Abstract
The present study provides the first comparative fatty acid profiling of the three Indian seabuckthorn species, collected from varying altitudes (2900–4300 masl) of Trans-Himalayas (Hippophae rhamnoides, H. tibetana) and Sikkim Himalayas (H. salicifolia) regions. Gas chromatography–mass spectrometry analysis showed variability in fatty acid composition of different seabuckthorn populations. Sikkim populations showed higher (1.28–1.6 folds) palmitic acid than Trans-Himalayan populations which possess higher linoleic (1.3–1.5 folds) and linolenic (1.6–1.8 folds) acids. Interestingly, a strong altitudinal gradient associated positive correlation was observed with the degree of unsaturation and PUFA content while negative correlation was observed with saturated fatty acids content of different seabuckthorn populations. H. salicifolia collected from Sikkim showed healthy ω-6:ω-3 ratio (closer to 1:1) of functional lipids exhibiting its better nutraceutical potential than other commonly used seed oils. Interestingly, H. tibetana from Losar showed higher (5.81) degree of unsaturation than Sikkim populations (3.5) suggesting its better stress tolerance trait. Chemo-taxonomic diversity analysis also formed two broad clusters of Trans-Himalayan and Sikkim populations which correlated with earlier taxonomic studies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hippophae (seabuckthorn) a cold hardy shrub is native to Europe and Asia. The plant is well adapted to grow in harsh environments such as drought, salinity and extreme temperatures. In India, genus Hippophae comprises of Hippophae rhamnoides, H. salicifolia and H. tibetana, which grows naturally in high-altitude (2391–4300 masl) Himalayas (Raina et al. 2011). H. rhamnoides has gained wide attention as a functional food with high pharmacological value, owing to the presence of > 200 bioactive compounds (Suryakumar and Gupta 2011; Zielinska and Nowak 2017). Several studies highlighting its health benefits are available but the remaining two species H. salicifolia and H. tibetana remain relatively unexplored and underutilized.
Seabuckthorn fruit, pulp, and seed oils are extensively used in food, health care, medicinal and cosmetic industries (Zielinska and Nowak 2017), yet studies pertaining to lipids and fatty acids are limited for Indian seabuckthorn (Fatima et al. 2013; Ding et al. 2018). In general, H. rhamnoides pulp is a source of higher palmitoleic (32–53%) and palmitic (25–35%) acid while seeds possess higher linoleic (30–40%) and α-linolenic (20–35%) acids (Zheng et al. 2017). Interestingly, H. rhamnoides is a rich source of essential fatty acids and antioxidants (tocopherols, tocotrienols, phytosterols, polyphenols, flavonoids, carotenoids and organic acids). Further, it is also known to prevent oxidative stress associated diseases like diabetes, rheumatoid arthritis, ulcerative colitis, cancer, cardiovascular, Crohn’s and autoimmune diseases (Zheng et al. 2017; Shukla et al. 2017) suggesting that seabuckthorn could be beneficial in promoting human health.
Seabuckthorn being a non-cultivated plant, exhibit higher variability even amongst naturally growing population (Dolkar et al. 2017a). However, due to its high nutritional value and role in soil and water conservation, efforts have been made for large scale cultivation of seabuckthorn (Stobdan et al. 2017). Although, several studies are available on the nutraceutical relevance and stress tolerance (Gupta and Deswal 2012) of H. rhamnoides but studies on H. tibetana and H. salicifolia remains relatively less explored. Moreover, most of these studies are restricted to region-specific analysis of different populations. To the best of our knowledge, broader comparison of Trans-Himalayan (H. rhamnoides and H. tibetana), and Sikkim (H. salicifolia) populations is not available till date. Lack of species specific comparative information on elite germplasm with better nutraceutical and stress tolerance poses a major hindrance in seabuckthorn large-scale cultivation and breeding programmes (Dolkar et al. 2017a; Saikia and Handique 2013).
Plant membranes is the first organelle to sense environmental variations (temperature, salinity, drought and pathogen attack) which affect the fluidity, permeability and stretching of membrane due to lipid remodelling (Zheng et al. 2014). In plants growing in alpine and desert ecosystems, maintenance of membrane fluidity is of particular biological significance as these are exposed to frequent environmental fluctuations throughout the day (Zheng et al. 2014; Barrero-Sicilia et al. 2017). Alterations in membrane lipid composition along with increased fatty acid unsaturation contributes to acclimation ability. Further, effort is also made to know if fatty acid methyl esters (FAMEs) analysis could help in analyzing the biochemical and phenotypic diversity of seabuckthorn and could serve as a chemotaxonomic marker (Choudhary Kumar et al. 2017).
Being a stress tolerant plant, seabuckthorn may serve as a system to study resilience of Himalayan flora to extreme environmental conditions. Moreover, bioactive constituents vary depending on the species, cultivar, harvesting time of fruits, agro-climatic conditions and geographical locations (Yang and Kallio 2001). Interestingly, a recent ecophysiolomic study using naturally growing H. rhamnoides has shown that variations in climatic factors may alter proteins, metabolites and fatty acids. The results also indicated the multifactorial stress acclimation strategies of seabuckthorn in response to diverse environmental conditions (Sharma and Deswal 2019).
Therefore, the objective of the present study was to provide a comparative analysis of fatty acid composition in Indian seabuckthorn particularly Trans Himalayan (H. rhamnoides and H. tibetana) and Sikkim Himalayan (H. salicifolia) populations growing at different altitudes. Analysis of essential fatty acids would help in identification of elite populations with higher nutraceutical relevance or better stress tolerance for large scale cultivation and breeding in future.
Materials and methods
Plant material
Berries of all the three Indian seabuckthorn species from major seabuckthorn growing areas in Trans-Himalayan (H. rhamnoides and H. tibetana) and Sikkim (H. salicifolia) Himalayan regions of India were collected. H. rhamnoides orange (HrS1) and yellow (HrS2) colored ecotypes were collected from Shey (34°04′ N 77°37′ E; 3200 m asl) and Thiksey (34°31′ N 78°16′ E; 3530 m asl) respectively from Leh, Jammu & Kashmir, India. H. rhamnoides (HrS3, 31°22′ N 77°07′ E; 3300 m asl) and H. tibetana (HtS, 32°22′ N 78°08′ E; 4300 m asl) were collected from Kaza and Losar respectively, Lahaul and Spiti valley, Himachal Pradesh, India. Different ecotypes of H. salicifolia (HsS1 and HsS2) were collected from Lachen (HsS1, 27°43′ N 88°30′ E; 2391 m asl) and Lachung (HsS2, 27°41′ N 88°44′ E; 3111 m asl) valley, North Sikkim. Geographical locations and altitudes of all the natural populations were determined using a GPS device (eTrex Vista® HCx, Taiwan) (Fig. 1).
As per the phenological data reported previously, the tissue was collected at the same developmental stage i.e. mature berries in the month of September (Trans-Himalayan populations) and November (Sikkim populations) from morphologically comparable natural populations. Seabuckthorn not being cultivated shows a lot of variations. Therefore, to minimize the variations even in a phenotypically similar population growing at same geographical location, blocking of the area was done in three different parts using randomized block design. Samples (berries) were collected randomly from individuals (n = 50) distributed evenly at each site and pooled. These pooled samples collected from three different parts of the area were considered as biological replicates. Further, berries were washed, frozen in liquid nitrogen and stored at − 80 °C. For FAMEs analysis intact seeds were separated manually from these berries.
GC–MS for fatty acid methyl ester analysis (FAMEs)
Fatty acid methyl esters (FAMEs) were obtained from lipids using acid-catalyzed transesterification procedure as described previously with minor modifications (Choudhary Kumar et al. 2017). Seeds (10–15) were grounded to powder, transferred to Teflon lined screw-capped glass tube and incubated in 2% (w/v) HCl in methanol (1 mL) for 1 h at 80 °C. After cooling, FAMEs were extracted by addition of 0.9% NaCl and hexane (2 mL), followed by centrifugation to allow phase separation. Hexane layer was pooled, dried under nitrogen gas and resuspended in (100 μl) hexane. FAMEs were determined by gas chromatography-mass spectrometry (GC–MS), using an Agilent Technologies GC–MS (5977A MSD coupled with 7890B GC series) equipped with a DB-wax capillary column (30 m × 0.25 mm × 0.25 mm; Agilent Technologies). Initial oven temperature was 50 °C, increased to 230 °C at 3 °C min−1. The carrier gas used was nitrogen with a flow rate of 1.8 mL min−1 and injection temperature was 230 °C. Injection volume was kept at 1 μl with a split ratio of 18:1. FAMEs were identified through comparison of mass spectral data with the NIST 11 library. FA composition was expressed as the percentage (%) of total fatty acids.
Statistical analysis
FA composition data were analysed and data from triplicates is expressed as mean ± standard deviation. One-way ANOVA using the Tukey post hoc range test was performed to find significant statistical differences amongst the samples where p < 0.05 were considered significantly different (SPSS, STATS 21). Principle component analysis (PCA) was performed to study the relationship amongst different Hippophae species on the basis of their seed fatty acid composition. Heat maps were used to represent the fold changes (log10 transform) in FAs and agglomerative hierarchical clustering (AHC) using euclidean distance measure was performed to evaluate overall multivariate phylogenetic relationship amongst different seabuckthorn species. PCA, AHC and heatmaps were generated using XLSTAT 2018 (New York) software.
Results and discussion
Seabuckthorn is a stress tolerant shrub with immense medicinal and nutraceutical properties. Seabuckthorn seed oil is rich in essential fatty acids (linoleic, ω-6 and α-linolenic, ω-3) (Fatima et al. 2013). Despite a plentiful source of nutraceuticals, currently the area under seabuckthorn cultivation is limited which needs to be expanded. Naturally growing seabuckthorn populations face extreme environmental conditions and are exposed to multiple abiotic stress. To survive these extreme conditions, it exhibits multifactorial stress adaptation including the alterations in proteins, metabolites and fatty acids (Sharma and Deswal 2019).
Remodeling of membrane lipids, the essential components of cellular membranes via regulation of unsaturation is an interesting acclimation strategy adapted by plants. Alpine and desert plants show significant variations in lipid species during stress conditions thus, indicating their specific roles in signaling cascades and defense (Zheng et al. 2014; Barrero-Sicilia et al. 2017).
Most of the studies on Indian seabuckthorn are focused on H. rhamnoides (Dolkar et al. 2017a) till date, whereas H. tibetana and H. salicifolia populations remains relatively less explored (Ranjith et al. 2006). Thus, comparative study of different seabuckthorn species may provide insights into their differential stress tolerance or nutraceutical relevance.
Alterations in fatty acid profiles of seabuckthorn populations collected from H.P, Leh and Sikkim
It is well established that variable environmental variations (nutrient availability, salinity, moisture, rainfall, humidity, and daylight) and geographical locations (latitude, longitude, altitude) may contribute to alterations in the FA content for maintenance of appropriate membrane fluidity (Johansson et al. 2000). In the present investigation, H. tibetana (4300 masl), H. rhamnoides (3200–3530 masl), and H. salicifolia (2391, 3111 masl) berries were collected from three diverse geographical locations and agro-climatic zones across Indian Himalayas. Therefore, the prime interest of present study was to provide a broader comparison of fatty acid composition in three seabuckthorn species. Further, we also wanted to investigate the altitudinal gradient associated variations in fatty acid composition of these populations.
Distribution, altitude and geographical location of seabuckthorn species is detailed in Fig. 1. Mature seeds from berries of H. rhamnoides (HrS1 and HrS2, Leh); H. rhamnoides (HrS3, HP), and H. tibetana (HtS, H.P), and H. salicifolia (HsS1 and HsS2, Sikkim) were used for Gas chromatography-mass spectrometry (GC–MS) analysis. GC chromatogram of seabuckthorn seeds showed total eight major FAs including three saturated [Palmitic acid (16:0), Stearic acid (18:0), Eicosanoic acid (20:0)], three monounsaturated [Palmitoleic acid (16:1), Oleic acid (18:1Δ9), Cis-vaccenic acid (18:1Δ11)] and two polyunsaturated [Linoleic acid (18:2), α-Linolenic acid (18:3)] (Fig. S1).
Biochemical profiling of FA composition revealed significant variability within the seabuckthorn species collected from diverse geographical locations or agro-climatic conditions (Fig. 2a, b and Table 1). In the present investigation, linoleic acid (30–46%), α-linolenic acid (18–32%), palmitic (11–19%), and oleic (10–19%) acids were observed as major FA while minor FA included palmitoleic (1.4–6%), stearic (2–3.6%), cis-vaccenic (2.6–6%) and eicosanoic acid (0.5–0.85%) (Fig. 2). To the best of our knowledge, the present investigation is the first comparative study of Trans-Himalayan and Sikkim Himalayan seabuckthorn (seeds) FA and also the first report on H. tibetana seed FA profile. Trans-Himalayan H. rhamnoides populations showed comparable FA composition with the global H. rhamnoides populations containing major fatty acids palmitic (7–13%), palmitoleic (< 4%), oleic (< 4%), linoleic (33–43%) and α-linolenic acid (28–39%) in the defined range (Fig. 3).
Comparative FAMEs analysis showing the a fatty acid (FA) composition and b the ratio of saturated (SFA), monounsaturated (MUFA) and polyunsaturated (PUFA) fatty acids in seeds of different seabuckthorn species collected from Trans-Himalayan and Sikkim populations. Data correspond to averages ± SD of 3 replicates per sample. Mean values marked with different letters in each column are significantly different (p < 0.05)
Comparative analysis showing the variations in fatty acid composition of Global and Indian seabuckthorn seed populations. Samples shown in dotted box (red) shows the Indian Trans-Himalayan (H. rhamnoides, H. tibetana) and Sikkim Himalayan (H. salicifolia) seed populations analyzed in the present study. FAMEs data for global and other Indian germplasm was obtained from literature studies as mentioned in the text (color figure online)
Interestingly, comparative analysis showed higher palmitic (1.15–2 folds) and linoleic (1.06–1.8 folds) acid in Indian (Trans-Himalayan) H. rhamnoides than global populations [Romanian (Dulf 2012), German (Burcová et al. 2017) and Finnish (Yang and Kallio 2001) and Canadian (Fatima et al. 2013)] (Fig. 3). Moreover, the Trans-Himalayan populations growing at higher altitudes also contained higher linoleic (1.3–1.5 folds) and α-linolenic (1.5–1.8 folds) than Sikkim populations (Table 2). On the contrary, higher linoleic (1.76 folds), α-linolenic (1.5 folds) acids, PUFA (61–66%) and lower palmitoleic (fourfolds) and oleic (1.8–2.6 folds) content was observed for Trans-Himalayan H. rhamnoides (3200 and 3530 masl) than H. rhamnoides seeds from Tabo, Lahaul-Spiti, HP (3165 masl) thus supporting the altitude and geographical locations dependent variations in FA profile (Fig. 3).
Interestingly, H. salicifolia (HS1 and HS2) populations collected from Sikkim Himalayas showed higher content of saturated fatty acids including palmitic (1.28–6 folds) and palmitoleic (2.75–7.6 folds) acid than Trans-Himalayan populations (Table 2). Further, H. salicifolia from Sikkim also showed higher essential fatty acid content [palmitic (1.18 folds), palmitoleic (2.2 folds), stearic (2.36 folds), linoleic (1.25 folds) and linolenic (1.5 folds)] than reported previously for H. salicifolia collected from H.P (Kaushal and Sharma 2011) suggesting altitudinal gradient associated variations in FA composition (Fig. 3).
Similar quantitative variations were observed in Oliva cuspidate seeds [oleic (69.3–74.5%), linoleic (11.2–15.2%), palmitic (11.2–14.0%), linolenic (1.3–3.20%), palmitoleic (1.31–2.10%) stearic (0.1–0.2%), and linolenic acid (11.2–15.2%)] collected from different geographical locations in Pakistan (Gulfraz et al. 2009). Likewise, variations (14–17%) in total FAs including lauric, myristic, oleic and linoleic acids are reported in 44 different species of Cuphea due to adaptive radiation, speciation, and habitats (Graham et al. 2016). These observations indicate that both Trans and Sikkim Himalayan populations showed marked variations in their respective FA metabolic signatures which suggested altitudinal gradient associated differential acclimation responses.
Degree of unsaturation in seabuckthorn populations may contribute to differential stress adaptation
Increase in unsaturated fatty acids is an acclimation strategy known as homeoviscous acclimation, in response to deviations from optimal temperature to maintain appropriate membrane fluidity (Sakamoto and Murata 2002). Literature studies have shown higher unsaturation index (ratio of unsaturated and saturated fatty acid) to be positively correlated with the better stress tolerance. Therefore, comparative analysis of saturated (SFA), monounsaturated (MUFA) and polyunsaturated FA (PUFA) contents was also performed to find whether similar correlation exists between the fatty acid composition and differential stress tolerance in different seabuckthorn populations (Table 1).
Minimum temperature during the seed fill and maturation periods during soybean seed development is considered as major factor affecting FA composition, particularly relative amounts of saturated/unsaturated FA, due to stimulatory effect of lower temperatures on desaturases gene expression (Hou et al. 2006). The study also showed that different geographical locations with varying altitudes accounted for higher variability in PUFA (linoleic and linolenic) content than SFAs like palmitic, stearic, oleic acid (Hou et al. 2006).
Higher PUFA (1.2–1.4 folds) content was observed in Trans-Himalayan populations (HtS) growing at higher altitudes (4300 masl) than Sikkim (2391–3111 masl) populations. Higher PUFA may help in mitigating environmental stress-induced damage due to harsher climatic conditions prevalent at higher altitudes. On the contrary, higher (1.15–1.47 folds) saturated FA was observed in H. salicifolia from Sikkim growing at lower altitude than Trans-Himalayan populations (Fig. 2b, Table 1).
Changes in degree of unsaturation (ratio of UFA vs. SFA), of membrane lipids affect membrane fluidity with temperature fluctuations. Cold acclimation increases the degree of unsaturation. Multiple studies in Arabidopsis have shown that defects in desaturases display inhibited growth or even death at non-freezing low temperatures of 6–12 °C (Falcone et al. 2004). Similarly, over-expression of chloroplastic Cucurbita maxima and A. thaliana glycerol-3-phosphate acyltransferase (GPAT) gene involved in desaturation led to increase in UFAs with corresponding decrease in chilling sensitivity of genetically engineered tobacco (Upchurch 2008).
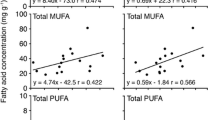
In the present study, unsaturation index correlated with altitudinal gradient where higher unsaturation was observed in Trans-Himalayan populations [HtS (5.81) > HrS3 (5.1) = HrS1 (5) > HrS2 (4.35)] than Sikkim [HsS1 (3.6) = HsS2 (3.5)] populations suggesting stress adaptive changes in membranes (Fig. 2b, Table 1). Further, linear regression (statistical) analysis was performed to strengthen the association between altitudinal gradients and the fatty acid composition of seabuckthorn populations. Interestingly, a strong altitudinal gradient associated correlation was observed with the unsaturation index (strong, R2 = 0.8707, p < 0.05), PUFA content (moderate, R2 = 0.77, p < 0.05) and unsaturated (strong, R2 = 0.96, p < 0.01) fatty acids content of different seabuckthorn populations. Similar, low temperature induced increase (15%) was also observed in degree of unsaturation due to PUFA accumulation in Cicer arietinum (Bakht et al. 2006). Likewise, cold acclimated potato (Solanum commersonii) showed higher (twofolds) linoleic acid in membrane glycerolipids of leaves, than non-acclimated potato (Solanum tuberosum) during cold stress (Upchurch 2008).
Interestingly, the above observations are in accordance with previous studies where altitudinal gradient associated variations in phenotypic (decrease in leaf size and area, increase in leaf thickness) and metabolic signatures (increase in chlorophyll and proline content) were observed in Trans-Himalayan H. rhamnoides populations (Dolkar et al. 2017b, 2019). These variations are considered as adaptive responses contributing to better stress tolerance to high altitudes plants growing in harsher environmental conditions.
Exploring the nutraceutical potential (functional lipids) of different seabuckthorn populations
Present-day hectic lifestyle and high levels of stress affects health negatively. Deficiency of essential fatty acids (EFAs) crucial for maintenance of good human health may occur during stress, due to inhibition of enzymes involved in formation of long chain PUFAs. EFAs (linoleic and α-linolenic acid), can only be supplied by diet as humans (due to lack of desaturase enzymes) are unable to synthesize these. Linoleic acid is precursor for functionally essential longer-chain ω-3, (eicosapentaenoic acid EPA and docosahexaenoic acid), ω-6 (Gamma linoleic acid) fatty acids and eicosanoids (prostaglandins, thromboxanes, and leukotrienes) biosynthesis in the human body (Alabdulkarim et al. 2012). Today’s diet is low in EFA while rich in unwanted saturated and trans fatty acids showing ω-6:ω-3 FAs ratio (1:10 to 1:50), higher than the recommended (1:1 to 4:1) ranges (Alabdulkarim et al. 2012).
Fish oils are considered abundant (60–70%) source of EFAs but growing concerns regarding accumulation of environmental pollutants in fish oil and sustainability of marine fish stock demands other alternative sources. Plant seeds (linseed, flaxseed) rich in ω-3 (40–50%) FAs may replace and offer a sustainable source of these lipids (Fatima et al. 2013). With the exception of flaxseed oil (> 50% α-linolenic acid), higher levels of ω-3 FAs are uncommon. Moreover, most of the seed oils have an undesirable ω-6:ω-3 ratio.
Seabuckthorn seed oils are considered excellent sources of PUFAs (48–69%), and EFAs due to unique (ω-6:ω-3 ratio close to 1:1) composition of linoleic (30–46%) and α-linolenic acids (18–32%) (Fatima et al. 2013). Proportions of PUFA from seeds obtained in this study were similar to those reported peviously for Finnish Hippophae ssp. sinensis and rhamnoides (Yang and Kallio 2001). Interestingly, both the seabuckthorn populations possess a healthy ω-6:ω-3 ratio of fatty acids in the range 1.19–2.56. These ratios lie within the approved USFDA range (closer to 1:1) suggesting better nutraceutical potential than commonly used seed oils including flaxseed oil (4:1), hemp oil (1:3), sunflower (0:65), safflower (0:75), corn (0:59), olive oil (0:8), Canola (1:4) and soybean (1:7) (Erasmus 1993).
Based on the unique composition of ω-6:ω-3 values closest to 1:1, Sikkim populations [HsS1 (1.19) and HsS2 (1.69)] exhibited better nutraceutical potential than other commonly used seed oils. Interestingly, Trans-Himalayan population particularly, HtS seeds also exhibited lower ω-6:ω-3 (1.68) ratio with higher degree of unsaturation (UFA:SFA ratio, 5.81) thus, representing its better nutraceutical value and better stress tolerance (Table 1).
Multivariate analysis for chemotaxonomic studies
In order to further understand the correlation between FA variables and different seabuckthorn populations, principal component analysis (PCA) was performed using eight FAs as variables. First two principal components (PC1, PC2) in the PCA model explaining 89.04% of the total data variance (Fig. 4a), were chosen on the basis of their eigenvalues (> 1, 5.436 and 1.687 respectively) (Fig. S2) indicating that these reflect majority of the information. Contribution of PC1 was higher (67.94%) than PC2 (21.09%) (Fig. 4a). Pearson correlation data matrix of FA variables for bi-plot demonstrated the correlation (p < 0.05) between each FA variable and seabuckthorn species (Fig. 4a, Table 2). PC1 elucidated high positive loading factor for C16:0 (0.891), C16:1 (0.934), C18:1△11 (0.966) and C20:0 (0.982) (Fig. 4a, Table S1). Hierarchical clustering analysis (HCA) revealed that HsS Lachen and HsS Lachung grouped together on the basis of higher abundances of C16:0, C16:1, C18:1△11 and C20:0 FAs. However, HrS HP and HrSO Leh exhibited closer correlations owing to presence of higher C18:0, C18:1△9 and C18:2 (Figs. 4a, S3A). Fatty acid clustering performed using heat-maps supported PCA analysis and demarcated the variations in FA composition of seabuckthorn species (Fig. S3B).
Chemo-diversity represented by FA patterns using multivariate analysis. a Scatter biplots showing principal component analysis of seed fatty acids from different seabuckthorn populations. b Dendrogram showing agglomerative hierarchical clustering depicting the relatedness of different seabuckthorn populations based on their fatty acid profiles
Algorithmic hierarchical clustering (AHC) dendrogram generated using Euclidean distance and Ward’s method showed marked intra and interspecies biochemical diversity dividing populations into two major clusters (Fig. 4b). Based on the degree of relatedness, cluster I represent Trans-Himalayan populations including both H. rhamnoides (HP and Leh) along with H. tibetana. On the contrary, cluster II represents Sikkim populations containing both the ecotypes of H. salicifolia from Lachen and Lachung. However, cluster I was further divided into two subclusters, wherein subcluster I include H. rhamnoides from both HP and Leh, while subcluster II contained H. tibetana from H.P (Fig. 4b). Interestingly, chemotaxonomic analysis correlated well with classical taxonomic studies and genetic diversity analysis using molecular phylogenetics (Raina et al. 2011). These results suggested that even a smaller subset of population is also retaining/supporting the genomic information thus indicating that FAMEs analysis could serve as a chemotaxonomic marker for validation of phylogenetic classification.
Conclusions
-
GC–MS showed variations in fatty acid composition of different seabuckthorn populations. Higher palmitic (12.5–14.8%) and linoleic (38–46%) acid in Trans-Himalayan H. rhamnoides populations than Canadian (7 and 33–42%), Romanian (8–13% and 33–43%), German (7.7 and 37.3%) and Finnish (7.4 and 39%) populations respectively, suggest its better nutraceutical potential.
-
Interestingly, Sikkim (H. salicifolia) populations showed higher content of essential fatty acids (palmitic, palmitoleic, stearic, linoleic and linolenic) than reported previously for H. salicifolia from H.P. Unique composition (ω-6:ω-3 ratio closer to 1:1) of essential fatty acids suggested the higher nutritional value of seabuckthorn than other seed oils.
-
Linear regression analysis confirmed the association between altitudinal gradient and fatty acid composition. Interestingly, increase in altitude showed a strong positive correlation with the unsaturation index and the PUFA content.
-
Higher PUFA (13–21%) content and highest unsaturation index (5.81) of H. tibetana (Trans-Himalayan) than H. rhamnoides [H.P (4.3), Leh (5)] and H. salicifolia populations (3.85) suggested its better stress tolerance potential at higher altitude (4300 masl).
-
Further, multivariate PCA analysis showed a positive correlation between chemo-taxonomical analysis and earlier taxonomic studies suggesting that FA profiling may be used as a chemotaxonomic marker not only for analysis of geographic origin associated diversity but also for nutritional value assessment for crop selection in breeding programs.
References
Alabdulkarim B, Bakeet ZAN, Arzoo S (2012) Role of some functional lipids in preventing diseases and promoting health. J King Saud Univ Sci 24:319–329. https://doi.org/10.1016/j.jksus.2012.03.001
Bakht J, Bano A, Dominy P (2006) The role of abscisic acid and low temperature in chickpea (Cicer arietinum) cold tolerance. II. Effects on plasma membrane structure and function. J Exp Bot 57:3707–3715. https://doi.org/10.1093/jxb/erl120
Barrero-Sicilia C, Silvestre S, Haslam RP, Michaelson LV (2017) Lipid remodelling: unravelling the response to cold stress in Arabidopsis and its extremophile relative Eutrema salsugineum. Plant Sci 263:194–200. https://doi.org/10.1016/j.plantsci.2017.07.017
Burčová Z, Kreps F, Schmidt Š, Jablonský M (2017) Composition of fatty acids and tocopherols in peels, seeds and leaves of Sea buckthorn. Acta Chim Slovaca 10:29–34. https://doi.org/10.1515/acs-2017-0005
Choudhary Kumar A, Sunojkumar P, Mishra G (2017) Phytochemistry fatty acid profiling and multivariate analysis in the genus Leucas reveals its nutritional, pharmaceutical and chemotaxonomic significance. Phytochemistry 143:72–80. https://doi.org/10.1016/j.phytochem.2017.07.007
Ding J, Ruan C, Guan Y, Krishna P (2018) Identification of microRNAs involved in lipid biosynthesis and seed size in developing sea buckthorn seeds using high-throughput sequencing. Sci Rep. https://doi.org/10.1038/s41598-018-22464-w
Dolkar P, Dolkar D, Angmo S, Kumar B, Stobdan T (2017a) Variability in phenolics, flavonoids and antioxidants in Seabuckthorn (Hippophae rhamnoides L.) seed from nine trans-Himalayan natural population. J Berry Res 7:109–116. https://doi.org/10.3233/JBR-170149
Dolkar P, Dolkar D, Kant A, Chaurasia OP, Stobdan T (2017b) Gender-specific seasonal pattern and altitudinal variation in freeze tolerance response of Seabuckthorn (Hippophae rhamnoides L.). J Berry Res 7:291–297. https://doi.org/10.3233/JBR-170165
Dolkar P, Dolkar D, Kant A, Chaurasia OP, Stobdan T (2019) Gender differences in phenotypic and adaptive response of Seabuckthorn (Hippophae rhamnoides L.) along an altitudinal gradient in trans-Himalaya. J Berry Res 9:1–10. https://doi.org/10.3233/JBR-170294
Dulf FV (2012) Fatty acids in berry lipids of six sea buckthorn (Hippophae rhamnoides L., subspecies carpatica) cultivars grown in Romania. Chem Cent J 6:1–12
Erasmus U (1993) Fats that heal, fats that kill: the complete guide to fats, oils, cholesterol, and human health. In: Health & fitness. Summertown, Tennessee
Falcone DL, Ogas JP, Somerville CR (2004) Regulation of membrane fatty acid composition by temperature in composition. BMC Plant Biol 4:1–15. https://doi.org/10.1186/1471-2229-4-17
Fatima T, Snyder CL, Schroeder WR et al (2013) Fatty acid composition of developing sea buckthorn (Hippophae rhamnoides L.) berry and the transcriptome of the mature seed. PlosOne 7:e34099. https://doi.org/10.1371/journal.pone.0034099
Graham SA, Coelho GP, Murad AM et al (2016) Patterns of fatty acid composition in seed oils of Cuphea, with new records from Brazil and Mexico. Ind Crop Prod 87:379–391. https://doi.org/10.1016/j.indcrop.2016.04.008
Gulfraz M, Kasuar R, Arshad G, Mehmood S et al (2009) Isolation and characterization of edible oil from wild olive. Afr J Biotechnol 8:3734–3738
Gupta R, Deswal R (2012) Low temperature stress modulated secretome analysis and purification of antifreeze protein from Hippophae rhamnoides, a Himalayan wonder plant. J Proteome Res 11:2684–2696. https://doi.org/10.1021/pr200944z
Hou G, Ablett GR, Pauls KP, Rajcan I (2006) Environment effects on fatty acid levels in soybean oil. J Am Oil Chem Soc 83:759–763
Johansson A, Laine T, Linna MM, Kallio H (2000) Variability in oil content and fatty acid composition in wild northern currants. Eur Food Res Technol 211:277–283. https://doi.org/10.1007/s002170000151
Kaushal M, Sharma PC (2011) Nutritional and antimicrobial property of seabuckthorn (Hippophae sp.) seed oil. J Sci Ind Res (India) 70:1033–1036
Raina SN, Jain S, Sehgal D et al (2011) Diversity and relationships of multipurpose seabuckthorn (Hippophae L.) germplasm from the Indian Himalayas as assessed by AFLP and SAMPL markers. Genet Resour Crop Evol 59:1033–1053. https://doi.org/10.1007/s10722-011-9742-1
Ranjith A, Kumar KS, Venugopalan VV (2006) Fatty acids, tocols, and carotenoids in pulp oil of three sea buckthorn species (Hippophae rhamnoides, H. salicifolia, and H. tibetana). JAOCS 83:359–364
Saikia M, Handique PJ (2013) Antioxidant and antibacterial activity of leaf, bark, pulp and seed extracts of seabuckthorn (Hippophae salicifolia D. Don) of Sikkim Himalayas. J Med Plant Res 7:1330–1338. https://doi.org/10.5897/JMPR12.1123
Sakamoto T, Murata N (2002) Regulation of the desaturation of fatty acids and its role in tolerance to cold and salt stress. Curr Opin Microbiol 5:206–210. https://doi.org/10.1016/S1369-5274(02)00306-5
Sharma B, Deswal R (2019) Ecophysiolomic analysis of stress tolerant Himalayan shrub Hipppophae rhamnoides shows multifactorial acclimation strategies induced by diverse environmental conditions. Physiol Plant. https://doi.org/10.1111/ppl.12942
Shukla S, Hegde S, Kumar A, Chaudhary G (2017) Fatty acid composition and antibacterial potential of Cassia tora (leaves and stem) collected from different geographic areas of India. J Food Drug Anal 26:107–111. https://doi.org/10.1016/j.jfda.2016.12.010
Stobdan T, Dolkar P, Chaurasia OP, Kumar B (2017) Seabuckthorn (Hippophae rhamnoides L.) in trans-Himalayan. Def Life Sci J 2:46–53
Suryakumar G, Gupta A (2011) Medicinal and therapeutic potential of Sea buckthorn (Hippophae rhamnoides L.). J Ethnopharmacol 138:268–278. https://doi.org/10.1016/j.jep.2011.09.024
Upchurch RG (2008) Fatty acid unsaturation, mobilization, and regulation in the response of plants to stress. Biotechnol Lett 30:967–977. https://doi.org/10.1007/s10529-008-9639z
Yang B, Kallio HP (2001) Fatty acid composition of lipids in sea buckthorn (Hippophae rhamnoides L.) berries of different origins. J Agric Food Chem 49:1939–1947. https://doi.org/10.1021/jf001059s
Zheng G, Tian B, Zhang F et al (2014) Plant adaptation to frequent alterations between high and low temperatures: remodeling of membrane lipids and maintenance of unsaturation levels. Plant Cell Environ 34:1431–1442. https://doi.org/10.1111/j.1365-3040.2011.02341.x.Plant
Zheng L, Shi L, Zhao C et al (2017) Fatty acid, phytochemical, oxidative stability and in vitro antioxidant property of sea buckthorn (Hippophaë rhamnoides L.) oils extracted by supercritical and subcritical technologies. LWT Food Sci Technol. https://doi.org/10.1016/j.lwt.2017.08.042
Zielińska A, Nowak I (2017) Abundance of active ingredients in sea-buckthorn oil. Lipids Health Dis 16:1–11. https://doi.org/10.1186/s12944-017-0469-7
Acknowledgements
This work was supported by financial assistance from Department of Biotechnology (IBSD/A1/P(PH-2)/4), Government of India to RD. BS is thankful to UGC and DBT for providing fellowship. Authors are thankful to Dr. Girish Mishra for kindly extending GC–MS facility for FAMEs analysis and critical suggestions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sharma, B., Arora, S., Sahoo, D. et al. Comparative fatty acid profiling of Indian seabuckthorn showed altitudinal gradient dependent species-specific variations. Physiol Mol Biol Plants 26, 41–49 (2020). https://doi.org/10.1007/s12298-019-00720-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-019-00720-1