Abstract
A simple, efficient, reproducible and comparatively genotype-independent in vitro plant regeneration protocol was developed for ten commercial Indian bread wheat cultivars using mature embryos as the explants. Three different auxins and five different combinations of growth regulators in a modified Murashige and Skoog’s basal medium were assessed for their effect on callus induction and plant regeneration, respectively, in a high yielding and widely grown cultivar, PBW-343. The optimized conditions were further evaluated with nine other commercial cultivars. A simple novel approach of physical isolation of regenerable calli from non regenerable structures during the early callus phase was used to improve plant regeneration. Callus induced on 2.0 mg-1 2,4-dichlorophenoxyacetic acid (2,4-D) showed a regeneration frequency of 86 % with 7.5 shoots per explants on hormone-free medium. A considerable improvement in the regeneration frequency (up to 97 %) and the average of shoots (19 shoots per explants) was obtained with a combination of thidiazuron (TDZ) and 2,4-D.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wheat (Triticum aestivum L.) is one of the most important staple food as well as feed crops in many parts of the world. Though India is the world’s second largest producer of wheat, with an overall production of 78 mMT (FAOSTAT 2008), its production, in recent years, is declining due to climate change which is intensifying various biotic as well as abiotic stresses (Joshi et al. 2007). Hence, there is an urgent need to produce wheat cultivars that are adaptable to diverse biotic and abiotic challenges. Traditional and newly discovered marker assisted selection (MAS) are unlikely to bring improvement in wheat breeding, because of the limited gene-pool (Vasil 2007). Thus, wheat improvement requires introduction of novel as well as alien genes by genetic transformation. However, genetic transformation of this cereal has been difficult and challenging due to its recalcitrant nature for in-vitro regeneration (Bhalla 2006; Ganeshan et al. 2006; Vasil 2007). Immature tissues like immature inflorescence and embryos are the most preferred explants for genetic transformation because of their high regeneration capacity (Jones 2005; Chauhan et al. 2007). However, use of immature tissues demands extra labour and expense for maintaining the donor plants. Additionally, their most suitable stage for culture also limits their use (Repellin et al. 2001). Mature embryos or tissues derived from them, has been used as an effective alternative to immature embryos because of their year around availability and easy isolation (Chugh and Khurana 2003; Patnaik et al. 2006; Ding et al. 2009). Furthermore, the physiological state of mature embryos shows minimal variability (Yu et al. 2008).
Zhou and Lee (1984) were the first to achieve successful plant regeneration from wheat mature embryos. Subsequently, several workers have used various techniques for explant preparation, such as endosperm-supported embryo (Ozgen et al. 1998; Filippov et al. 2006), thin mature embryo fragments (Delporte et al. 2001; Mendoza and Kaeppler 2002) and longitudinally bisected mature embryos (Yu et al. 2008) and pretreatment of embryo with high levels of 2,4-D prior to culture or during culture for callus induction and plant regeneration. Since high levels of 2,4-D cause somaclonal variation in cereal crops, it has been argued to develop protocols with low levels of auxin (Mendoza and Kaeppler 2002). Further, the effect of various factors, e. g., genotype (Ozgen et al. 1996, 1998; Zale et al. 2004), type and concentration of auxin (Mendoza and Kaeppler 2002; Filippov et al. 2006) and media components (Redway et al. 1990; Mendoza and Kaeppler 2002; Greer et al. 2009) either alone or in combinations has been evaluated for callus induction and subsequent regeneration. In spite of these studies, the regeneration has been found to be low (Ozgen et al. 1998; Delporte et al. 2001; Mendoza and Kaeppler 2002) and genotype dependent (Zale et al. 2004; Filippov et al. 2006; Yu et al. 2008). One of the important factors which has not been paid proper attention is the role of non-embryogenic (NE) callus in regeneration from mature embryos (Redway et al. 1990; Delporte et al. 2001; Mendoza and Kaeppler 2002). The different portions of the cereal embryo differ in their capacity to form embryogenic calli. Therefore, it is essential to identify and carefully clear the embryogenic calli from the surrounding non-embryogenic calli at the time of subculture in order to allow embryos to form (Mac Kinnon et al. 1987). The non-embryogenic calli, identified on the basis of their morphology and colour, are usually removed during callus subculture (Redway et al. 1990). However, their distinction on the basis of their morphology and colour is not always clear from those of the aged calli. Further, the colour of calli also changes with the cultivar and the type of auxin used for callus induction. In the present study, a novel approach of physical isolation of regenerable tissues from non-regenerating structures (NRS) formed during early callus induction phase has been used to improve callus induction and regeneration. We report here a highly reproducible, efficient and comparatively less genotype dependent plant regeneration protocol for a high yielding and widely grown bread wheat Indian cultivar, PBW-343 using mature embryo as the explants. The effect of different auxins either alone or in combination with other growth regulators has been optimized for callus induction and plant regeneration. Finally, the applicability of the protocol optimized for the cultivar PBW-343 has also been demonstrated for nine other Indian commercial cultivars.
Materials and methods
Plant material
Seeds of Indian bread wheat cultivars, C-306, WH-711, RAJ-3765, LOK-1, PBW-373, RRJ-19, RAJ-3077, PBW-502, HD-2329 and PBW-343 were procured from the National Bureau Plant Genetic Resources (NBPGR), New Delhi and the Directorate of Wheat Research, Karnal, India. Of these, a high yielding and widely grown cultivar, PBW-343 was used for optimization of tissue culture protocol. Seeds were surface sterilized with 70 % ethanol for 30 sec, followed by 0.2 % (w/v) aqueous mercuric chloride for 10–15 min, and then rinsed four-five times with sterile distilled water. After surface sterilization, seeds were soaked in sterile distilled water for 45 min. The mature embryos were dissected from the caryopsis with a fine scalpel under aseptic conditions and placed scutellum side up with plumule slightly embedded in the callus induction media in 90 mm Petri dishes (Tarson India Ltd., India) (Fig. 1a).
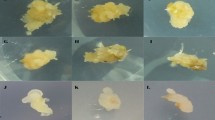
In vitro plant regeneration from mature embryo of wheat cultivar PBW-343 (a-l). Schematic representation of mature embryo culture on callusing medium (a), Identification of non-regenerable structures (NRS) and their removal as marked with thick lines from 14-d-old calli induced on 2,4-D (b), picloram (c) and on dicamba (d) containing media. Regeneration from calli with NRS (e, g, l) and without NRS (f, h, i, j, k). Non-regenerable callus (NC) with rhizogenesis (e) and regenerable callus (f), NC with chlorophyll deposition (g), multiple shoot formation stages (i, j), rooting of the shoots (k), stunted shoot like growth on NC (l), fertile mature plants established in the soil (m)
Callus induction, subculture and differentiation
A modified MS (Murashige and Skoog 1962) basal medium consisting of MS micro-, MS macro-nutrients with NH4NO3 (3,310 mgl−1), MS vitamins, maltose (30 gl−1) and solidified with 0.8 % agar (Himedia, Mumbai, India) was used for callus induction. In a preliminary experiment, the effect of three auxins, 2,4-dichlorophenoxyacetic acid (2,4-D), 3,6-dichloro-2-methoxybenzoic acid (dicamba) and 4-amino-3, 5-trichoropiclinic acid (picloram) at different concentrations (0–4.0 mgl−1) on callus induction was tested. All the auxins at concentrations, 2.0 and 4.0 mgl−1 were found to be good for callus induction. Hence, these concentrations were used for further experiments. Different medium components except growth regulators were dissolved and adjusted to pH 5.8, and autoclaved at 121 °C for 20 min. Plant growth regulators were filter-sterilized and added to autoclaved medium when the temperature was around 40–55 °C. Mature embryos were cultured in dark initially for two weeks and then the structures (named as non-regenerating structures, NRS) which lead to non embryogenic callus formation were identified (as marked with thick lines in Figs. 1b, c and d) and removed with a fine scalpel, and the remaining explants were further sub-cultured for 3 weeks more on the same medium containing the same auxin levels.
Shoot regeneration
For differentiation of callus into shoots, 5 weeks-old calli were transferred to growth hormone-free MS medium or MS medium supplemented with a very low concentration of 2,4-D in combination with zeatin or TDZ (see Table 1) under 16-h photoperiod of cool-fluorescent light of 80 μE m−2 s−1 intensity. Calli were sub-cultured every 2 weeks and the regenerated shoots which could develop roots were transferred to the pots containing soil whereas those without roots were transferred to MS basal medium containing 3.5 μM IAA in the culture tubes (25 × 150 mm) for rooting.
Transplantation and data analysis
Well-developed plantlets were taken out carefully from the culture tubes with the help of a forceps. Their roots were washed in running tap water and they were transplanted in pots containing soil. Each pot was covered with a polythene bag to maintain high humidity initially for the first few days. Subsequently the humidity was reduced gradually by making holes in the polythene bags to harden the plants. The hardened plants were grown under standard green house conditions. For callusing and regeneration, each experiment contained 20–25 explants per Petri-dish, three replicates were maintained for each treatment and each experiment was repeated thrice. The data regarding the number of explants forming calli, the number of calli forming shoots and the number of shoots per callus were recorded. The data were subjected to analysis of variance and significant treatment differences were selected by Newman-Keul’s multiple range tests.
Results and discussion
Modified MS basal medium containing ammonium nitrate (3,310 mgl−1) in double amounts and maltose (30 gl−1) as a carbon source was used for callus induction and plant regeneration. However, in most of the earlier studies, the basic nutrient medium was supplemented with a variety of adjuvant such as coconut water (Mathias and Simpson 1986), amino acid (glutamine) and organic nitrogen source (casein hydrolysate, CH) (Mendoza and Kaeppler 2002), glutamine, CH and proline (Ganeshan et al. 2006), CH and proline (Chauhan et al. 2007), glutamine, asparagine and CH (Bi and Wang 2008) and aspartic acid, glutamine, proline, tryptophan and CH (Yu et al. 2008). Modification of ammonium nitrate concentration in media has been shown to promote callus induction (Constabel and Shyluk 1994), somatic embryogenesis in cereals (Grimes and Hodges 1990; Kothari et al. 2004; Greer et al. 2009) and genetic transformation (Greer et al. 2009; Boyko et al. 2009). The beneficial effect of NH4NO3 on callus induction and regeneration has been probably by altering the sensitivity of the explants to auxin levels (Greer et al. 2009). Similarly, maltose has also been found to enhance callus growth and regeneration in wheat (Mendoza and Kaeppler 2002).
The effect of three different auxins (2,4-D, dicamba and picloram) on callus induction and plant regeneration was evaluated to identify the most effective auxin type and the concentration (Table 2). All the auxins, at the concentrations 2.0 and 4.0 mgl−1, induced callus in 100 % of the cultures. Callus initiation was observed after 2–3 days of culture with the swelling of the embryos. After 14 days, callus diameter was about 4–5 mm. With PBW-343, the callus induced on different auxins varied with regard to color, the site of origin and proliferation. Callus developed on dicamba containing media was white while those on picloram and 2,4-D media were yellowish in color. The radicle or coleorrhizal part of the explants showed maximum proliferation followed by mesocotyl or epicotyl on dicamba and picloram media. Radicular end of embryo turned into a rod- like structure, at the top of which hair- like growth could be seen (Figs. 1c and d) within a week time. On 2,4-D containing media, extension of the plumular end or coleoptile was the most common phenomenon (Fig. 1b). The structures developed at plumular or radicular end of embryo were named as non-regenerating structures (NRS) which showed rapid proliferation than other parts of the explant on sub-culture. The mass of non-regenerating structures (NRS) increased with increase in the concentration of the auxins. Overall, dicamba and picloram media showed high callus proliferation rate than on 2,4-D (data not shown).
The callus masses with or without NRS (removed as marked with thick lines in Figs. 1b, c and d) were subcultured further for three weeks more on the media containing same levels of auxin. The isolated NRS, and the calli obtained with or without NRS were transferred on hormone free-MS medium (RM) for regeneration. No regeneration was observed from NRS whereas calli propagated with NRS showed rhizogenic growth (Fig. 1e), chlorophyll deposition (Fig.1g) and emergence of few shoots (Fig. 1l) which could not be elongated even on prolonged subculture. The calli obtained without NRS were turned out to be compact, nodular (Fig. 1f) and developed green spots (Fig. 1h, termed as regenerable calli) which subsequently developed into shoots (Figs. 1i, j). Some of these shoots developed roots even on RM while others which could not develop roots on RM (Fig. 1j) were transferred on MS basal medium containing 3.5 μM IAA for rooting (Fig. 1k). Regenerable calli obtained on three auxins varied in their response for regeneration (Table 2). Increase in concentration of 2,4-D and picloram significantly decreased the mean number of shoots. 2,4-D at high concentration induced hair-like growth and necrotic calli after 4- to 5 days on regeneration medium. Increase in dicamba concentration non-significantly increased the frequency and the mean number of shoots. However, dicamba resulted in better regeneration frequency and the number of shoots than picloram. Overall, 2,4-D at 2.0 mgl−1 produced the highest regeneration frequency (86 %) and the mean number of shoots (7.5) per embryo followed by dicamba (6.9 shoots in 79 % cultures) and then picloram (4.4 shoots in 63 % cultures). Our results are consistent with others, 2,4-D at 2.0 mgl−1 alone is best for callus production and regeneration (Yasmin et al. 2001; Chauhan et al. 2007) while at concentrations higher than 2.0 mgl−1 proved detrimental for regeneration (Khurana et al. 2002).
Callus induced on 2,4-D (2.0 mgl−1) medium from mature embryo on transfer to MS basal medium containing different growth regulator(s) either alone or in combination differed significantly in terms of the regeneration frequency and the number of shoots per callus (Table 3). Callus induced on 2,4-D (2.0 mgl−1) resulted in good regeneration on hormone-free medium (RM). However, regeneration of such calli on medium containing 2,4-D (0.1 mgl−1) and zeatin (1.0 mgl−1) (RM1) did not differ significantly than those on RM. Addition of TDZ alone at low concentration (0.2 mgl−1) in MS medium during regeneration phase (RM 3) drastically decreased the regeneration frequency as well as the number of shoots over those on RM. This is in contrast to Chauhan et al. (2007) where lower concentration of TDZ during the regeneration phase proved more potent. However, TDZ in combination with a cytokinin (zeatin) (RM2) or an auxin (2,4-D) (RM4) significantly improved the regeneration potential over RM and RM3. TDZ in combination with auxin (2,4-D) (RM4) induced the highest regeneration frequency (97.3 %) with an average of 10 shoots per mature embryo. Similar beneficial effect of TDZ in combination of 2,4-D was observed on shoot regeneration from wheat immature embryo-derived callus by Biesaga-Kościelniak et al. (2010). Our results also corroborated the earlier work that TDZ should be used in combination with other growth regulators during the regeneration phase in mature embryo culture (Chauhan et al. 2007).
All the nine cultivars differed significantly in their ability to produce calli, regeneration capacity and the number of shoots regenerated (Table 4). However, all the cultivars responded beyond the regeneration frequencies on the medium optimized with PBW-343. The callus induction was highest in cultivars PBW-343, RRJ-19 and HD-2329 whereas the average number of the regenerated shoots was greatest from cultivars C-306, WH-711 and RAJ-3077. Thus, the callus induction was not significantly correlated with the average number of shoots regenerated suggesting independent control of callus formation and plant regeneration. The cultivar C-306 showed the highest proportion of shoot regeneration (19 shoots) followed by WH-711 with 18, and RAJ-3077 with 16 shoots. The regeneration response obtained in the present study is much better than most of the earlier reports where 0.2–0.3 shoots (Ozgen et al. 1996; Delporte et al. 2001), 8 shoots (Mendoza and Kaeppler 2002; Filippov et al. 2006) and 13 shoots per explant (Chauhan et al. 2007) were obtained. However, only in a single study a maximum of 64 % regeneration was obtained from calli on 1.0 mgl−1 2,4-D (Khurana et al. 2002). Further, the tissue culture response of the cultivars except PBW-343, HD-2329 has been examined for the first time.
All the plants regenerated through this protocol were fully-fertile (Fig. 1m) and were indistinguishable in morphology from the seed-raised plants. All of them set seeds with 100 % viability. The time required in regenerating the plantlets from initiation of culture to their establishment in soil is about 7–9 weeks. The higher regeneration response with low genotype dependency in the present study may be due to the physical isolation of regenerable tissues from non-regenerable structures (NRS) identified during the early callus phase and the use of an effective hormone combination during regeneration phase. NRS usually originated from the plumular and radicular ends of the embryo and showed high proliferation during subculture with no regeneration competency. The regenerable calli which developed from epicotyl and mesocotyl parts of the embryo were found to lose their regeneration potential drastically in the presence of NRS. This indicates that the different regions of the embryo differ in their regeneration potential. The novel approach used in present study with its inherent simplicity and low genotype dependency would allow the application of gene transfer methods to wheat cultivars of agronomical value. In conclusion, the present study has demonstrated that mature embryos are viable alternative to immature tissue derived explants for efficient shoot regeneration in a comparatively genotype independent manner on a simple basal medium containing 2,4-D and TDZ.
Abbreviations
- 2,4-D:
-
2,4-dichlorophenoxyacetic acid
- Dicamba:
-
3,6-dichloro-2-methoxybenzoic acid
- Picloram:
-
4-amino-3, 5-trichoropiclinic acid
- TDZ:
-
Thidiazuron
References
Bhalla PL (2006) Genetic engineering of wheat-current challenges and opportunities. Trends Biotechnol 24:305–311
Bi R, Wang H (2008) Primary studies on tissue culture from mature embryos in diploid and tetraploid wheat. Front Agric China 2:262–265
Biesaga-Kościelniak J, Kościelniak J, Janeczko A (2010) The impact of zearalenone and thidiazuron on indirect plant regeneration of oilseed rape and wheat. Acta Physiol Plant 32:1047–1053
Boyko A, Matsuoka A, Kovalchuk I (2009) High frequency Agrobacterium tumefaciens—mediated plant transformation induced by ammonium nitrate. Plant Cell Rep 28:737–757
Chauhan H, Desai SA, Khurana P (2007) Comparative analysis of the differential regeneration response of various genotypes of Triticum aestivum, Triticum durum and Triticum dicoccum. Plant Cell Tiss Org Cult 91:191–199
Chugh A, Khurana P (2003) Regeneration via somatic embryogenesis from leaf basal segments and genetic transformation of bread and emmer wheat by particle bombardment. Plant Cell Tiss Org Cult 74:151–161
Constabel F, Shyluk JP (1994) Initiation, nutrition and maintenance of plant cell and tissue cultures. In: Vasil IK, Thorpe TA (eds) plant cell and tissue culture. Kluwer Acad. Publ, Dordrecht, pp 3–15
Delporte F, Mostade O, Jacquemin JM (2001) Plant regeneration through callus initiation from thin mature embryo fragments of wheat. Plant Cell Tiss Org Cult 67:73–80
Ding L, Li S, Gao J, Wang Y, Yang G, He G (2009) Optimization of Agrobacterium-mediated transformation conditions in mature embryos of elite wheat. Mol Biol Rep 36:29–36
FAOSTAT (2008) (http://www.faostat.fao.org/)
Filippov M, Miroshnichenko D, Vernikovskaya D, Dolgov S (2006) The effect of auxins, time exposure to auxin and genotype on somatic embryogenesis from mature embryo of wheat. Plant Cell Tiss Org Cult 84:63–73
Ganeshan S, Chodaparambil SV, Baga M, Fowler DB, Huel P, Rossnagel B, Chibber RN (2006) In vitro regeneration of cereals based on multiple shoot induction from mature embryos in response to thidiazuron. Plant Cell Tiss Org Cult 86:63–73
Greer MS, Kovalchuk I, Eudes F (2009) Ammonium nitrate improves direct somatic embryogenesis and biolistic transformation of Triticum aestivum. New Biotechnol 26:1/2
Grimes HD, Hodges TK (1990) The inorganic NO −3 : NH +4 ratio influences plant regeneration and auxin sensitity in primary callus from immature embryos of indica rice (Oryza sativa L.). J Plant Physiol 136:362–367
Jones HD (2005) Wheat transformation: current technology and applications to grain development and composition. J Cereal Sci 41:137–147
Joshi AK, Mishra B, Chatrath R, Ortiz FG, Singh RP (2007) Wheat improvement in India: present status, emerging challenges and future prospects. Euphytica 157:431–446
Khurana J, Chugh A, Khurana P (2002) Regeneration from mature and immature embryos and transient gene expression via Agrobacterium-mediated transformation in emmer wheat (Triticum dicoccum Schuble). Indian J Exp Biol 40:1295–1303
Kothari SL, Agarwal K, Kumar S (2004) Inorganic nutrient manipulation for highly improved in vitro plant regeneration in finger millet (Elusine coracana L. gaertn.). In Vitro Cell Dev Biol Plant 40:515–519
Mac Kinnon C, Gunderson G, Nabors MW (1987) High efficiency plant regeneration by somatic embryogenesis from callus of mature embryo explants of bread wheat (Triticum aestivum L.) and grain sorgum (Sorghum bicolor). In Vitro Cell Dev Biol Plant 23:443–447
Mathias RJ, Simpson ES (1986) The interaction of genotypes and culture medium on the tissue culture responses of wheat (Triticum aestivum L.) callus. Plant Cell Tiss Org Cult 7:31–37
Mendoza MG, Kaeppler HF (2002) Auxin and sugar effects on callus induction and plant regeneration frequencies from mature embryo of wheat (Triticum aestivum L.). In Vitro Cell Dev Biol 38:39–45
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Ozgen M, Turet M, Ozcan S, Sancak C (1996) Callus induction and plant regeneration from immature and mature embryos of winter durum wheat genotypes. Plant Breed 115:455–458
Ozgen M, Turet M, Altmok S, Sancak C (1998) Efficient callus induction and plant regeneration from mature embryo culture of winter wheat (Triticum aestivum L.) genotypes. Plant cell Rep 18:331–335
Patnaik D, Vishnudasan D, Khurana P (2006) Agrobacterium-mediated transformation of mature embryos of Triticum aestivum and Triticum durum. Curr Sci 91:307–317
Redway FA, Vasil V, Lu D, Vasil IK (1990) Identification of callus types for long-term maintenance and regeneration from commercial cultivars of wheat (Triticum aestivum L.). Theor Appl Genet 79:609–617
Repellin A, Baga M, Jauhar PP, Chibbar RN (2001) Genetic enrichment of cereal crops via alien gene transfer: new challenges. Inorganic nutrient manipulation in the induction of embryogenic callus from immature embryos of wheat. Plant Cell Tiss Org Cult 64:159–183
Vasil IK (2007) Molecular genetic improvement of cereals: transgenic wheat (Triticum aestivum L.). Plant Cell Rep 26:1133–1154
Yasmin R, Javed F, Arfan M (2001) Somatic embryogenesis in callus culture of wheat (Triticum aestivum) accession 235/2. Int J Agri Biol 3:163–166
Yu Y, Wang J, Zhu ML, Wei ZM (2008) Optimization of mature embryo-based high frequency callus induction and plant regeneration from elite wheat cultivars grown in China. Plant Breed 127:249–255
Zale JM, Borchardt-Wier H, Kidwal KK, Stebar CM (2004) Callus induction and plant regeneration from mature embryos of a diverse set of wheat genotypes. Plant Cell Tiss Org Cult 76:277–281
Zhou MD, Lee TT (1984) Selectivity of auxin for induction and growth of callus excised embryo of spring and winter wheat. Can J Bot 62:1393–1397
Acknowledgements
The authors are grateful to UGC for the financial support and NBPGR, New Delhi and DWR, Karnal, India for the supply of seeds of wheat cultivars. SSP is thankful to CSIR, New Delhi, India for the award of the Senior Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Parmar, S.S., Sainger, M., Chaudhary, D. et al. Plant regeneration from mature embryo of commercial Indian bread wheat (Triticum aestivum L.) cultivars. Physiol Mol Biol Plants 18, 177–183 (2012). https://doi.org/10.1007/s12298-012-0101-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-012-0101-2