Abstract
An efficient in vitro protocol has been established for clonal propagation of elite plant of Spilanthes calva DC., an important source of spilanthol, an antimalarial larvicidal compound. Nodal explants excised from field grown plant of S. calva DC. when reared on Murashige and Skoog’s medium augmented with different cytokinins, viz. N6-Benzyladenine (BA), N6-(2-isopentenyl) adenine (2iP) and 6-furfuryl aminopurine (Kn), differentiated multiple shoots from the axils. BA at 10 μM proved optimum for elicitation of multiple shoots in 91.6 % cultures with an average of 7.12 shoots per explant within 6 weeks. The excised shoots rooted on half strength Murashige and Skoog’s medium supplemented with 0.1 μM IBA. Micropropagated plants were hardened and transferred to field for acclimatization, where 95 % plants survived and were phenotypically similar to the donor plant. Random amplified polymorphic DNA (RAPD) and inter-simple sequence repeat (ISSR) markers were employed to evaluate the genetic fidelity amongst the regenerants. Eleven individuals, randomly chosen amongst a population of 120 regenerants were compared with the donor plant. A total of 71 scorable bands, ranging in size from 100 bp to 1,100 bp were generated by a combination of the two markers in the aforesaid plants. All banding profiles from micropropagated plants were monomorphic and similar to those of mother plant. The similarity values amongst the aforesaid plants varied from 0.967 to 1.000. The dendrogram generated through UPGMA (Unweighted Pair Group Method with arithmetic mean) analysis revealed 98 % similarity amongst them, thus confirming the genetic fidelity of the in vitro clones.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spilanthes calva DC. commonly known as ‘Akarkara’ or toothache plant, belonging to the family Asteraceae is widely distributed in tropical and subtropical areas (Jansen 1985). In India, it is confined to South India, Chattisgarh and Jharkhand (Council of Scientific and Industrial Research 1989). “Akarkara” is a rich source of bioactive compounds: the Isobutylamides. The most active biomolecule is an antiseptic alkaloid, (2E, 6Z, 8E) –deca-2, 6, 8-trienoic acid N-isobutyl amide, commonly known as spilanthol (Yasuda et al. 1980). Due to this compound, the plant has immense applications in pharmaceuticals, food, health and body care products. In addition, the flower head of the plant is used against toothache, infections of gums and throat, paralysis of tongue, inflammation of jaw, psoriasis and dental caries. Spilanthol has been found harmless to majority of vertebrates and lethal to invertebrates (Watt and Brayer-Brandwijk 1962). Spilanthes extract is also effective against malarial parasites, specifically malarial spirochetes, either as a prophylactive or as a treatment for malarial paroxysms (Richard 1996). Besides, it is also known to possess antimicrobial (Prasad and Seenaya 2000), larvicidal (Saraf and Dixit 2002; Pandey et al. 2007) and insecticidal properties (Borges-Del-Castillo et al. 1984). This genus is also known for its anti-inflammatory and immune stimulating properties.
Conventional method of propagation of this species is through seeds, but viability of seeds is lost within a short period of time. Previous reports on this medicinal plant taxa regarding its conventional propagation reveals two limiting factors, viz. dependence upon season and slow germination rates thereby imparting hindrance in its commercial production (Singh and Chaturvedi 2010; Yadav and Singh 2011). Due to its multifold uses, the plant is being overexploited by the local population as well as pharmaceutical companies. It has therefore, become imperative to develop a protocol for mass propagation through tissue culture for large scale chemical extraction. Besides, genetic stability of micropropagated plantlets is yet another more important parameter for conservation of elite genotype (Haisel et al. 2001). Earlier reports on in vitro regeneration of Spilanthes were carried out through direct or indirect organogenesis in Spilanthes mauritiana (Bais et al. 2002) and Spilanthes acmella (Saritha et al. 2002; Haw and Keng 2003; Saritha and Naidu 2008; Pandey and Agrawal 2009; Singh et al. 2009a, b; Singh and Chaturvedi 2010). Though few reports (Senthilkumar et al. 2007; Amudha and Shanthi 2011; Devi et al. 2012) are available on regeneration of S. calva, they pertain to organogenesis through node or leaf derived calluses. Recently, Devi et al. 2012 have published paper on micropropagation through encapsulation of nodal explants which were derived from in vitro shoots. However, none of the above mentioned papers mentioned the fidelity of the regenerants.
Molecular techniques are at present powerful and valuable tools for assessing the genetic stability among in vitro micropropagated plants. Random amplified polymorphic DNA (RAPD) (Williams et al. 1990) and inter simple sequence repeat (ISSR) (Zietkiewicz et al. 1994) are polymerase chain reaction (PCR) based techniques widely used for the determination of the genetic fidelity of in vitro regenerated plantlets. Both RAPD and ISSR requires no prior sequence information, uses small amount of DNA and are easy to use, thus considered to be suitable techniques for assessment of genetic fidelity of in vitro regenerated plantlets. Present investigations, therefore have been carried out to develop efficient protocol for rapid clonal multiplication of S. calva DC. through in vivo nodal explants and establishment of genetic fidelity amongst the micropropagated plants employing RAPD and ISSR markers.
Materials and methods
Research material
Comprises nodal explants excised from field grown elite plant of Spilanthes calva DC. (Fig. 1a). Such plants were raised through seeds (procured from Jharkhand, India) in the Botanical Garden, Delhi University and the elite plant was selected on the basis of the optimum level of bioactive compound present (data not shown here). The nodal explants of the micro shoots raised in the axils of nodes of the aforesaid elite plant were employed for conducting different experiments.
Direct organogenesis through nodal explants of Spilanthes calva DC. after 6 weeks of culture (a) Field raised mature plant (4-month-old) of Spilanthes calva DC. (inset shows nodal explant at the time of inoculation). b Single axillary shoot after 6 weeks of culture on MS basal medium (c) Differentiation of multiple shoots from nodal explants within 6 weeks of culture on MS + 10 μM BA (d) Culture showing stunted shoots and formation of callus at the basal end of the nodal explant on MS + 20 μM BA (e) Excised in vitro shoot inducing root on MS (1/2) + 0.1 μM IBA (f) Tissue culture-derived plant acclimatized to soil in glass house (g) Three month old tissue culture raised plants in garden
Surface sterilization of explants
The excised nodal explants of S. calva were immediately dipped in freshly prepared 1 % (w/v) citric acid (SRL, Mumbai, India) solution to minimize browning. Surface sterilization was done by treating the explants with 5 % (v/v) teepol (detergent; Rickett and Colman India Ltd., Kolkata, India) and 0.5 % (w/v) bavistin solution (a systemic fungicide; BASF India Ltd., Mumbai, India) each for 15 mins, followed by 70 % (v/v) ethanol for 5 min, freshly prepared 0.1 % HgCl2 (SRL, Mumbai, India) and 150 mg/l cefotaxime solution for 5 min each with continuous shaking under a laminar flow cabinet. The nodal explants were finally washed four or five times with sterile distilled water on a laminar flow cabinet prior to implantation in semisolid media.
Culture medium and growth conditions
The MS (Murashige and Skoog 1962) basal medium was supplemented with N6-benzyladenine (BA), N6-(2-isopentenyl) adenine (2iP) and 6-furfuryl aminopurine (Kn) at different concentrations (0, 1, 5, 10, 15, 20 μM). For rhizogenesis, half strength MS (Murashige and Skoog 1962) basal medium with α-naphthalene acetic acid (NAA), indole-3-butyric acid (IBA) and indole-3-acetic-acid (IAA) at different concentrations (0, 0.1, 5, 10, 15, 20 μM) were employed. All plant regulators were obtained from Sigma Aldrich (USA). Salts and other chemicals were obtained from Qualigens, Glaxo and SRL, Mumbai (India). As carbon source, 3 % (w/v) sucrose (DCM, Daurala, India) was added to the media and the pH was adjusted to 5.8 using 0.1 N NaOH or 0.1 N HCl. Approximately, 20 ml media was dispensed in each 150 × 25 cm test tubes (Borosil, India), plugged with non-absorbent cotton wrapped in two-layered muslin cloth and sterilized by autoclaving at 1.06 kg cm−2 at 121 °C for 15 min. The nodal explants were cultured in test tubes containing semisolid medium in a vertical orientation. Cultures were maintained on continuous light (450–460 μW cm−2) by cool day light emitted from fluorescent incandescent tubes (40 W, Philips, Kolkata, India) of 16 h light followed by 8 h dark period. The cultures were maintained in a culture room at 25 ± 2 °C temperature with a relative humidity of 55 ± 5 %.
Acclimatization of regenerated plantlets
After rhizogenesis, healthy plantlets with well developed roots were removed from medium and were washed under running tap water to remove the adhering medium. They were treated with 1 % bavistin (BASF, Mumbai, India) solution to prevent any fungal infection, before being transferred to plastic pots (5 cm diameter) containing autoclaved soil. Subsequently, acclimatization was achieved by covering the plastic pots with polythene bags to maintain the humidity and the plants were irrigated with one-tenth of major salt solution of MS medium. After 1 week, 3–5 holes were made in the polythene bags and plants were irrigated after every 4 days. The potted plants were maintained inside the culture room. After 45 days, the plantlets were transplanted to earthen pots (25 cm diameter) containing garden soil and kept under shade in a net house for another 2 weeks before being transferred to field under full sun for developing into mature plants.
DNA isolation and RAPD and ISSR fingerprinting
For genetic fidelity studies, 11 hardened regenerated plants were chosen randomly amongst a population of 120 mature field transferred regenerated plants along with the elite donor mother plant of S. calva. Total genomic DNA of donor mother plant and the in vitro raised clones of S. calva were extracted from young leaf tissue (2 g) by using the cetyl trimethyl ammonium bromide (CTAB) method as described by Saghai-Maroof et al. (1984). A set of 20 decamer RAPD (Sigma Aldrich, USA) and 10 ISSR (Sigma Aldrich, USA) primers were screened for their repeatable amplification with the DNA from the aforesaid plants including the elite mother plant to assess the genetic stability of the regenerants. 10 RAPD and 7 ISSR primers were finally selected for the analysis on the basis of their amplification products for clear and scorable banding patterns. RAPD amplification was carried out in a total volume of 25 μl containing 20 ng of genomic DNA, 2.5 μl of 10X PCR buffer containing 15 mM MgCl2, 0.2 mM dNTPs, 1 unit Taq polymerase (Bangalore Genei, India) and 20 ng RAPD primer (Sigma Aldrich, USA). Amplification conditions using RAPD primers were performed as initial DNA denaturation at 94 °C for 4 min, followed by 45 cycles of 1 min denaturation at 94 °C, 1 min annealing at 36 °C and 2 min of extension at 72 °C with a final extension at 72 °C for 7 min. ISSR amplifications were performed in a total volume of 25 μl containing 20 ng of genomic DNA, 2.5 μl of 10X PCR buffer containing 15 mM MgCl2, 0.2 mM dNTPs, 1 unit Taq polymerase (Bangalore Genei, India) and 20 ng ISSR primer (Sigma Aldrich, USA). Amplification conditions using ISSR primers were performed as initial DNA denaturation at 94 °C for 4 min, followed by 45 cycles of 1 min denaturation at 94 °C, 1 min annealing at 40 °C and 2 min of extension at 72 °C with a final extension at 72 °C for 7 min. Amplification of DNA was performed in a 2720 Thermal cycler (Applied Biosystems, USA). The amplified DNA fragments were separated on a 1.2 % (w/v) agarose gel using 0.5X TBE buffer and stained with ethidium bromide (0.5 μg/ml). The size of the amplicons was estimated by comparing with 1 Kb DNA ladder (Bangalore Genei, India). Gels were visualized and photographed by an Alpha Imager Gel Documentation System (Alpha Innotech Corporation, USA). All the reactions were repeated twice and only the consistent reproducible bands were considered.
Recording of data and statistical analysis
The morphogenic response (caulogenesis) of explants was evaluated after 6 weeks of culture in terms of (1) percentage of explants producing single shoot or multiple shoots, (2) average shoot number per explant and (3) average shoot length per explant. For rhizogenesis, following parameters were considered: (1) percentage of shoots developing roots, (2) average number of roots per shoot and (3) average root length. For in vitro regeneration, the average number of shoots per explant, the average shoot length, the average number of roots per shoot and the average root length has been represented as mean values along with standard error (mean ± SE). The mean values were calculated on the basis of a minimum of 24 replicates in each experiment and repeated at least for once or twice. The data expressed as mean ± SE have been statistically analyzed using ANOVA (Analysis of Variance) through SPSS (Statistical Package for Social Sciences) version 16.0. The differences between means were tested for significance by Duncan’s multiple range test (DMRT) at p = 0.05.
For genetic fidelity studies, amplified DNA bands were recorded with all the selected RAPD and ISSR primers and only clear and scorable bands at a particular position were considered. Each band was treated as a marker and scoring of bands was done on the basis of their presence (‘1’) or absence (‘0’) in the gel. Intensity of the band was not considered while scoring. NTSYSpc (Numerical Taxonomy and Multivariate Analysis System) Version 2.1 software (Rohlf 2000) was used to perform the distance matrix and cluster analysis of the complete data set of the two markers employed. Genetic association amongst the different individuals was measured by the Jaccard’s similarity coefficient (Jaccard 1908) with the SIMQUAL (Similarity for qualitative data program in NTSYS) module of NTSYS-pc software. The similarity matrix was subjected to cluster analysis of UPGMA (unweighted pair group method with arithmetic mean) and a dendrogram was generated by using the SAHN (sequential, agglomerative hierarchical and nested clustering) module of NTSYS-pc.
Results
Shoot induction and elongation
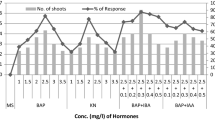
In order to establish an efficient in vitro micropropagation protocol for commercial exploitation of this important taxon, nodal explants of S. calva were inoculated on MS medium supplemented with various concentrations (0, 1, 5, 10 and 20 μM) of cytokinins (BA, KN and 2iP). The nodal segments cultured on growth regulator free MS medium showed minimum sign of bud break even after 2 weeks. The average number of shoots induced on MS basal medium was 0.083 with an average shoot length of 0.68 cm after 6 weeks of culture (Table 1).
However, addition of cytokinin was essential for differentiation of multiple shoots from the nodal explants. Of the three cytokinins tried, BA was most effective over the other two for induction of multiple shoots. The in vivo nodal segments responded by initial enlargement of dormant axillary buds followed by bud break within a week and multiple shoot induction and proliferation within 2 weeks of culture on BA containing media. 10 μM BA was optimum in inducing 91.6 % morphogenic cultures with an average of 7.12 shoots per explant having an average shoot length of 6.18 cm after 6 weeks of culture (Table 1, Fig. 1c). BA at 1 or 5 μM displayed poor morphogenic response both in terms of average number of shoots and average shoot length (Table 1; Fig. 1b). No significant differences on shoot growth and proliferation were observed after subculture of the explants at an interval of 3 weeks. At higher concentrations (15 or 20 μM) of BA, induction of multiple shoots was comparatively low and average shoot length too decreased (Table 1; Fig. 1d). In addition, formation of greenish brown callus was also observed at the base of explants at higher concentrations of BA. Contrary to BA, all the tried concentrations of Kn or 2iP revealed poor morphogenic response in terms of percentage responding cultures inducing multiple shoots as well as average number of induced shoots and shoot length (Table 1).
Root induction
Among the three different types of auxins employed for root induction on in vitro excised shoots of S. calva, IBA was the most effective. A maximum of 100 % shoots induced an average of 12.54 roots with an average root length of 8.72 cm after 3 weeks on half strength MS medium augmented with 0.1 μM IBA (Table 2). The roots were induced directly from the shoot base without callus formation at this concentration. On half strength MS basal medium, an average of 5.41 roots per shoot with an average root length of 7.36 cm was recorded only in 33.3 % of the cultures (Table 2; Fig. 1e). However, at higher concentrations (5, 10 and 15 μM) of IBA, though the number of roots were high, but a decline in root length was seen with concomitant callus formation (Table 2). Compared to IBA, poor rooting response was observed with all the tried concentrations of NAA and IAA (Table 2). The tissue culture derived plantlets (Fig. 1f) were acclimatized in field with 95 % survival. Such micropropagated plants were found to be phenotypically similar to the mother plant (Fig. 1g).
RAPD analysis
In order to test the genetic fidelity of micropropagated plants of S. calva, PCR based fingerprinting technique of RAPD was employed in 11 randomly chosen micropropagated plants along with the mother plant. A total of 20 arbitrary decamers (RAPD primers) were used for initial screening out of which 10 produced a total of 47 clear, distinct and reproducible scorable bands with an average of 4.7 bands per primer (range 2–8) with size ranging from 100 bp to 1,100 bp (Table 3). All the banding profile from micropropagated plants were monomorphic and similar to those of mother plant except three primers, viz. VAH-12, VAH-15 and VAH-20 which displayed polymorphic bands in two of the regenerants (Table 3). Four RAPD primers (VAH-04, VAH-06, VAH-07 and VAH-19) displayed prominent monomorphic bands amongst the in vitro derived regenerants and the elite donor mother plant (Fig. 2).
ISSR analysis
In case of ISSR analysis, 10 UBC primers were initially screened, out of which 7 primers developed 24 distinct and scorable bands with an average of 3.4 bands per ISSR primer with size ranging from 100 bp to 1,000 bp (Table 4). All the bands displayed monomorphic patterns across the 11 regenerants and the mother plant analyzed (Table 4). Two ISSR primers UBC-801 and UBC-842 revealed the optimum monomorphic banding patterns (Fig. 3a and b). However, in case of ISSR, two polymorphic bands were also recorded with two of the primers, viz. UBC-819 and UBC-871 (Table 4).
Dendrogram analysis
The similarity values based on Jaccard’s similarity coefficient between the regenerants and the donor mother plant calculated through a combination of data of the aforesaid two markers varied from 0.967 to 1.000 (Table 5). The dendrogram generated through UPGMA analysis revealed 98 % similarity amongst the regenerants and the donor mother plant, dividing the 12 accessions of plants into two groups (Fig. 4).
Discussion
Development of protocol for direct shoot regeneration through tissue culture from field grown plants is one of the important requisition toward clonal mass multiplication for commercial/pharmaceutical application. Establishment of genetic fidelity among the regenerants using various molecular markers is yet another significant parameter toward this direction. In the present investigation, it is clearly established that the plantlets were regenerated directly from nodal explants of field raised elite plant of S. calva DC. These regenerants have been further revalidated as genetically uniform using two different markers namely, RAPD and ISSR. Of the three cytokinins, viz. BA, Kn and 2iP tried, BA proved to be the best both in terms of percentage morphogenic cultures as well as average shoot number per explant. Significance of BA in inducing multiple shoots has been already reported in several taxa e.g., Simmondsia chinensis (Link) Schneider (Agrawal et al. 2002), Cassia angustifolia Vahl (Agrawal and Sardar 2003), Hollarhena antidysenterica (L.) Wall (Kumar et al. 2005) and Spilanthes acmella L. (Pandey and Agrawal 2009). The promotory effect of BA over other cytokinins could be due to its easy permeability, increased affinity for active cell uptake, less resistance to the enzyme cytokinin oxidase, or receptor abundance in its perception apparatus which interacts with the coupling elements in the signal transduction chain (Burch and Stuchbury 1987). Moreover, BA if added exogenously shortens the duration of S-phase of cell division through recruitment of latent origin of DNA replication both in vitro or in vivo (Francis and Sorell 2001).
For induction of rhizogenesis in the excised in vitro raised shoots, IBA yielded the optimum response in terms of percentage of root induction and average number of roots per shoot. NAA and IAA when tried induced callusing prior to rooting. Indole-3-butyric acid (IBA) is the most widely used auxin to stimulate the rooting process in shoots because of its high ability to promote root initiation (Weisman et al. 1988), its weak toxicity and great stability in comparison to naphthalene acetic acid and indole-3-acetic acid (Zolman et al. 2000). Exogenous application of IBA under in vitro conditions may induce changes in enzyme activities (peroxidase and IAA oxidase) and in their effectors contents (phenolics) allowing the establishment of the favourable endogenous hormone balance for root initiation in excised shoots (Qaddoury and Amssa 2004). The effectiveness of IBA for rhizogenesis has already been mentioned in Spilanthes acmella (Pandey and Agrawal 2009; Yadav and Singh 2010).
Furthermore, establishment of the genetic fidelity amongst the micropropagated plants employing two types of PCR based marker is yet another work which has been done for the first time here. The banding profiles obtained amongst micropropagated plants shown by RAPD and ISSR markers revealed almost complete uniformity indicating a high level of genetic fidelity amongst the regenerants. The UPGMA analysis through a combination of data from the aforesaid two markers also revealed a high percentage (98 %) of similarity amongst the regenerants and donor mother plant.
In contrast to the numerous reports on the absence of genetic variability amongst the micropropagated plants and donor mother plants e.g., in Asparagus officinalis L. (Raimondi et al. 2001), Chlorophytum arundinaceum Baker (Latto et al. 2006) and Simmondsia chinensis (Link) Schneider (Kumar et al. 2011), there are also some reports of polymorphism in the bands while analyzing for genetic fidelity. In our current investigation, 2 % variation was observed amongst the regenerants and donor mother plant. These small genetic variation in DNA may be attributed to naturally occurring variation or due to the accumulation of mutation by various factors such as in vitro process and its duration, auxin to cytokinin ratio (hormonal balance), in vitro stress, induced by added biochemicals, or other nutritional conditions, all of which are known to induce somaclonal variation (Devarumath et al. 2002). Genetic variation induced by such genetic and epigenetic mechanism can be reflected in the banding profile developed by different marker system (Phillips et al. 1994). This further supports the need for testing micropropagated plantlets well before their actual planting in the field and confirming the reliability of the micropropagation protocol for large scale exploitation. Somaclonal variations were reported in Codonopsis lanceolate Benth et Hook (15.7 %; Guo et al. 2006) and Dactyospermum ovalifolium Wight. (3.92 %; Chandrika et al. 2008) using PCR based RAPD and ISSR markers. During present study, we adopted the use of two PCR-based techniques, RAPD and ISSR markers for evaluation of genetic fidelity amongst the regenerants of S. calva which revealed a very low (2 %) variation amongst the regenerants. Because of their simplicity and cost effectiveness, these methods have been chosen. In addition, the use of more than one DNA fingerprinting technique was reported to be advantageous in evaluating genetic variability (Palombi and Damiano 2002).
Abbreviations
- 2iP:
-
N6-(2-isopentenyl) adenine
- BA:
-
N6-benzyladenine
- CTAB:
-
Cetyl trimethyl ammonium bromide
- DMRT:
-
Duncan’s multiple range test
- IAA:
-
Indole-3-acetic acid
- IBA:
-
Indole-3-butyric acid
- ISSR:
-
Inter simple sequence repeat
- Kn:
-
6-furfuryl aminopurine
- MS:
-
Murashige and Skoog
- NAA:
-
α-naphthalene acetic acid
- NTSYS:
-
Numerical taxonomy and multivariate analysis system
- RAPD:
-
Random amplified polymorphic DNA
- SAHN:
-
Sequential agglomerative hierarchical and nested clustering method program in NTSYS
- SIMQUAL:
-
Similarity for qualitative data program in NTSYS
- SPSS:
-
Statistical package for social sciences
- UPGMA:
-
Unweighted pair group method with arithmetic mean
References
Agrawal V, Sardar P (2003) In vitro organogenesis and histomorphological investigations in senna (Cassia angustifolia) a medicinally valuable shrub. Physiol Mol Biol Plants 9:1–10
Agrawal V, Prakash S, Gupta SC (2002) Effective protocol for in vitro shoot production through nodal explants of Simmondsia chinensis. Biol Plant 45:449–453
Amudha P, Shanthi P (2011) Indirect organogenesis and in vitro layering of Acmella calva (DC.) R.K. Jansen. from various explants. J Agric Tech 7:637–648
Bais HP, Green JB, Walker TS, Okemo PO, Vivanco JM (2002) In vitro propagation of Spilanthes mauritiana DC., an endangered medicinal herb, through axillary bud cultures. In Vitro Cell Dev Biol Plant 38:598–601
Borges-Del-Castillo J, Vazquez-Bueno P, Secundino-Lucas M, Martinez-Martir AI, Joseph-Nathan P (1984) The N-2-Phenylethylcinnamide from Spilanthes ocymifolia. Phytochem 23:2671–2672
Burch LR, Stuchbury T (1987) Activity and distribution of enzymes that interconvert purine bases, ribosides and ribotides in the tomato plant and possible implications in cytokinin metabolism. Physiol Plant 69:283–288
Chandrika M, Thoyajaksha, Ravishankar Rai V, Ramachandra Kin K (2008) Assessment of genetic stability of in vitro grown Dictyospermum ovalifolium. Biol Plant 52:735–739
Council of Scientific Industrial Research (1989) The Wealth of India: a dictionary of Indian raw materials and industrial products, CSIR, New Delhi 10:11–12
Devarumath R, Nandy S, Rani V, Matimuthu S, Muraleedharan N, Raina S (2002) RAPD, ISSR and RFLP fingerprints as useful markers to evaluate genetic integrity of micropropagated plants of three diploid and triploid elite tea clones representing Camellia sinensis (China type) and C. assamica ssp. assamica (Assam-India type). Plant Cell Rep 21:166–173
Devi BC, Mahendran P, Wesley PS, Shibu BS, Vetrivel P, Malligavathi D (2012) Micropropagation of Acmella calva using encapsulated in vitro nodal explants. J Trop Med Plants 13:33–41
Francis D, Sorell DA (2001) The interphase between the cell cycle and plant growth regulators, a mini-review. Plant Growth Reg 33:1–12
Guo WL, Gong L, Ding ZF, Li YD, Li FX, Zhao SP, Liu B (2006) Genomic instability in phenotypically normal regenerants of medicinal plant Codonopsis lanceolate Benth et Hook as revealed by ISSR and RAPD markers. Plant Cell Rep 25:896–906
Haisel D, Hofman P, Vagneri M, Lipavska H, Ticha L, Schafer C, Capkova V (2001) Ex vitro phenotype stability is affected by in vitro culture. Biol Plant 44:321–324
Haw AB, Keng CL (2003) Micropropagation of Spilanthes acmella L. a bio-insecticide plant, through proliferation of multiple shoots. J Appl Hort 5:65–68
Jaccard P (1908) Nouvelles recherches sur Ia distribution Rorale. Bull Soc Vaudoise Sci Nat 44:223–270
Jansen RK (1985) Systematics of Acmella (Asterceae: Heliantheae). Syst Bot Mongr 8:115
Kumar R, Sharma K, Agrawal V (2005) In vitro clonal propagation of Holarrhena antidysentrica L. wall through nodal explants from mature trees. In Vitro Cell Dev Biol Plant 41:137–144
Kumar S, Mangal M, Dhawan AK, Singh N (2011) Assessment of genetic fidelity of micropropagated plants of Simmondsia chinensis (Link) Schneider using RAPD and ISSR markers. Acta Physiol Plant 33:2541–2545
Latto SK, Bamotra S, Saprudhar R, Khan S, Dhar AK (2006) Rapid plant regeneration and analysis of genetic fidelity of in vitro derived plants of Chlorophytum arundinaceum Baker–an endangered medicinal herb. Plant Cell Rep 25:499–506
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Palombi MA, Damiano C (2002) Comparison between RAPD and SSR molecular markers in detecting genetic variation in kiwifruit (Actinidia deliciosa A. Chev). Plant Cell Rep 20:1061–1066
Pandey V, Agrawal V (2009) Efficient micropropagation protocol of Spilanthes acmella L. possessing strong antimalarial activity. In Vitro Cell Dev Biol Plant 45:491–499
Pandey V, Agrawal V, Raghavendra K, Dash AP (2007) Strong larvicidal activity of three species of Spilanthes (Akarkara) against malaria (Anopheles stephensi Liston, Anopheles culicifacies, species C) and filarial vector (Culex quinquefasciatus Say). Parasitol Res 102:171–174
Phillips RL, Kaeppler SM, Olhoft P (1994) Genetic instability of plant tissue cultures: breakdown of normal controls. Proc Natl Acad Sci 91:5222–5226
Prasad MM, Seenaya G (2000) Effects of spices on growth of red halophilic cocci isolated from salt cured fish and solar salt. Food Res Nutr 33:793–798
Qaddoury A, Amssa M (2004) Effect of exogenous indole butyric acid on root formation and peroxidase and indole-3-acetic acid oxidase activities and phenolic contents in date Palm offshoots. Bot Bull Acad Sin 45:127–131
Raimondi JP, Camadro EL, Masuelli RW (2001) Assessment of somaclonal variation in Asparagus by RAPD fingerprinting and cytogenetic analyses. Sci Hortic 90:19–29
Richard (1996) A Spilanthes. http://www.chatlink.com/herbseed/Spilanthes
Rohlf FJ (2000) NTSYS-pc: numerical taxonomy and multivariate analysis system, version 2.1. Exeter Software, Setanket, New York
Saghai-Maroof MA, Soliman KM, Jorgensen RA, Allard RW (1984) Ribosomal DNA spacer-length polymorphisms in barley: Mendelian inheritance, chromosomal location and population dynamics. Proc Natl Acad Sci USA 81:8014–8018
Saraf DK, Dixit VK (2002) Spilanthes acmella Murr. Study on its extract spilanthol as larvicidal compound. Asian J Exp Sci 16:9–19
Saritha KV, Naidu CV (2008) Direct shoot regeneration from leaf explants of Spilanthes acmella. Biol Plant 52:334–338
Saritha KV, Prakash E, Ramamurthy N, Naidu CV (2002) Micropropagation of Spilanthes acmella Murr. Biol Plant 45:581–584
Senthilkumar P, Paulsamy S, Vijayakumar KK, Kalimuthu K (2007) Regeneration the herb Nilgiri shola, Acmella calva L. from leaf derived callus. Plant Tiss Cult Biotech 17:109–114
Singh M, Chaturvedi R (2010) Optimization of Spilanthes acmella L. cultivation by in vitro nodal segment culture. Acta Hortic 865:109–114
Singh SK, Rai MK, Asthana P, Pandey S, Jaiswal VS, Jaiswal U (2009a) Plant regeneration from alginate-encapsulated shoot tips of Spilanthes acmella (L.) Murr. a medicinally important and herbal pesticidal plant species. Acta Physiol Plant 31:649–653
Singh SK, Rai MK, Asthana P, Sahoo L (2009b) An improved micropropagation of Spilanthes acmella L. through transverse thin cell layer culture. Acta Physiol Plant 31:693–698
Watt PM, Brayer-Brandwijk MC (1962) The medicinal and poisonous plants of Southern and Eastern Africa, 2nd edn. E and S Livingstone Edinburgh
Weisman Z, Riov J, Epstein E (1988) Comparison of movement and metabolism of indole-3-acetic acid in mung bean cuttings. Physiol Plant 74:556–560
Williams JGK, Rubelik AR, Livak KJ, Rafalski A, Tingey SV (1990) DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res 18:6531–6535
Yadav K, Singh N (2010) Micropropagation of Spilanthes acmella Murr.—an important medicinal plant. Nature Sci 8:5–11
Yadav K, Singh N (2011) In vitro flowering of shoots regenerated from cultured nodal explants of Spilanthes acmella Murr.—an ornamental cum medicinal herb. An Univ Oradea Fasc Biol 18:66–70
Yasuda I, Takeya K, Itokawa H (1980) The geometric structure of spilanthol[J]. Chem Pharm Bull 28:2251–2253
Zietkiewicz E, Rafalski A, Labuda D (1994) Genome fingerprinting by simple sequence repeat (SSR) anchored polymerase chain rection amplification. Genomics 20:176–183
Zolman BK, Yoder A, Bartel B (2000) Genetic analysis of indole-3-butyric acid responses in Arabidopsis thaliana reveals four mutant classes. Genetics 156:1323–1337
Acknowledgements
Authors are grateful to the Indian Council of Medical Research, New Delhi for providing financial assistance to Veena Agrawal in the form of a Major Research Project and University Grants Commission, Delhi and University of Delhi, Delhi for sanctioning funds for Research and Development.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Razaq, M., Heikrujam, M., Chetri, S.K. et al. In vitro clonal propagation and genetic fidelity of the regenerants of Spilanthes calva DC. using RAPD and ISSR marker. Physiol Mol Biol Plants 19, 251–260 (2013). https://doi.org/10.1007/s12298-012-0152-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-012-0152-4