Abstract
Drought is considered one of the major constraints of plant growth and productivity worldwide. Plants respond to drought through different mechanisms including physiological, biochemical, and gene expression modulation. Studying these mechanisms will provide better understanding of drought response mechanisms and will help breeders in developing new cultivars. In this study, growth, biochemical, and molecular responses of four wheat (Triticum durum Desf.) landraces to drought stress (300 mM mannitol) were investigated at the seedling stage. Reverse transcription-polymerase chain reaction was used to assess gene expression level for a drought stress responsive gene (DHN15.1). Germination percentage, shoot length, root length, and root number for all T. durum landraces were decreased significantly under drought stress. However, drought stress caused an increase in proline content, lipid peroxidation level, and DHN15.1 transcript level. According to the studied traits, the Karak landrace showed long shoots (48% relative to its control), the longest roots (45% relative to its control) and the highest proline content (483% relative to its control). The results indicate that from the landraces studied, Karak may be selected as the most tolerant wheat landrace and may help in wheat breeding programs for adaptation to drought-prone environments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant growth is influenced by different factors including biotic and abiotic stresses. Abiotic stresses are a major limiting factor of plant growth and productivity. Salinity, water deficit, and extreme temperatures are examples of abiotic stresses (Xiong and Zhu 2001; Rizhsky et al. 2002; Mittler 2006). As a response to stress conditions, several signaling pathways are activated which convert the stress signals to a biochemical response as a result of modulation of stress responsive gene expression. In turn, this response helps the plant to acclimate to the current stress (Rizhsky et al. 2002; Rampino et al. 2012).
Wheat (Triticum spp.) is one of the most produced cereal crops in the world. The genus Triticum is a widely cultivated crop with a short growing season and plays an essential role in world trade (Vasil 2007; Food and Agriculture Organization of the United Nations (FAOSTAT) 2012).
A landrace is defined as a local variety of a plant species that has adapted to its natural environment over time (Acquaah 2007) and maintained by traditional farmers to meet their environmental, economic, and cultural requirements (Belay et al. 1995). Modern crop cultivars are often genetically similar, with a narrow genetic distance whereas landraces (which result from both natural and farmer selection (Belay et al. 1995)) have a broader genetic base and consequently are a valuable source for breeding programs (Zou and Yang 1995; AL-Shalabi 2015).
Landraces provide an opportunity to use an evolutionary approach to study adaptation and survival in arid and semi-arid environments (Brown 2000). Both natural and farmer selection has led to the emergence of genotypes with different combinations of traits, such as time to heading, time to maturity, drought, heat, and cold tolerance (Masood et al. 2005). Bread and durum wheat landraces have largely been replaced by monocultures of pure genotypes (Ehdaie and Waines 1989; Jaradat 2006). This has resulted in loss of genetic diversity for survival under biotic and abiotic stresses (Ali Deb et al. 1992).
The goal of developing new varieties from landraces is to improve landraces traits, such as high nutrient levels under stress conditions. Further, increasing demands on wheat products and changing climatic conditions necessitate enhancing yield potential, and more importantly tolerance to biotic and abiotic stresses (Ehdaie and Waines 1989; Koshgoftarmanesh et al. 2010).
The Mediterranean climate in Jordan is characterized by dry hot summers with regional variation in temperature and mild wet winters with extreme variability in annual rainfall (Black 2009). These climate conditions create abiotic stresses that affect plant growth and productivity (Rizhsky et al. 2002; Mittler 2006; AL-Shalabi 2015). Wheat is a major crop plant on which the effects of abiotic stress have been investigated at the morphological, phenotypical, and physiological levels in Jordan, but there are limited studies at molecular level (Jaradat 1991).
Water stress occurs as drought (water deficit) stress or flooding (overwatering) stress. However, drought is the main environmental factor that influences plant growth (Mahajan and Tuteja 2005; Fleury et al. 2010). Plants in warm regions are subjected to drought, since the rate of water loss through transpiration is higher than consumption rate by water uptake from the soil (Chaves et al. 2003). The effects of drought on plants differ from species to species, and from one developmental stage to another. The effects also depend on the duration of the drought stress (Bartels and Sourer 2004).
When plants are subjected to an unfavorable growth factor (stress), several signaling pathways are activated in order to convert the physical stress into a biochemical response and each signaling pathway promotes the expression of certain stress-responsive genes, such as dehydrin genes (DHN) under drought stress (Xiong and Zhu 2001). In plants, Dhn genes exist as multi-gene families. They act as stabilizers of membranes or proteins under unfavorable conditions. Rampino et al. (2006) found that DHN15.1 expression is enhanced in wheat after drought stress. Therefore, an effective way to increase stress tolerance is manipulation of the transcription of stress responsive genes (Hu et al. 2006).
The aim of the present study is to investigate the effect of drought stress on phenotypes and gene expression level in four durum wheat landraces collected from different regions in Jordan.
Materials and methods
Plant material, growth conditions and stress treatments
Wheat landraces (Triticum durum Desf.) used in this study were collected from four (Kofr yuba, Wasteyah, Ramtha and Karak) geographical and climatic regions in Jordan.
Seeds of the collected landraces were sterilized by immersing for 20 min in sterilization solution (10% sodium hypochlorite containing 0.3% (v/v) Tween 20) and rinsed three times with sterile distilled water under a laminar air flow hood. Seeds were allowed to germinate on filter paper in 9 cm diameter Petri dishes, moistened with distilled water (control) or 300 mM mannitol (test solution). Plates were incubated in the growth chamber at 23(±1) °C with a 16-h-light/8-h-dark photoperiod. The Petri dishes were arranged in completely randomized design (CRD) with four replicates for each treatment.
Germination percentages were recorded on the 4th day of planting. Seed showing radical extrusion by ≥ 2 mm long were considered to be germinated seed (Montaña et al. 2014). Growth parameters such as shoot length, root length, and root number for the seedlings were recorded. Relative germination (%) was calculated according to the equation proposed by Smith and Dobrenz (1987), as follows: number of germinated seeds in stress medium/number of germinated seeds in control medium × 100.
ISSR-PCR and gel electrophoresis
Genetic variation between landraces was assessed using 7 ISSR primers (Table 1). Genomic DNA was extracted from 7-day old seedlings using DNA extraction kit (Promega, Madison Wis, USA) following the manufacturer’s instructions. To check the quality of isolated DNA, 5 μl of DNA were mixed with 2.5 μl of bromophenol blue and loaded on a 1% agarose gel. The gel was run at 90 V for 60 min in 1× Tris Borate EDTA buffer (TBE). DNA quantity and quality was measured using NanoDrop (NanoDrop 2000, Thermo Scientific).
DNA amplification was carried out in a thermal cycler in a total volume of 25 μl containing 12.5 μl of master mix (1× Amplicon), 1.5 μl primer, 2 μl DNA, and 9 μl DNase free water. PCR amplification was performed with the following cycling profile: an initial denaturation at 94 °C for 5 min, followed by 45 cycles of 59 s at 94 °C, 59 s at 49 °C, and 1 min at 72 °C, with a final extension for 5 min at 72 °C.
Amplified products were detected by mixing 7 μl of PCR product with 3 μl of loading dye, and then loaded on a 1% agarose gel in 1× of TBE buffer at 90 V for 120 min. DNA fragments were visualized under UV light, and the intensities of bands were observed. Different banding patterns are associated with polymorphisms between the landraces.
Proline content assay
Proline content was measured according to the Bates et al. (1973) method. 0.5 g of plant material was homogenized in 10 ml of 3% aqueous sulfosalicylic acid and the homogenate was filtered. 2 ml of filtrate was transferred to a test tube containing 2 ml acid ninhydrin and 2 ml glacial acetic acid, then the mixture was incubated in boiling water for 1 h. To stop the reaction, test tubes were transferred to an ice bath. 4 ml of toluene was added and mixed vigorously for 15–20 s in order to extract the chromophore. The test tubes were then incubated at room temperature until the two phases separated. Afterwards, the chromophore, which contains toluene, was transferred to another test tube and measured at 520 nm. Proline concentration was determined by using a standard curve in the range of 20–100 µg/ml and it was calculated on a fresh weight basis as follows:
Lipid peroxidation content assay
Lipid peroxidation (LPO) level was measured in terms of malondialdehyde (MDA) content (a product of lipid peroxidation) according to Carmak and Horst’s (1991) method. 0.5 g of fresh tissue was homogenized in 10 ml of 0.1% trichloroacetic acid (TCA), and the resulting homogenate was centrifuged at 15,000g for 10 min. A 2 ml aliquot of the supernatant was mixed with 4 ml 20% TCA containing 0.5% thiobarbituric acid (TBA). The mixture was then transferred to a 95 °C water bath for 30 min and left to boil. Afterwards, the mixture was cooled in an ice bath, then centrifuged at 10,000g for 10 min. Finally, the supernatant was measured at a wavelength of 532 nm. The content of MDA was calculated using an extinction coefficient of 155/mM per cm.
RT-PCR analysis
The reverse transcription PCR (RT-PCR) technique was used to study the effect of drought stress on DHN15.1 transcript level. Shoots of 7-day old seedlings grown under control and drought stress (300 mM mannitol) conditions were used for RNA extraction and RT-PCR analysis. Total RNA was isolated from leaves using RNA extraction kit (RNeasy plant mini-kit, Qiagen) following the manufacturer’s instructions. The quantity of extracted RNA was measured using NanoDrop (NanoDrop 2000, Thermo Scientific). The quality of extracted RNA was checked by mixing 5 μl of RNA with 2.5 μl of bromophenol blue, and loading on a 1% agarose gel, which was run at 90 V for 60 min.
cDNA was synthesized using reverse transcriptase (Thermoscientific revertaid first strand cDNA synthesis). Sequence specific primers (F:ATGGAGTTCCAAGGGCAGCA, R:TCAGTGCTGTCCCGGCAG, transcript size: 450 bp) were used to amplify the cDNA of TdDHN15.1, which is the target gene. Α-tubulin was used as an internal control gene of T. durum, where specific primers (F: CATTACACCATTGGCAAGGA, R: AAGGCCAGAGCCAGTTC, transcript size: 575 bp) were used to amplify the cDNA. PCR products were separated on 1% agarose gel stained with ethedium bromide. Gel was run in 1× TBE buffer at 90 V for 120 min, and band intensities were measured using ImageJ Inc., version 2.0 software.
Statistical analysis
A completely randomized design with four replications was used in all experiments. Each experiment was repeated twice. Mean and standard error (SE) values were determined for each treatment. Data for each experiment was subjected to analysis of variance (ANOVA) using the Statistical Product and Service Solution (SPSS Inc., version 19, released 2010). Mean comparison was performed using the Least Significant Difference (LSD) method. A significance level of 5% was used for all statistical analysis.
Results
ISSR-PCR and gel electrophoresis
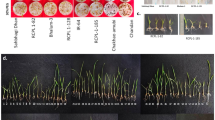
In order to assess the genetic variation among collected T. durum landraces, ISSR was used to generate molecular markers. Seven day old seedlings from each landrace were used for DNA extraction. Seven ISSR primers were used to amplify DNA. The banding pattern profile resulting from amplification was screened for the presence of polymorphisms. Two ISSR primers (UBC 828 and 841) didn’t reveal a considerable difference in banding pattern profiles. However other primers, such as UBA 834 (Fig. 1a), revealed the presence of genetic variation between wheat landraces.
ISSR amplification profile of 4 wheat (T. durum Desf.) landraces [lane 1 ‘Kofr yuba’, 2 ‘Wasteyah’, 3 ‘Ramtha ‘, 4 ‘Karak’], using primer UBC 834 (a). Germination percentage of wheat (T. durum Desf.) landraces under drought stress (300 mM mannitol) after 4 days (b), 7-day old seedlings of wheat (T. durum Desf.) under drought stress landraces (c). Vertical bars denote standard error. Numbers above bars represent response relative to its control. Means followed by the same letter are not significantly different at the p ≤ 0.05 according to Tukey’s test
Effect of drought stress on growth parameters
Germination percentage of the four wheat landraces differed significantly (p < 0.01) (Table 2) in their response to drought stress (Fig. 1b, c). Karak landrace showed the highest germination percentage (92% relative to its control) under drought stress (300 mM mannitol), while Kofr yuba landrace had the lowest one (64% relative to its control). This indicates that at the germination stage, Karak landrace is the most drought tolerant of the tested landraces.
All wheat landraces were affected by drought stress and displayed a significant (p < 0.01) reduction in shoot length compared to the control (Table 2; Fig. 2a). The interaction between landraces and mannitol was significant which indicates that wheat landraces differ in their responses to drought stress (Table 2). The highest shoot lengths under drought stress were recorded for Karak (48% relative to its control) (Figs. 1c, 2a), while Kofr yuba was the most affected landrace by drought stress and produced the shortest shoot length (29% relative to its control).
Effect of drought stress (300 mM mannitol) on shoot length (a), root length (b) and root number (c). Vertical bars denote standard error. Numbers above bars represent response relative to its control. Means followed by the same letter are not significantly different at the p ≤ 0.05 according to Tukey’s test
Root length for the tested wheat landraces was significantly decreased (p < 0.05) under drought stress compared to its control (Table 2; Fig. 2b). Under 300 mM mannitol, the longest roots were observed for Karak (45% relative to its control), while the shortest roots were observed for the Wasteyah landrace (29% relative to its control). This indicates that, in terms of root length, Karak landrace was the most drought tolerant landrace while Wasteyah was the most drought sensitive landrace.
Root number of the wheat landraces was affected significantly (p < 0.01) (Table 2) by drought stress (Fig. 2c). Drought significantly decreased root number of the Ramtha and Wasteyah landraces (72 and 77% relative to its control, respectively). However, the root number of the Karak landrace was not affected by drought stress.
Proline content
Seven day old seedlings grown under control or drought stress (300 mM mannitol) conditions were used for proline content analysis. Proline accumulation was increased significantly (p < 0.01) under drought stress (Table 2; Fig. 3a) for all tested landraces. Proline content in the Karak landrace under drought stress showed the highest accumulation rate (483%) among the studied landraces, while Ramtha landrace showed the lowest relative proline accumulation rate, 182% relative to its control.
Effect of drought stress (300 mM mannitol) on proline content (a) and malondialdehyde (MDA) concentration (b) of wheat (T. durum Desf.) landraces. Vertical bars denote standard error. Numbers above bars represent proline content percentage of each landrace relative to its control. Means followed by the same letter are not significantly different at the p ≤ 0.05 according to Tukey’s test
Lipid peroxidation level
In this study, LPO level was measured in terms of MDA concentration for 7-day old seedlings grown under control and drought stress conditions. Results showed that LPO level was increased significantly (p < 0.01) (Table 2) by drought stress for the studied landraces (Fig. 3b). Under 300 mM mannitol, the highest LPO level was observed for the Wasteyah landrace (194% relative to its control). On the other hand, Karak landrace showed the lowest LPO level under drought stress (147% relative to its control). This indicates that the minimum cell membrane damage among the studied landraces was in the Karak landrace.
Effect of drought stress on DHN15.1 transcript level
In the present study, RT-PCR analysis was used to study the effect of drought stress on DHN15.1 transcript level. Shoots of 7-day old seedlings grown under control and drought (300 mM mannitol) conditions were used for RNA extraction and RT-PCR analysis. Results showed that DHN15.1 transcript level was significantly (p < 0.01) (Table 2) induced by drought stress (Fig. 4a, b). Under drought stress conditions, the maximum relative DHN15.1 up regulation level was observed for the Karak landrace (6.6 fold), while, the lowest up regulation level (around 2 folds) was observed for the Ramtha landrace.
Effect of drought stress (300 mM mannitol) on DHN15.1 transcript level of wheat (T. durum Desf.) landraces. Reverse transcription PCR (RT-PCR) products for 7-day old seedlings (ladder band is 500 bp) (a), quantification of DHN15.1 transcript level (DHN15.1 values normalized to A-tubulin level). Vertical bars denote standard error (n = 5). Numbers above bars represent DHN15.1 transcript level of each landrace relative to its control. Means followed by the same letter are not significantly different at the p ≤ 0.05 according to Tukey’s test (b)
Discussion
Understanding plant response mechanisms to drought stress will help breeders to develop new drought tolerant cultivars. Landraces have a broader genetic diversity than modern cultivars (Belay et al. 1995), and hence, may play an important role in development of drought tolerant cultivars. Results presented in this study, show that the collected landraces respond differently to drought stress and some of them show a good tolerance to drought stress with respect to biochemical, and molecular responses.
Drought stress negatively affects the growth parameters of wheat landraces and decrease germination percentage, shoot length, root length, and root number. This reduction in growth might be due to low osmotic potential and a decrease in cellular expansion (Mohammadkhani and Heidari 2008) as well as cell division and elongation (Fraser et al. 1990). The data on growth parameters under induced drought stress (Fig. 2), indicate that Karak can be classified as the most drought tolerant of the landraces examined. Compared to other landraces, under drought stress the Karak landrace had high germination percentage, the longest shoot and root length, and the highest root number relative to its control.
Proline content of all tested wheat landraces was increased sharply under drought stress, Karak showed the highest Proline accumulation rate and Ramtha was the lowest among the tested landraces. It is well known that proline plays an important role in plant tolerance against environmental stress. Hayano-Kanashiro et al. (2009) and Rampino et al. (2006) found that proline content in maize and wheat increased under stress conditions. Proline accumulation is believed to occur as a consequence of stress (particularly water deficit) resistance (Aspinall and Paleg 1981). Proline accumulation is higher in drought tolerant plants than drought sensitive ones (Mahajan and Tuteja 2005). Proline had a major role in maintaining low water potential through osmoregulation (Hanson and Hitz 1982; Mahajan and Tuteja 2005; Ashraf and Foolad 2007). The increase in proline content under drought stress protects plant cell by balancing the osmotic potential of cytosol with that of vacuole and external environment (Johari-Pireivatlou 2010). Proline accumulation under stress also supplies energy for survival and growth and thereby helps plants to tolerate stress condition (Kumar et al. 2011). Proline content is a good indicator for screening drought tolerant varieties under water stress conditions (Lum et al. 2014).
In addition to proline content, MDA level in all studied landraces was also increased when plants were subjected to water stress. Wasteyah, Kofr yuba and Ramtha landraces showed high levels of MDA content which indicates more lipid peroxidation level and more membrane permeability and are considered more susceptible to drought stress than Karak which produces less MDA level under water stress conditions that indicates higher capability for drought stress tolerance. This is in agreement with Hasheminasab et al. (2012) who found that under drought stress, susceptible wheat genotypes produce more LPO than tolerant ones. Lipid peroxidation is considered the most challenging effect of water stress to cell membranes (Sairam et al. 1997). In Phaseolus acutifolius, LPO level was higher in drought-sensitive genotypes than in drought-tolerant genotypes (Turkan et al. 2005).
In this study, DHN15.1 transcript level (Fig. 4) was found to associate with drought tolerance. Karak showed the highest DHN15.1 expression level among the tested landraces, on the other hand, Ramtha showed the lowest. The up regulation of DHN expression level in drought tolerant genotypes compared to drought sensitive ones under drought stress conditions in the present study is in agreement with Hassan et al. (2015). Stress factors that affect plant growth are associated with plant cell dehydration. Plant cells respond to dehydration in several ways including accumulation of osmotically active compounds such as Dehydrins. In wheat, Dehydrins are induced under dehydration conditions (including drought and salt), in addition to abscisic acid (ABA), due to occurrence of many abscisic acid-responsive elements (ABRE) in Dehydrin promoters (Choi et al. 1999). Shakirova et al. (2016) found that wheat seedlings grown under drought stress accumulated high levels of low molecular mass dehydrins compared to the control seedlings. Furthermore, it has been shown that dehydrins are regulated either by ABA-dependent or ABA independent pathways under drought stress conditions (Kosova et al. 2014; Shakirova et al. 2016). The role of dehydrins in drought stress tolerance is found to be through membrane and enzymes protection, and inhibition of reactive oxygen species formation (Graether and Boddington 2014).
Based on correlation analysis (Table 3), results show that under drought stress (300 mM mannitol), shoot length and proline content showed high positive correlation (r = 0.99, p < 0.01). Accumulation of proline under water stress helps plants maintain osmosis balance (Fedina et al. 2002), reduce water losses (Yokota et al. 2006), and also supplies energy for survival and growth (Kumar et al. 2011). Therefore, the result of this study shows that proline content is a good indicator for drought tolerance and can be used to screen wheat varieties at early stages of development. Significant positive correlation was also found between DHN15.1 transcript level and germination percentage (r = 0.95, p < 0.05). Accumulation of dehydrin proteins is associated with drought tolerance in many plants (Close et al. 1993; Su et al. 2013). Several types of dehydrins in wheat are induced by cold and drought as well as by ABA (Choi et al. 1999; Tommasini et al. 2008; Wang et al. 2014). These results are consistent with the increase in DHN15.1 transcript level and its correlation with germination percentage that we observe.
In conclusion, the studied wheat landraces were found to have variation in their physiological, biochemical, and molecular responses to drought stress. Wheat landraces that exhibited more proline, higher DHN15.1 transcript level, and lower LPO level were more tolerant to drought stress. Some of the studied parameters, such as proline content, could be used as a prescreening method for drought tolerance in wheat genotypes, and it will be worthwhile to examine the effectiveness of this screening under field conditions. The Karak landrace appeared to be a good donor for drought tolerant genes due to its drought tolerance mechanisms showed in this study.
References
Acquaah G (2007) Principles of plant genetics and breeding. Blackwell Publishing Ltd., Malden
Ali Deb T, Monneveux P, Araus JL (1992) Adaptation à la sécheresse et notion d’idéotype chez le blé dur. II. Caractères physiologiques d’adaptation. Agronomie 12:381–393
AL-Shalabi A (2015) Differential gene expression in Jordanian durum wheat landraces exposed to drought, temperature stress and combination of drought and heat stress. M.Sc. thesis, Department of Biological Sciences, Yarmouk University, Jordan
Ashraf M, Foolad M (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59(2):206–216
Aspinall D, Paleg LG (1981) The physiology and biochemistry of drought resistance in plants. In: Paleg LG, Aspinall D (eds). Academic Press, Sydney, pp 205–240
Bartels D, Sourer E (2004) Molecular responses of higher plants to dehydration. Plant Response Abiotic Stress 4:9–38
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39(1):205–207
Belay G, Tesemma T, Bechere E, Mitiku D (1995) Natural and human selection for purple-grain tetraploid wheat in the ethiopian highlands. Genet Resour Crop Evol 42(4):387–391
Black E (2009) The climate change on daily precipitation statistics in Jordan and Israel. Atmos Sci Lett 10(3):192–200
Brown AHD (2000) The genetic structure of crop landraces and the challenge to conserve them in situ on farms. In: Brush SB (ed) Genes in the field: on-farm conservation of crop diversity. Lewis Publishers, Boca Raton, pp 29–48
Carmak I, Horst JH (1991) Effects of aluminum on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol Plant 83:463–468
Chaves MM, Maroco JP, Pereira JS (2003) Understanding plant responses to drought from genes to the whole plant. Funct Plant Biol 30:239–264
Choi DW, Zhu B, Close TJ (1999) The barley (Hordeum vulgare L.) dehydrin multigene family: sequences, allele types, chromosome assignments, and expression characteristics of 11 Dhn genes of cv Dicktoo. Theor Appl Genet 98(8):1234–1247
Close TJ, Fenton RD, Moonan F (1993) A view of plant dehydrins using antibodies specific to the carboxy terminal peptide. Plant Mol Biol 23(2):279–286
Ehdaie B, Waines JG (1989) Genetic variation, heritability, and path-analysis in landraces of bread wheat from southwestern Iran. Euphytica 41:183–190
Fedina IS, Georgieva K, Grigorva I (2002) Light-dark changes in proline content of barley leaves under salt stress. Biol Plant 45:59–63
Fleury D, Jefferies S, Kuchel H, Langridge P (2010) Genetic and genomic tools to improve drought tolerance in wheat. J Exp Bot 61(12):3211–3222
Food and agriculture organization of the United Nations (FAOSTAT) (2012) Retrieved November, 3, 2013 from the World Wide Web: http://faostat.fao.org/site/339/default.aspx
Fraser TE, Silk WK, Rost TL (1990) Effects of low water potential on cortical cell length in growing regions of maize roots. Plant Physiol 93(2):648–651
Graether S, Boddington K (2014) Disorder and function: a review of the dehydrin protein family. Front Plant Sci 5:576
Hanson AD, Hitz WD (1982) Metabolic responses of mesophytes to plant water deficits. Annu Rev Plant Biol 33:163–203
Hasheminasab H, Assad MT, Aliakbari A, Sahhafi SR (2012) Influence of drought stress on oxidative damage and antioxidant defense systems in tolerant and susceptible wheat genotypes. J Agric Sci 4(8):20–30
Hassan N, El-Bastawisy Z, El-Sayed A, Ebeed H, Nemat Alla M (2015) Roles of dehydrin genes in wheat tolerance to drought stress. J Adv Res 6:179–188
Hayano-Kanashiro C, Calderón-Vázquez C, Ibarra-Laclette E, Herrera-Estrella L, Simpson J (2009) Analysis of gene expression and physiological responses in three Mexican maize landraces under drought stress and recovery irrigation. PLoS ONE 4(10):1–19
Hu H, Dai M, Yao J, Xiao B, Li X et al (2006) Overexpressing a NAM, ATAF, and CUC (NAC) transcription factor enhances drought resistance and salt tolerance in rice. Proc Natl Acad Sci USA 103:12987–12992
Jaradat AA (1991) Phenotypic divergence for morphological and yield-related traits among landrace genotypes of durum wheat from Jordan. Euphytica 52(3):155–164
Jaradat AA (2006) Phenotypic divergence in the meta-population of the Hourani durum wheat landrace. J Food Agric Environ 4:186–191
Johari-Pireivatlou M (2010) Effect of soil water stress on yield and proline content of four wheat lines. Afr J Biotechnol 9(1):36–40
Koshgoftarmanesh AH, Schulin R, Chaney RL, Daneshbakhsh B, Afyuni M (2010) Micronutrient-efficient genotypes for crop yield and nutritional quality in sustainable agriculture. Agron Sustain Dev 30:83–107
Kosova K, Vítámvás P, Prášil IT (2014) Wheat and barley dehydrins under cold, drought, and salinity—what can LEA-II proteins tell us about plant stress response? Front Plant Sci 5:343
Kumar RR, Karajol K, Naik GR (2011) Effect of polyethylene glycol induced water stress on physiological and biochemical responses in pigeonpea (Cajanus cajan L. Millsp.). Recent Res Sci Technol 3(1):148–152
Lum MS, Hanafi MM, Rafii YM, Akmar AS (2014) Effect of drought stress on growth, proline and antioxidant enzyme activities of upland rice. J Anim Plant Sci 24(5):1487–1493
Mahajan S, Tuteja N (2005) Cold, salinity and drought stresses: an overview. Arch Biochem Biophys 444:139–158
Masood MS, Javaid A, Rabbani MA, Anwar R (2005) Phenotypic diversity and trait association in bread wheat (Triticum aestivum L.) landraces from Baluchistan, Pakistan. Pak J Bot 37:949–957
Mittler R (2006) Abiotic stress, the field environment and stress combination. Trends Plant Sci 11(1):15–19
Mohammadkhani N, Heidari R (2008) Drought-induced accumulation of soluble sugars and proline in two maize varieties. World Appl Sci J 3:448–453
Montaña FR, Magnitskiy S, Zuluaga G (2014) Effect of NaCl salinity on seed germination and seedling emergence of purple passion fruit (Passiflora edulis Sims). Agron Colomb 32:188–195
Rampino P, Pataleo S, Gerardi C, Mita G, Perrotta C (2006) Drought stress response in wheat: physiological and molecular analysis of resistant and sensitive genotypes. Plant Cell Environ 29(12):2143–2152
Rampino P, Mita G, Fasano P, Borrelli GM, Aprile A et al (2012) Novel durum wheat genes up-regulated in response to a combination of heat and drought stress. Plant Physiol Biochem 56:72–78
Rizhsky L, Liang H, Mittler R (2002) The combined effect of drought stress and heat shock on gene expression in tobacco. Plant Physiol 130:1143–1151
Sairam RK, Shukla DS, Saxena DC (1997) Stress induced injury and antioxidant enzymes in relation to drought tolerance in wheat genotypes. Biol Plant 40(3):357–364
Shakirova F, Allagulova C, Maslennikova D, Fedorova K, Yuldashev R et al (2016) Involvement of dehydrins in 24-epibrassinolide-induced protection of wheat plants against drought stress. Plant Physiol Biochem 108:539–548
Smith SE, Dobrenz AK (1987) Seed age and salt tolerance at germination in alfalfa. Crop Sci 27(5):1053–1056
Su K, Moss JQ, Zhang G, Martin DL, Wu Y (2013) Bermudagrass drought tolerance associated with dehydrin protein expression during drought stress. J Am Soc Hortic Sci 138(4):277–282
Tommasini L, Svensson JT, Rodriguez EM, Wahid A, Malatrasi M, Kato K, Wanamaker S, Resnik J, Close TJ (2008) Dehydrin gene expression provides an indicator of low temperature and drought stress: transcriptome-based analysis of barley (Hordeum vulgare L.). Funct Integr Genomics 8(4):387–405
Turkan I, Bor M, Ozdemir F, Koca H (2005) Differential response of lipid peroxidation and antioxidants in the leaves of drought-tolerant P. Acutifolius Gray anddrought-sensitive P. vulgaris L. subjected to polyethylene glycol mediated water stress. Plant Sci 168:223–231
Vasil I (2007) Molecular genetics improvement of cereals: transgenic wheat (Triticum aestivum L.). Plant Cell Rep 26:1133–1154
Wang Y, Xu H, Zhu H, Tao Y, Zhang G, Zhang L, Zhang C, Zhang Z, Ma Z (2014) Classification and expression diversification of wheat dehydrin genes. Plant Sci 214:113–120
Xiong L, Zhu J (2001) Abiotic stress signal transduction in plants: molecular and genetic perspectives. Physiol Plant 112:152–166
Yokota A, Takahara K, Akashi K (2006) Physiology and molecular biology of stress tolerance in plants. In: Raghavendra MK, Janardhanreddy K (eds). Springer, Berlin, pp 15–40
Zeven AC (1998) Landraces: a review of definitions and classifications. Euphytica 104(2):127–139
Zou ZT, Yang W (1995) Development of wheat germplasm research in Sichuean province. Crop Genet Resour 2:19–20
Acknowledgements
The authors would like to thank the deanship of research at Yarmouk University/Jordan for funding this project as M.Sc. thesis for Ala’a Al Shalabi (31/2015).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Al Khateeb, W., Al Shalabi, A., Schroeder, D. et al. Phenotypic and molecular variation in drought tolerance of Jordanian durum wheat (Triticum durum Desf.) landraces. Physiol Mol Biol Plants 23, 311–319 (2017). https://doi.org/10.1007/s12298-017-0434-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-017-0434-y