Abstract
A set of experiments was conducted to provide significant insights of micro-algal consortia regarding chromium adsorption. Four monocultures; Scenedesmus dimorphus, Chlorella sp., Oscillatoria sp., and Lyngbya sp., and their synthetic consortia were evaluated initially for chromium bio-adsorption at four different regimes of hexavalent chromium i.e. 0.5, 1.0, 3.0 and 5.0 ppm. Based on findings, only 1.0 and 5.0 ppm were considered for future experiments. Consequently, three different types of monoculture and consortia cells namely; live cells, heat-killed cells, and pre-treated cells were prepared to enhance their adsorption potential. Maximal adsorption of 112% was obtained at the dose of 1.0 ppm with 0.1% SDS pre-treated consortia cells over live consortia cells. In support, atomic absorption spectroscopy, laser induced breakdown spectroscopy, pulse amplitude modulated chlorophyll fluorescence, and scanning electron microscopy were performed to assess the structural and functional changes within consortia and their utilization in mitigation of elevated chromium levels.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Oxygenic photosynthesis and pollutant degradation by green algae and cyanobacteria have invigorated the dual concept of heavy metal-affected wastewater treatment and biomass production (Patel et al. 2014). These organisms have the good potential towards water purification by mitigation of the elevated levels of heavy metal toxicity (Khalida et al. 2012; Cardinale 2011; Weis et al. 2008; Cardinale et al. 2007). Individually, these organisms are not able to completely remove the heavy metals; therefore, considering the novelty of microalgae monocultures, efforts are ongoing to develop their suitable synthetic consortia that can be able to alleviate the enhanced levels of heavy metal pollution in different water reservoirs. In addition, regular practices to produce the high-quality microalgae biomass from such wastewater treatment plants is also in progress (Cardinale et al. 2006). Utilization of these biological agents as designer consortia for nutrient removal, pollutant degradation and biomass production from wastewater may enhance the chances of their acceptance by public (Power and Cardinale 2009). Different microalgae have varying inherent traits that define their morphological appearance and functional roles in nature as well as in the heavy metal-affected environment. High concentrations of organic pollutants like styrene and phenolic compounds inhibit the growth of microbial community due to their low aqueous solubility. Consequently, heavy metals such as chromium, mercury, copper and lead are soluble in water up to a high extent. Therefore, utilization of such heavy metal polluted water in plant irrigation or direct intake by population is the major cause behind a number of serious metabolic disorders or diseases (He et al. 2012). Amongst the monocultures, some species have very strong potential while others have limited or negligible efficiency towards the heavy metal detoxification.
Furthermore, their ability towards the detoxification may vary alone or within a group of similar or related taxa (Bose et al. 2011). These variations are due to the alterations in the structure and functioning of entire consortia. Sometimes, combinatorial activities of these monocultures either in fresh or in contaminated water may lead to enhance rates of CO2 mitigation, heavy metal adsorption and biomass production. Therefore, selection of novel species that can have the higher potential for heavy metal degradation is the target of present day biotechnologists. In this aspect, a little success has been achieved (Ruffing and Trahan 2014; Stevenson 2014; Singhvi and Chhabra 2013; Spolaore et al. 2006; Tilman et al. 1996). However, utilizing a single species for microbial degradation of the pollutants has some bottlenecks; therefore, it requires a coordinated approach involving both biochemical and molecular tools to investigate the novel insights into the microalgal consortia for heavy metal polluted water restoration. In comparison to the introduction of genes or enzymes in a single organism which requires their integration within the regulatory and metabolic network for the proper expression (Silva-Rocha and de Lorenzo 2010), engineering of the microbial consortium is comparatively easier, achievable and acceptable. Developing consortia on the basis of the division of labour by a combination of tasks of constituent members could provide a better understanding of the natural assemblages of microbial communities (Patel et al. 2017). It could be a possible way to get the microalgal consortia with enhanced abilities towards the trial missions of pollutant degradation, mitigation of CO2 by its dark photosynthetic fixation reactions, and to get increased biomass yields for commercial production of metabolites of different biotechnological importance (Ortiz-Marquez et al. 2013).
Chromium, a highly poisonous chemical that is responsible for a number of metabolic disorders and incurable serious ailments, being deposited excessively in most of the water reservoirs from industrial sectors such as leather tanning, textile industries, battery, and electroplating in relatively larger amounts. Leaching of rocks and topsoil are natural sources of chromium entry in different water bodies. Most common sources of groundwater chromium contamination are improper disposal of wastes from chromate processing units. According to World Health Organization (WHO), Geneva (1988) Environmental Health Criteria No. 61, levels of chromium in drinking water should not be more than 0.05 ppm. On the other hand, its level in rainwater, seawater, surface water, groundwater (irrigation water) should be between 0.2–1.0, 0.04–0.5, 0.5–2.0 and 1.0–10 µg L−1, respectively. Water purification tanks are being planted to purify chromium contaminated water reservoirs; however, a little success has been achieved (Berg et al. 2009). A number of physical and chemical methods such as coagulation followed by filtration, membrane filtration, adsorption, and ion-exchange are being used to remove the increased levels of chromium from drinking water and non-drinking water. Yet, these methods have a number of limitations which does not allow them to meet the standards with their application. However, coagulation–filtration is still being used as a common method for chromium removal. Membrane technology has been found efficient in removing both Cr6+ and Cr3+. In the absence of proper technology, still a number of efforts are ongoing, and biotechnologists are searching the potential microalgae species that could be actively engaged in chromium-contaminated water purification processes. Moreover, a number of studies have been done with monocultures, especially with green algae Chlorella, and cyanobacteria Oscillatoria and Lyngbya; globally, the role of microalgae consortia in chromium adsorption is underway globally. According to the Safe Drinking Water Act (SDWA) (1991), maximum contaminated level of water with hexavalent chromium should not be more than 100 ppb i.e. 0.1 ppm; Consequently, its toxicity level has increased beyond this limit in industrial sectors, and some of which is contaminating the rivers that are causing the serious water issues in human beings and other animals. Therefore, several water purification programs are ongoing, which majorly involve cyanobacteria and green algae cultures. Considering all these facts that may provide improved water quality and higher biomass yields, this study was proposed, which processes of microalgal consortia were assessed in mitigation of chromium toxicity under different regimes of hexavalent chromium to see that how the changes in microalgal community structure could affect its processes (Volland et al. 2013). Green algae Chlorella sp., Scenedesmus dimorphus, cyanobacteria Oscillatoria sp., and Lyngbya sp. were in vitro evaluated to alleviate the levels of chromium in BG11+ broth. Monocultures as well as consortia of these organisms were investigated in their natural habitat and chromium enriched environment to develop a successful micro-algal technology which could reduce the elevated chromium levels in heavy metal enriched fresh water systems, and can produce biomass (Berg et al. 2009; Renuka et al. 2013). Consortia of these photosynthetic organisms could provide not only less polluted water but also sustainable biomass which can be utilize for other purposes after complete recycling of its adsorbed chromium (Khalida et al. 2012); otherwise, it may further create the problem of bio-accumulation or toxicity. Four organisms were selected; Chlorella, S. dimorphus, Oscillatoria and Lyngbya for this study, since these were previously tested by a number of research groups and identified as potential agents in chromium removal. Therefore, evaluating them in consortia could provide their better exploitation in chromium removal (Renuka et al. 2013).
Materials and methods
Microalgae cultures
The members of family Chlorophyceae and Cyanophyceae; Chlorella sp., S. dimorphus, Oscillatoria sp., Lyngbya sp, and their consortia were selected for the study. These cultures were available at the Phycology laboratory, Centre of Biotechnology, University of Allahabad, India. Axenic cultures were maintained on BG11+ agar media (pH 7.8) supplemented with 10 mM sodium thiosulphate (Behera et al. 2015; Gross et al. 2014; Cardinale et al. 2007).
Culture conditions
Microalgae cultures were incubated at 27 ± 1 °C and daylight fluorescent lamps were used for providing illumination at the irradiance of 92.5 µmol photons m−2 S−1 in 14:10 light–dark diurnal cycle. Furthermore, at the start of experiments and for various physico-chemical studies, equal number of cells of above mentioned cultures of Chlorophyceae and Cyanophyceae (optimized through corresponding O.D. and cell counts) were inoculated from the late log phase in equal volume of BG11+ culture broth under two different experimental setups; first set involved cultivation with 0.5, 1.0, 3.0 and 5.0 ppm doses of chromium while second set was deficient in chromium (Shukla et al. 2012).
Experimental designs
Experiments were performed in three replicates, a Complete Randomized Design (CRD) was used to elucidate the effects of Cr6+ on the microalgae consortia consisting Chlorella sp., S. dimorphus, Oscillatoria sp. and Lyngbya sp. Hexavalent chromium stock of 100 ppm was prepared by dissolving 2.830 g potassium dichromate (K2Cr2O7) in 1000 mL sterile distilled water. Consequently, the stock was used to make the further dilutions of 0.5, 1.0, 3.0 and 5.0 ppm. Before the start of experiment, inoculums were grown up to the late log phase. An equal number of cells of green algae and cyanobacteria were mixed to get the microalgae consortia. Based on our previous standardizations (O.D. vs. cell counts), the cells and filaments were mixed. In each treatment, equal numbers of cells of monocultures or consortia having equal cells of monocultures were inoculated in separate flasks. Thus, initially, each flask contained 1 × 107 cells mL−1 of culture broth (BG11+ media supplemented with or without different doses of Cr6+. The flasks without chromium treatments were considered as experimental controls. To nullify the experimental error, the optical density of un-inoculated BG11+ supplemented with chromium was normalized with O.D730 of natural BG11+. The chromium-induced stress effects and changes in physico-chemical parameters of monocultures as well as of consortia were evaluated under four different regimes including; 0.5, 1.0, 3.0 and 5.0 ppm for S. dimorphus, Chlorella sp., Oscillatoria sp. and Lyngbya sp. in consortia of BG11+ media (Fox, 2004). Chromium treated cultures along with respective experimental controls (BG11+) were incubated up to 25 days under above described culture conditions. Consequently, different controls (different microalgae cultivated in BG11+ individually as well as in consortia) were compared with treated monocultures and consortia to record changes in the original physiological consequences and role of different regimes of chromium stress on their biomass production ability.
Analytical methods
Bio-sorption of chromium by different species and consortia
Bio-sorption of chromium by different microalga sp. and their consortia was studied by three different treatment methods; in first the one, living mono culture cells and consortia were used to monitor the rates of uptake of chromium heavy metal by supplementing the basal media with different concentrations (1.0 and 5.0 ppm) of chromium stock solutions. In the second treatment, heat-killed cells were prepared by taking fresh biomass and incubating it in water at 100 °C for 5 min. In third treatment, pre-treated cells were made by suspending the fresh biomass in 0.1 N NaOH and 0.01% SDS separately followed by incubating these cells at room temperature (R.T.) for 20 min. The biomass obtained from each treatment was later suspended in solutions containing different concentrations of chromium. The biomass was left for 1 h in the respective chromium containing solutions of 1.0 and 5.0 ppm; cell-free supernatants were collected from each, and analyzed for chromium bio-sorption by measuring the optical density at 540 nm wavelength (Jayashree et al. 2012, Micheli et al. 2014).
Calculation of LD50
The LD50 of Cr6+ was determined calorimetrically to decide the optimal dose at which various monocultures and consortia could perform effectively. Simultaneously, the maximal dose at which major processes of these cells get inhibited was also selected for comparative analyses (Stohs et al. 2001).
Chlorophyll-a (chl-a) fluorescence
The chl-a fluorescence of various microalgae and their consortia under different experimental conditions was monitored by a dual modulation kinetic fluorometer FL3500/F (PSI, Brno, Czech Republic, version 3.7.0.1). The monocultures and their consortia were pre-adapted for 15 min in the dark before examining their fluorescence rates (Maxwell and Johnson 2000; Patel et al. 2016).
Microscopy
The microscopic observations were made at the end of cultivation to study the structural changes within control and treated cells of monocultures and consortia. The cells were harvested and washed with sterile distilled water two times, and suspended to the original volume by adding the fresh BG11+ media. Two drops of the cultures were placed on the neat and clean slides and observed under 40× objective of the compound microscope.
Scanning electron microscopy (SEM)
The high resolution micrographs of control and adsorbed samples of micro-algal consortia were obtained under the Scanning Electron Microscope (SEM) through FEI Quanta 200. Consortia grown in original BG11+ media, 1.0 and 5.0 ppm were harvested by centrifugation at 2000 rpm for 5 min, washed three times with Sorenson’s buffer. Subsequently, each micro-algal consortia sample was fixed with a fixing solution containing 4.0% v/v formaldehyde and 2.5% glutarldehyde for 4 h at R.T. Fixing solution was prepared in 0.2 M Sorenson’s phosphate buffer. Fixed samples were washed with 0.2 M Sorenson’s phosphate buffer three times with 15 min for each wash. Consequently, each sample was dehydrated in a series of sequential series of 20, 40, 60, 80 and 100% absolute ethanol (Bharti et al. 2016). Finally, each sample was dried in a critical point dryer under CO2 enriched environment and examined by using the FEI Quanta 200.
Laser induced breakdown spectroscopy (LIBS)
To perform the LIBS, control consortia and consortia treated with 1.0, and 5.0 ppm Cr6+ were grown in 2000 mL of BG11+ media in 5 L Conical flasks up to 25 days. Proper light and dark treatments was maintained and hand shaking was provided timely to mix the content throughout cultivation. Each sample was centrifuged and filtered with Whatman filter paper (2.0 µm pore size), and oven-dried to remove moisture completely. Equal biomass of 1.0 g from each sample was further considered for the preparation biomass pellets by the help of KBR-press device. Furthermore, pellets were used for LIBS analysis. Considering the sensitive nature of microalgae biomass pellets and destruction by Laser-pulse irradiation induced thermal shock, shot to shot protocol was used to minimize any destruction and to get the high degree of reproducibility. The peaks obtained after the LIBS, were compared with the CN bands available at NIST (National Institute of Standards and Technology, Gaithersburg, Maryland) to get the presence or absence of chromium (Kumar et al. 2014).
Atomic absorption spectroscopy (AAS)
Atomic Absorption Spectrophotometer was used to perform the analysis by following the method suggested by Singh et al. 1989. To perform the AAS, equal dried biomass of each consortia control, consortia 1.0 and 5.0 ppm Cr6+ treated samples were washed by double distilled water two times and oven-dried, and equal dried biomass of 1.0 g was considered for analysis. Single acid digestion was done with HNO3, and samples were kept in hot air dry-oven for complete drying. Acid digested samples were then mixed in 0.5% HNO3, and final volume was made to 100 mL. Quantitative determination of Cr6+ was done by AAS of Perkin Elmer at scientific and Industrial research organization, Centre of Food technology, University of Allahabad, Allahabad. The experimental standards were prepared by dissolving the Cr6+ in similar way as done for various algal samples in 0.5% HNO3. The results were compared to standards to get the concentrations of Cr6+ adsorbed in consortia biomass by available list of metal spectra in NIST database.
Statistics
Culture related experiments were performed in three replications while analytical evaluation through biochemical and spectrophotometric methods were done in six replications. Various experimental data were analyzed by either one way or two way ANOVA or mixed ANOVA as where needed by using either Graph Pad Prism 5.0 or Origin 8.5 statistical software (Loreau et al. 2001). The Null hypothesis was rejected or accepted on the basis of Fisher ratios (F value) and probability (p ≤ 0.05) at 95% confidence levels.
Results and discussion
Chromium induced changes in microalgae community
Exogenous supplementation of different doses of chromium significantly affected the structure and functioning of various monocultures and consortia. In order to find out the tolerance limits of these cyanobacteria, green algae and their consortia against chromium, their growth media was supplemented with four concentrations of Cr6+ in different experimental flasks up to 25 days. In natural environment, these photosynthetic microbes had different behavior towards chromium when compared to other treatments. Highly drastic morphological distortions were seen under microscope at higher doses of Cr6+. Almost all the cells of Oscillatoria were lost at the 5.0 ppm of Cr6+ whilst Chlorella, Lyngbya sp. and Scenedesmus showed comparatively better stress tolerance in consortia. Microscopic observations showed that at the 0.5 ppm Cr6+, the cell morphology of monocultures and consortia cells was not affected significantly (Adinath et al. 2015; Pereira et al. 2013; Tchounwou et al. 2012). On the other hand; appearance of these photosynthetic organisms was altered at higher doses of Cr6+. However, at the lower doses of 0.5 and 1.0 ppm, most of the cells were slightly swollen. Since, swollen cells have smaller surface to volume ratios, therefore, it may be a reason for slightly increased physiological performance at lowest dose (0.5 ppm) of Cr6+. In addition, cell surfaces of different algae and cyanobacteria get blocked by adsorption of Cr6+ which alternatively affected the original rate of exo/endo-osmosis of important metabolites. Therefore, natural physiology of chromium treated cells gets altered that highly affected the growth rates and biomass yields (Fig. 1a–e).
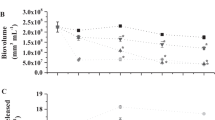
Similar trends were recorded for the consortia at different doses of Cr6+ (Fig. 2). The monocultures in control consortia showed the following order of tolerance as observed by cell counts at the time of harvest; Oscillatoria sp. <S. dimorphus < Chlorella < Lyngbya sp. respectively. Therefore, within consortia Lyngbya sp. was found to be dominant sp. Based on the structural changes and calculated LD50 values of various monocultures and consortia under stress, we selected only 1.0 and 5.0 ppm doses for future studies. At 1.0 ppm Cr6+, cells performed optimally whilst at highest dose of 5.0 ppm highly diminished physiology was obtained for each culture (Fig. 3). Under stressed environment, assemblages of cyanobacteria and green alage produce unusual compounds to protect them from adverse environmental effects. In another study by Dixit et al. (2017), Jaishankar et al. (2014), they isolated two strains of Oscillatoria sp. RBD01 and Leptolyngbya sp. RBD05 were isolated from nutrient and heavy metal polluted stretch of Ganga River, and characterized them as producer of microcystin. These compounds highly affect the growth rates of other individuals of consortia (Table 1).
Fluorescence response of chlorophyll
Cumulative interactions of these photosynthetic monoculture cyanobacteria and green alage in consortia towards the chromium tolerance were highly reduced at the 5.0 ppm (Govindjee 2005; Holt et al. 2004). However, Chl. Fluorescence of consortia was less affected than monocultures. Among the monocultures, maximum Fv/Fm ratio was obtained for the Lyngbya sp. followed by Chlorella sp., S. dimorphus and Oscillatoria sp. respectively. Moreover, with respect to various monoculture controls 2.802, 0.813, 10.886, 4.583, 42.51, 70.49, 15.44, 44.87% inhibition in the rate of Chl-a fluorescence was obtained at the 1.0 and 5.0 ppm Cr6+ respectively for Chlorella sp., Oscillatoria sp. Lyngbya sp., and S. dimorphus. In contrary, only 14.57 and 7.32% inhibition was recorded for consortia when compared to the consortia control (Table 2). Therefore, enriching the consortia of these organisms with 1.0 ppm Cr6+ enriched water reservoirs could be a possible way to get the more photosynthetic yields. Beyond that, these organisms would have very neglible contribution in reducing the Cr6+ toxicity. At the higher concentrations, all the cultures had lower cell counts and decreased chl-a fluorescence. It might be a reason for the increased perennation on the surface of culture broth in stressed environments. Rate of perennation was increased with increasing in the level of Cr6+ stress. It indicates that they were reached their succession stages much earlier than the respective controls. Micrographs and Fv/Fm ratios of various cultures collectively showed that Oscillatoria sp. was highly affected whilst Lyngbya was dominating species within consortia. Since, both at the 1.0 and 5.0 ppm Cr6+, the percentages inhibition in chl-fluorescence was found to be lowest in the case of Lyngbya followed by Chlorella, Scenedesmus and Oscillatoria respectively. Therefore, Lyngbya was photo-synthetically most active in Cr6+ rich environment. For the natural habitats affected with chromium, similar trends can be expected with some deviations, since in natural water reservoirs there are several other independent factors or variables which affect the structure and function of natural assemblages of green algae and cyanobacteria. These variables may be of biological, physical or chemical nature. Therefore, above 1.0 ppm Cr6+, these microalgae lose their original features. The measurement of fast kinetics and multiphase (OJIP) transitions showed that after an adaptation of algae in dark, the fluorescence yield from the transition ‘O’ (F0) is the emission of light energy and excitement before going to the reaction center by chlorophyll antenna of PSII (Iara and Hendrik 2009). At this level, the reaction centers are opened and the QA is completely oxidized, because the energy is not sufficient to induce the separation of charge. Normally, the yield of F0 is constant; however, the performance change of F0 is an alteration of pigment-protein complexes associated with PSII. Decreased surfaces to volume ratios of stressed cells are directly related to the size of intracellular contents. At lower doses of 0.5 and 1.0 ppm Cr6+, due to increased cell surfaces the antenna complex surface may increased that can be a major cause for slightly increased or unaltered rate of transitions of electrons.
Scanning electron microscopy (SEM)
SEM generated micrographs of the control, 1.0 and 5.0 ppm treated consortia are shown in Fig. 4a–c respectively. From micrographs, it is evident that with increased doses of Cr6+ up to 1.0 and 5.0 ppm, morphology of constituent cells of consortia gets highly distorted. It clarifies that how the increment in the concentrations of Cr6+ can create morphological changes. It was reported that in the presence of excess Cr6+, due to hydrolysis of polysaccharides more number of hydroxyl ions get accumulated on the surface of consortia and decreases overall effective sizes of cells (Shukla et al. 2012; Zhang et al. 2016). Possibly, negative charges were distributed throughout the cell surface of cyanobacteria and algae which provided an enormous surface area for binding of the positively charged chromium ions.
Bio-sorption of chromium by various monocultures and their consortia
The bio-sorption of Cr6+ was performed by preparing three types of cells; live cells, heat killed cells, and pre-treated cells. 0.1% SDS pre-treated consortia cells showed maximal metal uptake of 84.5% at 1.0 ppm. In contrary, 0.1 N NaOH pre-treated consortia cells could uptake 83.6% (Fig. 2). In general, metal uptake by pre-treated cells was found to be more than live cells or heat-killed cells. Sodium hydroxide and sodium dodecyl sulphate are anionic detergents having negatively charged –OH groups. Therefore, when these detergents come in contact with positively charged cations such as Cr6+, Cd, Pb etc., they get bind to them. So, it may be a cause behind the optimum adsorption of positively charged Cr6+ cations to NaOH and SDS pre-tretaed cyanobacterial and green algae cells (Fisher et al. 1984). In a study by Rabsch and Elbracher (1980), they observed that heat-killed cells of Coscinodiscus granii could accumulate three times more zinc than its live cells. The uptake of zinc metal by microalgal consortia is comparatively higher (Ahuja et al. 1999). Some studies showed that in immobilized monocultures or consortia on alginate have greater metal uptake potential (Brun et al. 1998; Gloaguen et al. 1996; Ye et al. 1997).
Laser induced breakdown spectroscopy (LIBS)
To prove the presence of Cr6+ in microalgal consortia, different treated samples were subjected for LIBS analyses. LIBS is an emerging and most suitable laboratory analytical technique, being routinely used for wide range elemental analysis of solid, liquid, gaseous samples. It is a rapid and non-distractive technique which requires no to minimal sample preparation. With their qualitative and quantitative analysis, LIBS can simultaneously analyze the presence of all the elements which are present in the sample. Analytical results of LIBS of algal consortia favor for the presence of elements C, Mg, Ca, Na, O, N, H, K and Cr (Fig. 5a–d) respectively for control and with Cr6+ treated consortia. Peaks obtained during studies showed the spectral signature for the elements C, Mg, Ca, Na, O, N, H, K and Cr (Kumar et al. 2014) as compared with available NIST database list (Table 1). Published literature showed that CO2 assimilation and photosynthesis of algal consortia is highly decreases due to the toxic effects of Cr6+. In addition, PSII reaction center is highly sensitive to any damage caused by heavy metal stress which alternatively affects the photosynthetic carboxylation reactions, photosystem II (PS II) electron transport system (ETS) and oxygen-evolving complex. Therefore, experimental results of the present study clearly demonstrate that Cr6+ affected the cell morphology and physiological parameters. Presence of spectral peak of Cr6+ was obtained at 357.8, 359.5, and 360.4 nm in the LIBS spectra for the stressed cells of consortia. LIBS spectra are shown in Fig. 5a–e for control and treated consortia. Presence of peak in treated cells clearly indicates that consortia cells had adsorbed the Cr6+. In contrary, complete absence of spectral peak in control consortia clarify the absence of chromium in the sample.
Atomic absorption spectroscopy (AAS)
Micro-algal consortia can perform functions which are difficult or even impossible for the individual species (Brenner et al. 2008; Perales-Vela et al. 2006). Living together may provide robustness to the environmental fluctuations, ability of cumulative metabolite production, and resistance to invasion by other species. A number of proof-of-principle studies on the consortia of cyanobacteria/microalgae–bacteria for pollutant can be retrieved from the published literature. The results of this study indicated that the biomass of consortia could be developed as a suitable technology for the efficient removal of chromium from the waste water. Present results showed that highest amounts of 92.0 and 84.0% of Cr6+ metal could be adsorbed at 1.0 and 5.0 ppm respectively by the consortia of cyanobacteria and green algae (Fig. 6).
Conclusions
Increasing concentration of heavy metals in different water reservoirs, especially drinking water has generated the serious issues of bio-accumulation at higher tropic levels. Chromium in excess creates a number of serious health issues; therefore, regular efforts to remove it from water are underway. Different approaches of microbial environmental biology are being tried to reach the target. In this aspect cyanobacteria and green algae are identified as potential agents. In natural habitats these organisms are in different assemblages. Consortia based mitigation with increased diversity may lead to enhanced water purification (Zimmerman and Cardinale 2014; Chekroun and Baghour 2013; Chakraborty et al. 2011; Dwivedi et al. 2010; Cervantes et al. 2001) through mitigating the increased chromium levels.
Biomass yields in control community was highest than all the monocultures, however, in the presence of Cr6+ perturbation at different regimes, ranging from 1.0 to 5.0 ppm, overall growth was inhibited. As Cr6+ affects the photosynthetic apparatus of algae, at its lowest dose of 5.0 ppm there was an increase in the fluorescence ‘FO’. Consequently, at slightly higher dose of 1.0 ppm Cr6+ due to altered light harvesting antennae complex of PSII, we noticed a decrease in the fluorescence. It shows the degradation of electron transfer system at higher doses. Therefore, it could be said that as a consequence of damage to PSII the Fv/Fm ratio was decreased with increased concentration of Cr6+. Conclusively the use of chlorophyll fluorescence as an indicator of toxicity of chromium is a simple and fast method, it gives us an indication of the photosynthetic apparatus of the algae, therefore, we could use this as bioassays to detect environmental stress. Simultaneously, SEM proved the presence of Cr6+ due to altered morphological appearances. In the same direction, LIBS analysis of control consortia revealed the presence of elements C, Mg, Ca, Na, O, N, H and K, however, consortia with Cr6+ contained the spectral signature of elements C, Mg, Ca, Na, O, N, H, K and Cr. In fact, the pre-treated cells have shown best results to the remove chromium at various concentrations. Therefore, these studies could be a possible tool to reach the physiological consequences in consortia under Cr6+ stress.
References
Adinath RA, Singh SK, Dixit K, Sundaram S (2015) Chromium induced alterations in different individual microalga and their consortia. IJONS 6(32)
Ahuja P, Gupta R, Saxena RK (1999) Zn2+ biosorption by Oscillatoria anguistissima. Process Biochem 34:77–85
Behera S, Singh R, Arora R, Sharma NK, Shukla M, Kumar S (2015) Scope of algae as third generation biofuels. Front Bieng Biotechnol 2:1–13
Berg AK, Lyra C, Sivonen K, Paulin L, Suoimalainen S, Tuomi P, Rapala J (2009) High diversity cultivable heterotrophic bacteria in association with cyanobacterial water blooms. ISME J 3:314–325
Bharti N, Pandey SS, Barnawal D, Patel VK, Kalra A (2016) Plant growth promoting rhizobacteria Dietzia natronolimnaea modulates the expression of stress responsive genes providing protection of wheat from salinity stress. Sci Rep 6:34768
Bose SC, Suresh R, Ramakrishnan B, Megharaj M, Venkateshwarlu K, Naidu R (2011) Consortia of cyanobacteria/microalgae and bacteria: biotechnological potential. Biotechnol Adv 29(6):896–907
Brenner N, Joseph B, Jones M (2008) Theorizing sociospatial relations. Environ Plan D Soc Sp 26:389–401
Brun LA, Maillet J, Richarte J, Herrmann P, Remy JC (1998) Relationship between extractable copper, soil properties and copper uptake by wild plant in vineyard soils. Environ Pollut 102:151–161
Cardinale BJ (2011) Biodiversity improves water quality through niche partitioning. Nature 472:86–89
Cardinale BJ, Srivastava DS, Duffy JE, Wright PJ, Downing LA, Sankaran M, Jouseau C (2006) Effect of biodiversity on the functioning of trophic groups and ecosystems. Lett Nat 443(7114):989–992. doi:10.1038/nature05202
Cardinale BJ, Wright JP, Cadotte MW, Carroll IT, Hector A, Srivastava DS, Loreau M, Weis JJ (2007) Impacts of plant diversity on biomass production increase through time because of species complementarity. PNAS 104(46):18123–18128
Cervantes C, Campos-García J, Devars S, Gutierrez-Corona F, Loza-Tavera H, Torres-Guzmán JC, Moreno-Sánchez R (2001) Interactions of chromium with microorganisms and plants. FEMS Microbiol Rev 25:335–347
Chakraborty N, Banerjee A, Pal R (2011) Bio monitoring of lead, cadmium and chromium in environmental water from Kolkata, North and South 24-Parganas using algae as bioreagent. J Algal Biomass Utln 2(3):27–41
Chekroun KB, Baghour M (2013) The role of algae in phytoremediation of heavy metals: a review. J Mater Environ Sci 4(6):873–880
Dixit RB, Patel AK, Toppo K, Nayaka S (2017) Emergence of toxic cyanobacteria species in the Ganga River, India, due to excessive nutrient loading. Ecol Indic 72:420–427
Dwivedi S, Srivastava S, Mishra S, Kumar A, Tripathi RD, Rai UN, Dave R, Tripathi P, Charkrabarty D, Trivedi PK (2010) Characterization of native micro-algal strains for their chromium bioaccumulation potential: phytoplankton response in polluted habitats. J Hazard Mater 173(1–3):95–101
Fisher NS, Bohw M, Teyssie JL (1984) Accumulation and toxicity of Cd, Zn, Hg and Ag in the marine phytoplanktons. Mar Ecol Prog Ser 18:201–204
Fox JW (2004) Effect of algal and herbivore diversity of the partioning of biomass within and among trophic levels. Ecol Ecol Soc Am 85(2):549–559
Gloaguen V, Morvan H, Hoffmann L (1996) Metal accumulation by immobilized cyanobacterial mats from a thermal spring. J Environ Sci Health A31(10):2437–2451
Govindjee (2005) Announcement: advances in photosynthesis and respiration, volume 19: ‘Chlorophyll a fluorescence: a signature of photosynthesis’, edited by George C. Papageorgiou and Govindjee. Photosynth Res 83:101–105
Gross K, Cardinale BJ, Fox WJ, Gonzalez A, Loreau M, Polly WH, Reich BP, Ruiwen VJ (2014) Species richness and the temporal stability of biomass production: a new analysis of recent biodiversity experiments. Am Nat 183:1–12
He Z, Piceno Y, Deng Y, Xu M, Lu Z, De Santis T, Andersen G, Hobbie ES, Reich BP, Zhou J (2012) The phylogenetic composition and structure of soil microbial communities shifts in response to elevated carbon dioxide. ISME J 6:259–272
Holt NE, Fleming GR, Niyogi KK (2004) Toward an understanding of the mechanism of nonphotochemical quenching in green plant. Biochemistry 43(26):8281–8289
Iara R, Hendrik K (2009) Chromium and copper-induced inhibition of photosynthesis in Euglena gracilis analysed on the single-cell level by fluorescence kinetic microscopy. New Phytol 181:405–420
Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN (2014) Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol 7(2):60–72
Jayashree S, Thangaraju N, Gnanadoss JJ (2012) Toxic effects of chromium on the aquatic cyanobacterium Oscillatoria spand removal of chromium by biosorption. J Exp Sci 3(5):28–34
Khalida Z, Amar Y, Boutiba Z, Zemri M, Popovic R (2012) Use of chlorophyll fluorescence to evaluate the effect of chromium on activity photosystem II at the alga Scenedesmus obliquus. IJRRAS 12(2):304–314
Kumar R, Tripathi DK, Devanathan A, Chauhan DK, Rai AK (2014) In-situ monitoring of chromium uptake in different parts of the wheat seedling (Triticum aestivum) using laser-induced breakdown spectroscopy. Spectros Lett 47(7):554–563
Loreau M, Naeem S, Inchausti P, Bengtsson J, Grime JP, Hector A, Hooper DU, Huston MA, Raffaelli D, Schmid B, Tilman D, Wardle DA (2001) Biodiversity and ecosystem functioning: current knowledge and future challenges. Sci 294:804–808
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence: a practical guide. J Exp Bot 51(345):659–668
Micheli C, Cianchi R, Paperi R, Belmonte A, Pushparaj B (2014) Antarctic cyanobacteria biodiversity based on its and TRNL sequencing and its ecological implication. Open J Ecol 4:456–467
Ortiz-Marquez JCF, Do Nascimento M, Zehr JP, Curatti L (2013) Genetic engineering of multispecies microbial cell factories as an alternative for bioenergy production. Trends Biotechnol 31(9):521–529
Patel VK, Maji D, Singh AK, Sussela MR, Sundaram S, Alok K (2014) A natural plant growth promoter, caliterpenone, enhances growth and biomass, carbohydrate and lipid production in cyanobacterium Synechocystis PCC 6803. J Appl Phycol 26:279–286
Patel VK, Maji D, Pandey SS, Rout PK, Sundaram S, Kalra A (2016) Rapid budding EMS mutants of Synechocystis PCC6803 producing carbohydrate or lipid enriched biomass. Algal Res 16:36–45
Patel VK, Sahoo NK, Patel AK, Rout PK, Naik SN, Kalra A (2017) Exploring microalgae consortia for biomass production: a synthetic ecological engineering approach towards sustainable production of biofuel feedstock. In: Gupta SK et al (eds) Algal biofuels. Springer, New York. ISBN 978-3-319-51009-5
Perales-Vela HV, Pena-Castro JM, Canizares-Villanueva RO (2006) Heavy metal detoxification in eukaryotic microalgae. Chemosphere 64:1–10
Pereira M, Bartolome MC, Sanchez FS (2013) Bio-adsorption and bioaccumulation of chromium trivalent in Cr(III)-tolerant microalgae: a mechanism for chromium resistance. Chemosphere 93(6):1057–1063
Power DL, Cardinale BJ (2009) Species richness enhances both algal biomass and rates of oxygen production in aquatic microcosms. Oikos 118:1703–1711
Rabsch V, Elbracher R (1980) Cadmium and zinc uptake growth and primary production in coscinodiscus granii cultures containing low level of cells and dissolved organic carbon. Helgol Wiss Meeresuter 33:79–88
Renuka N, Sood A, Ratha KS, Prasanna R, Ahluwalia SA (2013) Evaluation of micro algal consortia for treatment of primary treated sewage effluent and biomass production. J Appl Phycol 25:1529–1537
Ruffing AM, Trahan CA (2014) Biofuel toxicity and mechanisms of biofuel tolerance in three model cyanobacteria. Algal Res 5:121–132
Shukla D, Padma SV, Srivastava SK (2012) Bioremediation of hexavalent chromium by a cyanobacterial mat. Appl Water Sci 2:245–251
Silva-Rocha R, de Lorenzo V (2010) Noise and robustness in prokaryotic regulatory network. Annu Rev Microbiol 64(257–275):2010
Singhvi P, Chhabra M (2013) Simultaneous chromium removal and power generation using algal biomass in a dual chambered salt bridge microbial fuel cell. J Bioremed Biodegrad 4:190
Spolaore P, Joannis CC, Duran E, Isambert A (2006) Commercial applications of microalgae. J Biosci Bioeng 101:87–96
Status of Chromium and copper from water pipes Lea d and Copper rule under SDWA (Safe Drinking Water Agency) (1991)
Stevenson J (2014) Ecological assessments with algae: a review and synthesis. J Phycol 50:437–461
Stohs JS, Bagchi D, Hassoun E, Bagchi M (2001) Oxidative mechanisms in the toxicity of chromium and cadmium ions. J Environ Pathol 19(3):201–213
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Heavy metals toxicity and the environment. EXS.101, pp 133–164
Tilman D, Wedin D, Knops J (1996) Productivity and sustainability influenced by biodiversity in grassland ecosystem. Nature 379(6567):718
Volland S, Elisabeth B, Verena B, Ancuela A, Cornelius L, Evelyn S, Ursula LM (2013) Rescue of heavy metal effects on cell physiology of the algal model system micrasterias by divalent ions. J Plant Physiol 171:154–163
Weis JJ, Madrigal SD, Cardinale JB (2008) Effects of algal diversity on the production of biomass in homogeneous and heterogeneous nutrient environments: a microcosm experiment. PLoS ONE 3(7):e2825. doi:10.1371/Journal.pone.0002825
World Health Organization, Geneva (1988) Chromium in Drinking Water. (Criteria No. 61) p 4
Ye ZH, Baker AJM, Wong MH, Willis AJ (1997) Zinc, lead and cadmium tolerance, uptake and accumulation in population of Phragmites australis (Cav.) Trin. Ex Steudel. Ann Bot 80:363–370
Zhang X, Zhao X, Wan C, Chen B, Bai F (2016) Efficient biosorption of cadmium by the self-flocculating microalga Scenedesmus obliquus AS-6-1. Algal Res 16:427–433
Zimmerman KE, Cardinale JB (2014) Is the relationship between algal diversity and biomass in North American lakes consistent with biodiversity experiments? Oikos 123:267–278
Acknowledgements
Adinath is thankful to UGC New Delhi, India for providing him UGC-D.Phil research fellowship.
Author’s contributions
Shanthy Sundaram and Adinath designed the experiments, Adinath performed all the experiments. P K Tiwari and AK Rai helped Adinath in elemental analysis of chromium. Adi Nath and Shanthy Sundaram drafted the manuscript, Shanthy Sundaram reviewed the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nath, A., tiwari, P.K., Rai, A.K. et al. Microalgal consortia differentially modulate progressive adsorption of hexavalent chromium. Physiol Mol Biol Plants 23, 269–280 (2017). https://doi.org/10.1007/s12298-017-0415-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-017-0415-1