Abstract
Cadmium (Cd), poisoning has been reported from all around the World, causing many deaths annually. Cd is a toxic heavy metal, and is widely present in environment. It has been reported that chronic Cd exposure is associated with kidney disease, osteoporosis, cardiovascular diseases and cancer. Smoking causes exposure to significantly higher Cd levels in humans. Tobacco smoke transports Cd into the lungs. Blood then transport it to the rest of the body where it increases effects by potentiating Cd that is already present from Cd-rich food. Other high exposures of Cd can occur with people, who live near hazardous waste sites, or factories that release Cd into the air and people who work in the metal refinery industry. Breathing of Cd can severely damage the lungs and may even cause death. Multiple studies have shown an association between environmental exposure to hazardous chemicals including toxic metals and obesity, diabetes, and metabolic syndrome. At the same time, the existing data on the impact of Cd exposure on obesity and diabetes are contradictory. On the converse, results of epidemiologic studies linking Cd exposure and Osteoporosis, overweight or obesity are far less consistent and even conflicting, also depending on differences in exposure levels. In turn, laboratory studies demonstrated that Cd adversely affects adipose tissue physiopathology through several mechanisms, thus contributing to increased insulin resistance and enhancing diabetes. However, intimate biological mechanisms linking Cd exposure with human diseases are still to be adequately investigated. Therefore, the aim of the present review was to explore the impact of Cd exposure and status on the risk of Cd in human diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd) is present everywhere in the environment. A wide range of disease can occur through Cd exposure in water and food as well as occupational sources. Cd is an important environmental pollutant and a very toxic heavy metal, which causes poisoning in various tissues of animals and humans [1]. Cd enters into the living-organism through water or food, it binds to albumin and erythrocytes in the blood and then is transferred into tissues and organs, where it is bound to proteins of low molecular mass producing metallothioneins (Cd-MT) by the induction of metallothionein mRNA synthesis [2]. And these Cd-MT complexes generate reactive oxygen species (ROS) such as hydrogen peroxide (H2O2), superoxide (O2–) and hydroxyl radical (OH–), nitric oxide (NO–) [3] and can reduce the level of the main antioxidant compounds in the cells by inactivating enzymes and other antioxidant molecules [4] and as a result, lipid peroxidation (LPO) is generated that ultimately cause cell death and disease [5, 6] (Fig. 1). Cd always occurs in combination with zinc and mainly found in the earth’s crust. Cd is also found as an inevitable by-product of zinc, lead and copper extraction in the industries. Cd is found in manures and pesticides through which it enters into the environment mainly by the ground.
A very large amount of Cd about 25,000 tons a year is released into the environment naturally. Some portion of this Cd is released into air through volcanoes and forest fires and about half portion of Cd is discharged into rivers through weathering of rocks. The rest portion of this Cd is discharged into the human activities. No Cd ore is mined for the metal, because more than enough is produced as a byproduct of the smelting of zinc from its ore, sphelerite (ZnS), in which cadmium sphelerite (CdS) is a significant impurity, making up as much as 3%. The main mining areas of Cd are those associated with zinc. However, world production of cadmium is around 14.000 tons per year. The main Cd producing country is Canada, with the USA, Australia, Mexico, Japan and Peru also being the major suppliers. Cd has a high toxicity potential with non-essential heavy metal. The major sources for the exposure of Cd to humans are diet, polluted air, and cigarette smoke. The exposure of Cd associated with increased risk of chronic diseases, including diabetes, hypertension, nephropathy and atherosclerosis, all of which could be attributable to dysfunctional endothelial and smooth muscle cells. Cd toxicity is associated with increased reactive oxygen formation which leads to the depletion of antioxidants, resulting in an oxidative stress. Chelation of Cd has proved useful in the removal of the Cd burden but several chelating agents cause side effects in clinical usage.
Furthermore, Cd is toxic at very low levels and has acute and chronic effects on health. The most dangerous characteristic of Cd is that it accumulates throughout one’s lifetime. Cd has a long biological half-life of 17–30 years in humans. Evidence suggests that food and cigarette smoke are the major sources of non-occupational Cd exposure in the general population [7]. Occupational exposure results mainly from Cd fume inhalation in the cadmium-nickel battery industry, as well as from coating and plating of metals and in the production of stabilizers for plastic and paint pigments. Cd has been implicated in the pathogenesis of age-related macular degeneration and hearing loss [8,9,10]. However, the most commonly affected organ systems by Cd toxicity are the kidney, lung, bone and skeletal, cardiovascular, and nervous systems [11]. Observational studies indicate that chronic Cd exposure is associated with an increased risk of cardiovascular disease, including hypertension, atherosclerosis, nephropathy, and diabetes [12,13,14,15,16]. Therefore in this review we will elucidate the toxic effects of Cd on various human diseases.
Cadmium Risk and Exposure
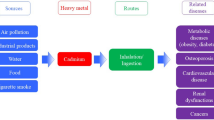
Some of the occupations that directly involve Cd and carry a higher risk of exposure include: battery manufacture, jobs involving welding or soldering, smelting, mining, textile work, Cd alloy manufacture or use, manufacture of materials that contain Cd, such as certain paints and plastics, jewelry making, glassware decorated with Cd and municipal solid waste recovery workers. Sources of exposure to Cd are (Fig. 2).
Cigarette Smoking
Cigarettes contain Cd and smokers inhale Cd when they smoke. Smokers may receive the double dose of Cd daily as compared to non-smokers. People breathing cigarette smoke can also inhale Cd.
Food
Vegetables, like potatoes and leafy vegetables, and cereal grains grown in contaminated soils with high levels of Cd may contain small amounts of Cd. Kidneys and livers of animals and shellfish also contain higher levels of Cd than other foods.
Industrial Areas
Some industrial processes, such as metal smelting, release Cd into the air. To the ensure protection of public health, controls are placed on industry to limit emission levels.
Fertilized Soils
In agricultural areas, phosphate fertilized soils may contain higher levels of Cd than unfertilized soils.
Inhalation of Cadmium
Inhalation is the main way workers are exposed to Cd. To protect the health of workers many safety levels and standards of exposure are enforced. Breathing air with high levels of Cd over a short period of time is initially like the flu with chills, fever and muscle pain, then later can cause shortness of breath, lung damage, cough and chest pain, which may lead to death in severe cases.
Breathing lower levels of Cd over a longer period of time can lead to kidney disease and cause bones to become weaker.
Ingestion of Cadmium
Eating food or drinks, contaminated with high levels of Cd can cause stomach irritation, abdominal cramps, nausea, vomiting and diarrhea. Swelling of the throat and tingling hands, headaches and flu-like symptoms may also occur. Only a small amount of Cd remains in the body after eating food contaminated with Cd, but if consumed over a long period of time, Cd can lead to kidney diseases and can cause bones to become weaker. Large amounts of Cd can damage the kidney, liver and heart and in severe cases may cause death.
Health Effects of Cadmium
Foodstuffs are the major sources for the uptake of Cd in human. Cd concentration in human body increases greatly by the foodstuffs that are rich in Cd. Example are dried seaweed, shellfish, mushrooms, mussels, cocoa powder and liver. An exposure to significantly higher Cd levels occurs when people smoke. Tobacco smoke transports Cd into the lungs. Blood then transport it through the rest of the body where it increases effects by potentiating Cd that is already present from Cd-rich food. Other high exposures can occur with people who live near hazardous waste sites or factories that release Cd into the air and people that work in the metal refinery industry. When people breathe in Cd it can severely damage the lungs. This may even cause death. The bloodstream transport Cd first to the liver and then Cd is bond to proteins to form complexes that are transported to the kidneys. Cd accumulates in kidneys, where it damages filtering mechanisms. This results the excretion of essential sugars and proteins from the body which may lead to kidney damage. It takes a very long time before Cd, which has accumulated in kidneys to get it excreted from a human body. Other health effects that can be caused by Cd are: Diarrhea, stomach pains and severe vomiting, Bone fracture Reproductive failure and even infertility, psychological disorders, damage to the immune system, damage to the central nervous system, possibly DNA damage or cancer development (Fig. 3).
The Chronic Impact of Cadmium on Human Health Around Asia
The effect of Cd-induced lung damage in workers was first reported in the 1930s and later, the effects of Cd on bone proteinuria was reported in the 1940s [17]. After World War II, a form of Cd-induced renal osteomalacia called itai–itai disease and characterized by fractures and severe pain and it was identified in the Cd-polluted Jinzu River basin in Toyama, Japan [18]. Subsequently, international warnings of health risks from Cd pollution were issued in the 1970s. In the 1990s, the toxic effect of Cd on skeletal, renal and reproductive systems was reported in Chinese populations who were exposed to Cd via consumption of contaminated rice [19]. Long-term environmental exposure to Cd was also reported to cause renal dysfunction, which occurred initially as tubular damage, followed by glomerular damage. World Health Organization (WHO) confirmed that the kidney is the most important target organ for Cd toxicity, and a crude quantitative evaluation was conducted in 1992 (WHO 1992). Previous studies showed a relationship between dietary Cd contaminated rice and the presence of chronic renal failure [20, 21]. A high concentration of Cd in the urine was detected in non-smoking farmers who worked on sludge farms in Hyderabad, India [22]. Osteoporosis and nephropathy caused by Cd exposure were found in populations living in polluted areas of southeast China [23].
A high prevalence of renal failure was also detected among the farmers in the agricultural area under irrigation in the North Central Province, Sri Lanka [24]. Chronic Cd toxicity is a significant health concern among workers engaged in zinc smelting, battery production and silver jewellery industries, particularly in developing countries. In Thailand, environmental Cd contamination was first reported in some rural villages of Mae Sot District, Tak Province, and Northwestern Thailand [25]. These issues soon become a public health concern, and Cd toxicity got monitored in residents living in those contaminated areas. Cd contaminations in those areas were primarily associated with mining of zinc ores. In particular, Cd contamination may result from irrigation of paddy fields by two creeks (Mae Tao and Mae Ku) which pass through an area where a zinc mine has actively operated for more than 20 years [26]. People who live in contaminated areas are likely to be exposed to Cd via consumption of rice and other crops that are grown locally in those areas [27].
Cadmium and Cardiovascular Dysfunction
In the cardiovascular system, Cd-induced cardiac damage is associated with alteration of antioxidant defense by increased generation of ROS [28, 29], which causes reduced coronary blood flow [30], and inhibition of the electron transport chain in cardiomyocytes [29]. The vascular endothelium is believed to be the primary site at which the deleterious effects of high blood pressure, high glucose levels in diabetes and high plasma lipid concentrations which may lead to the impairment of endothelial function. Therefore, the endothelium is probably affected by Cd. Several lines of evidence suggest that vascular endothelium is an important target of Cd toxicity [31]. The cellular mechanisms by which Cd might contribute to the development of hypertension include Cd-stimulated release of proinflammatory mediators (e.g. tumor necrosis factor-alpha) and antithrombolytic agents (e.g. plasminogen activator inhibitor-1) from vascular endothelial cell culture [32, 33].
Cd also facilitates the adhesion of peripheral human polymorphonuclear leukocytes to the endothelium [34]. It was reported that Cd stimulates the secretion of endothelin-1 and angiotensin II from the cultured coronary microvascular endothelial cells [35]. Regarding the microvascular effect of Cd, it was found that Cd alters the function of a Ca2 + -dependent cell adhesion molecule, the so-called vascular endothelial-cadherin (VE-cadherin), thereby leading to a disruption of endothelial barrier integrity [15]. The effect of Cd on VE-cadherin has been suggested to be involved in the development of atherosclerosis [36]. The other mechanism of Cd-induced hypertension suggested by Satarug et al. [37] is that Cd deposited in human kidney leads to alterations in metal homeostasis and redox state, changes in gene expression profiles, and ultimately tubular injury. Increased risk of hypertension is found in humans with nephropathy caused by environmental Cd exposure [38].
Cadmium and Inflammation
Increasing data show that in vivo and/or in vitro exposure to Cd results in activation of specific cell types (e.g. Kupffer cells in the liver), organ infiltration with neutrophils and enhanced release of proinflammatory and anti-inflammatory factors by monocytes/macrophages [39]. Many inflammatory biomarkers, released upon Cd exposure have been linked to different diseases—atherosclerosis, cardiovascular diseases, etc. The immunomodulatory role of the metal and in addition its differential effects on cytokine production reveals the effects of Cd on pro- and anti-inflammatory cytokines. Even in micromolar concentrations (1–10 μM) [40] Cd exhibits pro-inflammatory properties and up-regulates the expression of IL-1, IL-6, IL-8, TNF-α and different chemokines in various types of cells [41, 42]. Of the immune system, cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) are early response genes in the inflammatory process and are commonly used as inflammatory markers [43].
Cadmium and Osteoporosis
High exposure to cadmium was the cause of the feared itai–itai (ouch–ouch) disease in Japan, where cadmium contaminated rice was ingested by the local farmers leading to osteoporosis and osteomalacia [44, 45] clear evidence that long-term exposure to cadmium chloride produced marked abnormalities in bone biomarkers and increasing risk of fracture. In recent years, cross sectional examinations of people living in areas of Japan polluted by cadmium have shown evidence of lowered bone calcium and reduced concentrations of kidney activated vitamin D [46,47,48] A few studies have shown a pattern similar to that of patients with itai–itai among workers exposed to high concentrations of cadmium [45, 49] Animal experiments have shown that cadmium in much lower doses may cause osteoporosis [50]. Animal experiments have shown that bone resorption due to cadmium exposure can occur at B-Cd concentrations similar to those reported for people occupationally exposed to cadmium and for people who smoke cigarettes (27–80 nmol/l). Moreover, bone diseases may occur early before the development of tubular damage [50]. The discrepancy found between di Verent parts of the skeleton (low BMD in the forearm, normal in spine and hip) is not unusual.
Although post-menopausal osteoporosis and cortisone induced osteoporosis initially dominate in trabecular bone, bone loss due to hyperparathyroidism and senile osteoporosis, for example, is most marked in the cortical bone of the forearm. It should be noted that forearm BMD is a good predictor of fractures, regardless of location. One possible link between cadmium accumulation in the kidney and bone demineralization may be through a decreased renal activation of vitamin D to calcitriol. Reports from two of the areas of Japan polluted by cadmium show significant associations between renal damage induced by cadmium in the form of ß2-microglobulinuria, or increased serum creatinine, and lowered plasma concentrations of calcitriol [47, 48]. Cadmium concentrations in urine and blood were taken as exposure biomarkers, and ß2-microglobulin, retinol binding protein and albumin were used as biomarkers of effect. A marked dose response relationship between these markers of exposure and effect was shown. Hypercalciuria, which may advance to osteoporosis, has been taken as a sensitive renal-tubular biomarker of a low level of cadmium exposure. Cadmium may also act specifically on bone. Animal studies have shown that cadmium is responsible to stimulate the development and activity of osteoclasts, separating the collagen network in bone. Osteoporosis is the primary cause of cracks in post-menopausal ladies, a common occurrence worldwide, giving rise to disability and a high cost to health services. The identification of cadmium, an environmental pollutant, as one causal factor is highly significant in helping to control the incidence of this complex condition [51].
Cadmium and Diabetes
Diabetes and diabetes-related kidney disease are serious health problems that are the cause of growing concern in many parts of the world. It is estimated the total number of people with diabetes worldwide will rise from 171 million in 2000 to an estimated 366 million by the year 2030 [52]. The projected increase is mainly due to an epidemic of type II diabetes that has been attributed to environmental factors such as greater urbanization and industrialization as well as longer life expectancies, and this increase is likely to occur even if the prevalence of obesity remains constant [52]. In this context, the growing volume of evidence suggesting that Cd may play a role in the development and progression of diabetes and diabetes-related kidney disease could be especially significant.
Cd is an important nephrotoxic pollutant, that poses increasing risks to populations in many parts of the world [53,54,55]. With chronic exposure, Cd accumulates in the epithelial cells of the proximal tubule of the kidney. When a threshold concentration of 150–200 μg/g tissue is reached, Cd causes a generalized dysfunction of the proximal tubule characterized by polyuria, low molecular weight proteinuria and glucosuria [20, 56]. As a result of the extensive use of Cd in industry and its extensive dissemination in the environment, numerous studies have focused on the identification of the early stages of Cd-induced kidney injury in exposed human populations [57]. In some cases, the results of these epidemiological studies have suggested a possible link between Cd exposure and diabetes [58].
Results on human studies suggest an association between Cd exposure, elevated blood glucose levels and the development of diabetes and diabetes-related kidney disease. In addition, many epidemiological and experimental studies suggest that Cd potentiates or exacerbates diabetic nephropathy. While the link between Cd exposure and elevated blood glucose levels has been established, the mechanisms responsible for Cd-induced changes in blood glucose levels have yet to be elucidated. Of the many diverse mechanisms by which Cd may increase blood glucose levels outlined above, there is considerable evidence that Cd can injure cells within the islets of Langerhans and subsequently influence insulin levels. Study also showed that significant elevations of blood glucose levels occur prior to changes in overt renal dysfunction. This would suggest that Cd-induced elevation in fasting blood glucose levels may in fact contribute to, the etiology of Cd-induced renal dysfunction.
Conclusion
In conclusion, the results from previous studies, suggests the toxic effect of Cd in various human diseases. Cd is a persistent and widespread pollutant that affects the structure and function of several organs by generating oxidative stress. Exposure of Cd associated with a number of illnesses including cardiovascular diseases, early atherosclerosis, kidney disease, and hypertension have been reported. Although studies show a significant correlation between cadmium exposure and occurrence of disease in human populations, a necessary molecular mechanism has not yet been identified. One hypothesis holds that Cd is an endocrine disruptor and some experimental studies have shown that it can interact with different hormonal signaling pathways. For example, cadmium can bind to the estrogen receptor alpha, and affect signal transduction along the estrogen and MAPK signaling pathways at low doses Cd affects the cardiovascular system by altering cardiovascular structure and function. Endothelial and vascular smooth muscle cells are the major targets of Cd toxicity. The most common single source of Cd exposure in the general population is the Tobacco smoking. An estimated 10% of the Cd content of a cigarette is inhaled through smoking. Absorption of Cd through the lungs is more effective than through the gut, and as much as 50% of the Cd inhaled in cigarette smoke may be absorbed. On average, Cd concentrations in the blood of smokers are 4 times 5 times greater and in the kidney, 2–3 times greater than non-smokers. However, evidence to support the Biochemical mechanism and its pathways in the toxicity of Cd in human diseases is still lacking. Therefore, further studies are needed to explore the exact mechanisms underlying the toxic effects of Cd, in various human diseases.
References
Bernhoft RA. Cadmium toxicity and treatment. Sci World J. 2013;2013:394652.
Sato M, Kondoh M. Recent studies on metallothionein: protection against toxicity of heavy metals and oxygen free radicals. Tohoku J Exp Med. 2002;196:9–22.
Hassoun EA, Stohs SJ. Cadmium-induced production of superoxide anion and nitric oxide, DNA single strand breaks and lactate dehydrogenase leakage in J774A.1 cell cultures. Toxicology. 1996;112:219–26.
Filipic M, Fatur T, Vudrag M. Molecular mechanisms of cadmium induced mutagenicity. Hum Exp Toxicol. 2006;25:67–77.
Koedrith P, Seo YR. Advances in carcinogenic metal toxicity and potential molecular markers. Int J Mol Sci. 2011;12:9576–95.
Rikans LE, Yamano T. Mechanisms of cadmium mediated acute hepatotoxicity. J Biochem Mol Toxicol. 2000;14:110–7.
Lin YS, Caffrey JL, Chang MH, Dowling N, Lin JW. Cigarette smoking, cadmium exposure and zinc intake on obstructive lung disorder. Respir Res. 2010;11:53.
Erie JC, Good JA, Butz JA, Hodge DO, Pulido JS. Urinary cadmium and age-related macular degeneration. Am J Ophthalmol. 2007;144:414–8.
Castellanos MJ, Fuente A. The adverse effects of heavy metals with and without noise exposure on the human peripheral and central auditory system: a literature review. Int J Environ Res Public Health. 2016;13:1223.
Chantarawong W, Takeda K, Sangartit W, Yoshizawa M, Pradermwong K, Shibahara S. Microphthalmiaassociated transcription factor as the molecular target of cadmium toxicity in human melanocytes. Biochem Biophys Res Commun. 2014;454:594–9.
Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN. Toxicity mechanism and health effects of some heavy metals. Interdiscip Toxicol. 2014;7:60–72.
Puri VN. Cadmium induced hypertension. Clin Exp Hypertens. 1999;21:79–84.
Satarug S, Nishijo M, Ujjin P, Vanavanitkun Y, Moore MR. Cadmium-induced nephropathy in the development of high blood pressure. Toxicol Lett. 2005;157:57–68.
Eum KD, Lee MS, Paek D. Cadmium in blood and hypertension. Sci Total Environ. 2008;407:147–53.
Prozialeck WC, Edwards JR, Nebert DW, Woods JM, Barchowsky A, Atchison WD. The vascular system as a target of metal toxicity. Toxicol Sci. 2008;102:207–18.
Messner B, Bernhard D. Cadmium and cardiovascular diseases: cell biology, pathophysiology and epidemiological relevance. Biometals. 2010;23:811–22.
Nordberg GF. Cadmium and health in the 21st century: historical remarks and trends for the future. Biometals. 2004;17:485–9.
Aoshima K. Itai-itai disease: cadmium-induced renal tubular osteomalacia. Nihon Eiseigaku Zasshi. 2012;67:455–63.
Cai SW, Yue L, Hu ZN, Zhong XZ, Ye ZL, Xu HD, et al. Cadmium exposure and health effects among residents in an irrigation area with ore dressing wastewater. Sci Total Environ. 1990;90:67–73.
Satarug S, Moore MR. Adverse health effects of chronic exposure to low-level cadmium in foodstuffs and cigarette smoke. Environ Health Perspect. 2004;112:1099–103.
Simmons RW, Pongsakul P, Saiyasitpanich D, Klinphoklap S. Elevated levels of cadmium and zinc in paddy soils and elevated levels of cadmium in rice grain downstream of a zinc mineralized area in Thailand: implications for public health. Environ Geochem Health. 2005;27:501–11.
Srikanth R, Khanam A, Rao V. Cadmium levels in the urine of male sewage sludge farmers of Hyderabad. India J Toxicol. Environ Health. 1994;43:1–6.
Jin T, Nordberg G, Ye T, Bo M, Wang H, Zhu G, et al. Osteoporosis and renal dysfunction in a general population exposed to cadmium in China. Environ Res. 2004;96:353–9.
Bandara JM, Wijewardena HV, Bandara YM, Jayasooriya RG, Rajapaksha H. Pollution of River Mahaweli and farmlands under irrigation by cadmium from agricultural inputs leading to a chronic renal failure epidemic among farmers in NCP Sri Lanka. Environ Geochem Health. 2011;33:439–53.
Limpatanachote P, Swaddiwudhipong W, Mahasakpan P, Krintratun S. Cadmium-exposed population in Mae Sot District Tak Province: 2. Prevalence of renal dysfunction in the adults. J Med Assoc Thai. 2009;92:1345–53.
Songprasert N, Sukaew T, Kusreesakul K, Swaddiwudhipong W, Padungtod C, Bundhamcharoen K. Additional burden of diseases associated with cadmium exposure: a case study of cadmium contaminated rice fields in Mae Sot District, Tak Province, Thailand. Int. J. Environ. Res Public Health. 2015;12:9199–217.
Swaddiwudhipong W, Limpatanachote P, Mahasakpan P, Krintratun S, Padungtod C. Cadmium-exposed population in Mae Sot District, Tak Province: 1. Prevalence of high urinary cadmium levels in the adults. J Med Assoc Thai. 2007;90:143–8.
Baek K, Chung I. Cadmium exposure is associated with monocyte count and monocyte to HDL ratio, a marker of inflammation and future cardiovascular disease in the male population. J Korean Med Sci. 2017;32:1415–22.
Tellez-Plaza M, Guallar E, Howard BV, Umans JG, Francesconi KA, Goessler W, et al. Cadmium exposure and incident cardiovascular disease. Epidemiology. 2013;24:421–9.
Kisling GM, Kopp SJ, Paulson DJ, Tow JP. Cadmium-induced attenuation of coronary blood flow in the perfused rat heart. Toxicol Appl Pharmacol. 1993;118:58–64.
Kukongviriyapan U, Pannangpetch P, Kukongviriyapan V, Donpunha W, Sompamit K, Surawattanawan P. Curcumin protects against cadmium-induced vascular dysfunction hypertension and tissue cadmium accumulation in mice. Nutrients. 2014;6:1194–208.
Yamamoto C, Kaji T, Sakamoto M, Kozuka H. Cadmium stimulation of plasminogen activator inhibitor-1 release from human vascular endothelial cells in culture. Toxicology. 1993;83:215–23.
Szuster-Ciesielska A, Lokaj I, Kandefer-Szerszen M. The influence of cadmium and zinc ions on the interferon and tumor necrosis factor production in bovine aorta endothelial cells. Toxicology. 2000;145:135–45.
Hernandez M, Macia M. Free peripheral sulfhydryl groups, CD11/CD18 integrins, and calcium are required in the cadmium and nickel enhancement of human-polymorphonuclear leukocyte adherence. Arch Environ Contam Toxicol. 1996;30:437–43.
Kusaka Y, Kelly RA, Williams GH, Kifor I. Coronary microvascular endothelial cells cosecrete angiotensin II and endothelin-1 via a regulated pathway. Am J Physiol Heart Circ Physiol. 2000;279:1087–96.
Pereira FE, Coffin JD, Beall HD. Activation of protein kinase C and disruption of endothelial monolayer integrity by sodium arsenite: potential mechanism in the development of atherosclerosis. Toxicol Appl Pharmacol. 2007;220:164–77.
Satarug S, Nishijo M, Lasker JM, Edwards RJ, Moore MR. Kidney dysfunction and hypertension: role for cadmium, p450 and heme oxygenases? Tohoku J Exp Med. 2006;208:179–202.
Wu H, Liao Q, Chillrud SN, Yang Q, Huang L, Bi J, et al. Environmental exposure to cadmium: health risk assessment and its associations with hypertension and impaired kidney function. Sci Rep. 2016;6:29989.
Sundblad BM, Ji J, Levänen B, Midander K, Julander A, Larsson K, et al. Extracellular cadmium in the bronchoalveolar space of long-term tobacco smokers with and without COPD and its association with inflammation. Int J Chron Obstruct Pulmon Dis. 2016;11:1005–13.
Olszowski T, Baranowska-Bosiacka I, Gutowska I, Chlubek D. Pro-inflammatory properties of cadmium. Acta Biochim Pol. 2012;59:475–82.
Odewumi C, Latinwo L, Sinclair A, Badisa V, Abdullah A, Badisa R. Effect of cadmium on the expression levels of interleukin-1α and interleukin-10 cytokines in human lung cells. Mol Med Rep. 2015;12:6422–6.
Papa V, Wannenes F, Crescioli C, Caporossi D, Lenzi A, Migliaccio S, et al. The environmental pollutant cadmiuminduces homeostasis alteration inmuscle cells in vitro. J Endocrinol Investig. 2014;37:1073–80.
Ramyaa P, Krishnaswamy R, Padma V. Quercetin modulates OTA-induced oxidative stress and redox signalling in HepG2 cells-up regulation of Nrf2 expression and down regulation of NF-κB and COX-2. Biochim Biophys Acta. 2014;1840:681–92.
Kido T, Nogawa K, Yamada Y, Honda R, Tsuritani I, Ishizaki M, et al. Osteopenia in inhabitants with renal dysfunction induced by exposure to environmental cadmium. Int Arch Occup Environ Health. 1989;61:271–6.
Kjellström T. Mechanism and epidemiology of bone e Vects of cadmium. IARC Sci Publ. 1992;118:301–10.
Kido T, Nogawa K, Honda R, Tsuritani I, Ishizaki M, Yamada Y, et al. The association between renal dysfunction and osteopenia in environmental cadmium-exposed subjects. Environ Res. 1990;51:71–82.
Aoshima K, Kasuya M. Preliminary study on serum levels of 1,25-dihydroxyvitamin D and 25-hydroxyvitamin D in cadmium-induced renal tubular dysfunction. Toxicol Lett. 1991;57(91–9):438.
Tsuritani I, Honda R, Ishizaki M, Honda R, Yamada Y, Ishizaki M. Impairment of vitamin D metabolism due to environmental cadmium exposure, and possible relevance to sex-related diVerences in vulnerability to the bone damage. J Toxicol Environ Health. 1992;37:519–33.
Kazantzis G. Renal tubular dysfunction and abnormalities of calcium metabolism in cadmium workers. Environ Health Perspect. 1979;28:155–9.
Bhattacharyya MH, Sacco-Gibson NA, Peterson DP. Cadmium-induced bone loss: increased susceptibility in female beagles after ovariectomy. IARC Sci Publ. 1992;118:279–86.
Kazantzis G. Cadmium, osteoporosis and calcium metabolism. Biometals. 2004;17:493–8.
Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53.
Cackovic M, Kalinic N, Vadjic V, Pehnec G. Heavy metals and acidic components in total deposited matter in sibenik and national park kornati, croatia. Arch Environ Contam Toxicol. 2009;56:12–20.
Yang QW, Li H, Long FY. Heavy metals of vegetables and soils of vegetable bases in Chongqing, Southwest China. Environ. Monit. Assess. 2007;130:271–9.
Diawara MM, Litt JS, Unis D, Alfonso N, Martinez L, Crock JG, et al. Arsenic, cadmium, lead, and mercury in surface soils, Pueblo, Colorado: implications for population health risk. Environ Geochem Health. 2006;28:297–315.
Jarup L. Cadmium overload and toxicity. Nephrol Dial Transplant. 2002;17:35–9.
Prozialeck WC, Vaidya VS, Liu J, Waalkes MP, Edwards JR, Lamar PC, et al. Kidney injury molecule-1 is an early biomarker of cadmium nephrotoxicity. Kidney Int. 2007;72:985–93.
Bernard A. Renal dysfunction induced by cadmium: biomarkers of critical effects. Biometals. 2004;17:519–23.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fatima, G., Raza, A.M., Hadi, N. et al. Cadmium in Human Diseases: It’s More than Just a Mere Metal. Ind J Clin Biochem 34, 371–378 (2019). https://doi.org/10.1007/s12291-019-00839-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12291-019-00839-8