38.3.393
Li P, Weng J, Zhang Q, Yu L, Yao Q, Chang L, Niu Q (2018) Physiological and biochemical responses of Cucumis melo L. chloroplasts to low-phosphate stress. Front Plant Sci 9:1525. https://doi.org/10.3389/fpls.2018.01525
Liu T, Ren T, White PJ, Cong R, Lu J (2018) Storage nitrogen co-ordinates leaf expansion and photosynthetic capacity in winter oilseed rape. J Exp Bot 69:2995–3007. https://doi.org/10.1093/jxb/ery134
Makino A, Sakuma H, Sudo E, Mae T (2003) Differences between maize and rice in N-use efficiency for photosynthesis and protein allocation. Plant Cell Physiol 44:952–956. https://doi.org/10.1093/pcp/pcg113
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence -a practical guide. J Exp Bot 51:659–668. https://doi.org/10.1093/jexbot/51.345.659
Nunes MA, Ramalho JC, Dias MA (1993) Effect of nitrogen supply on the photosynthetic performance of leaves from coffee plants exposed to bright light. J Exp Bot 44:893–899. https://doi.org/10.1093/jxb/44.5.893
Pan C, Ahammed GJ, Li X, Shi K (2018) Elevated CO2 improves photosynthesis under high temperature by attenuating the functional limitations to energy fluxes, electron transport and redox homeostasis in tomato leaves. Front Plant Sci 9:1739. https://doi.org/10.3389/fpls.2018.01739
Rascher U, Liebig M, Lüttge U (2000) Evaluation of instant light-response curves of chlorophyll fluorescence parameters obtained with a portable chlorophyll fluorometer on site in the field. Plant Cell Environ 23:1397–1405. https://doi.org/10.1046/j.13653040.2000.00650.x
Sakai H, Hasegawa T, Kobayashi K (2006) Enhancement of rice canopy carbon gain by elevated CO2 is sensitive to growth stage and leaf nitrogen concentration. N Phytol 170:321–332. https://doi.org/10.1111/j.1469-8137.2006.01688.x
Sun H, Qian Q, Wu K, Luo J, Wang S, Zhang C, Ma Y, Huang Liu Q, Yuan XQ (2014) Heterotrimeric G proteins regulate nitrogen-use efficiency in rice. Nat Genet 46:652. https://doi.org/10.1038/ng.2958
Terashima I, Evans JR (1988) Effects of light and nitrogen nutrition on the organization of the photosynthetic apparatus in spinach. Plant Cell Physiol 29:143–155. https://doi.org/10.1093/oxfordjournals.pcp.a077461
Tremblay N, Wang Z, Cerovic ZG (2012) Sensing crop nitrogen status with fluorescence indicators. A review. Agron Sustain Dev 32:451–464. https://doi.org/10.1007/s13593-011-0041-1
Usuda H (1985) The activation state of ribulose 1, 5-bisphosphate carboxylase in maize leaves in dark and light. Plant Cell Physiol 26:1455–1463. https://doi.org/10.1093/oxfordjournals.pcp.a077047
Wang X, Kolattukudy PE (1996) Isolation of a protein containing covalently linked large and small subunits of ribulose-1, 5-bisphosphate carboxylase/oxygenase from Botryococcus braunii. Plant Physiol 111(2):441–445. https://doi.org/10.1104/pp.111.2.441
Ware MA, Belgio E, Ruban AV (2014) Comparison of the protective effectiveness of NPQ in Arabidopsis plants deficient in PsbS protein and zeaxanthin. J Exp Bot 66(5):1259–1270. https://doi.org/10.1093/jxb/eru477
Xiaochuang C, Chu Z, Lianfeng Z, Junhua Z, Hussain S, Lianghuan W, Qianyu J (2017) Glycine increases cold tolerance in rice via the regulation of N uptake, physiological characteristics, and photosynthesis. Plant Physiol Biochem 112:251–260
Yang J, Gong W, Shi S, Du L, Sun J, Song S, Chen B, Zhang Z (2016) Analyzing the performance of fluorescence parameters in the monitoring of leaf nitrogen content of paddy rice. Sci Rep 6:28787. https://doi.org/10.1038/srep28787
Yokoya NS, Necchi O, Martins AP, Gonzalez SF, Plastino EM (2007) Growth responses and photosynthetic characteristics of wild and phycoerythrin-deficient strains of Hypnea musciformis (Rhodophyta). J Appl Phycol 19:197–205. https://doi.org/10.1007/s10811-006-9124-9
Yousuf PY, Abdallah EF, Nauman M, Asif A, Hashem A, Alqarawi AA, Ahmad A (2017) Responsive proteins in wheat cultivars with contrasting nitrogen efficiencies under the combined stress of high temperature and low nitrogen. Genes 8:356. https://doi.org/10.3390/genes8120356
Zhao LS, Li K, Wang QM, Song XY, Su HN, Xie BB, Zhang XY, Huang F, Chen XL, Zhou BC (2017) Nitrogen starvation impacts the photosynthetic performance of Porphyridium cruentum as revealed by chlorophyll fluorescence. Sci Rep 7:8542. https://doi.org/10.1038/s41598-017-08428-6
Zhong C, Cao X, Bai Z, Zhang J, Zhu L, Huang J, Jin Q (2018) Nitrogen metabolism correlates with the acclimation of photosynthesis to short-term water stress in rice (Oryza sativa L.). Plant Physiol Biochem 125:52–62. https://doi.org/10.1016/j.plaphy.2018.01.024
Zhou X, Sun C, Zhu P, Liu F (2018) Effects of antimony stress on photosynthesis and growth of Acorus calamus. Front Plant Sci 9:579. https://doi.org/10.3389/fpls.2018.00579
Živcak M, Olsovska K, Slamka P, Galambosova J, Rataj V, Shao HB, Kalaji HM, Brestic M (2014) Measurements of chlorophyll fluorescence in different leaf positions may detect nitrogen deficiency in wheat. Zemdirbyste 101:437–444. https://doi.org/10.13080/z-a.2014.101.056
Funding
This work was supported by research Grants from DBT-NEWS-India-UK (BT/IN/UK-VNC/44/NR/2015-16).
Author information
Authors and Affiliations
Contributions
A. Ahmad conception and design of the study, acquisition of data, analysis, and interpretation of data, drafting the article and final approval of the submitted version. The author takes responsibility for the integrity of the article as a whole. aahmad.bo@amu.ac.in. A.Y. Tantray conception and design of the study, acquisition of data, interpretation of data, drafting the article, final approval. S.S. Bashir conception of the study, acquisition of data, analysis, and interpretation of data, final approval.
Corresponding author
Ethics declarations
Conflict of interest
All authors have declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tantray, A.Y., Bashir, S.S. & Ahmad, A. Low nitrogen stress regulates chlorophyll fluorescence in coordination with photosynthesis and Rubisco efficiency of rice. Physiol Mol Biol Plants 26, 83–94 (2020). https://doi.org/10.1007/s12298-019-00721-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-019-00721-0