Abstract
Nano carriers have greatly revolutionized the treatment of most diseases recently. One of these nano carriers, liposomes, has got particular significance. On the other hand, Artemisinin which is used as an effective anticancer drug has some side effects. To reduce such side effects, liposomes can be employed. In order to prepare pegylated nanoliposomal artemisinin, particular proportions of phosphatidylcholine, polyethylene glycol 2000 and artemisinin were combined. As a result, the mean diameter of nano liposomes is 455 nm. Besides, the encapsulation efficiency and the drug release from pegylated nanoliposomes for pegylated nanoliposomal artemisinin are respectively 91.62 ± 3.5 and 5.17 %. The results also show that IC50 of the produced formulation is less than that of the standard drug. This study reveals that the amount of artemisinin cytotoxicity compared to standard drug is increased by pegylated nanoliposomal formulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nanotechnology has revolutionized the diagnosis and treatment of cancer [1]. Various carriers in nano scale are used in clinical application. Nano carriers cause an increase in drug solution, drug release control, decreased side effects and improved natural distribution of drug [2]. In recent decades, lipid based drug delivery has attracted considerations more than before [3]. A kind of these drug carriers are liposomes which consist of one or more semi core lipids that are formed by water and lipid sources which are found between two layers of lipids [4, 5].
Chemotherapy, radiotherapy and surgery are the conventional breast cancer therapies [6]. One of the drugs used in cancer treatment is artemisinin which is a lactone taken from the leaves of Artemisia annua [7, 8]. Artemisinin molecule causes molecular damage and ultimately makes the cells die [7, 9]. This drug, in spite of being effective, has some side effects in long-term usage. One of the controversial issues in nano treatment is reducing or completely eliminating the side effects. Nanotechnology is one of the modern and successful ways of decreasing such side effects.
In this study, artemisinin was nanoliposomed and pegylated in order to have better treatment efficiency and reduce side effects.
Materials and Methods
Materials
Artemisinin, phosphatidylcholine, cholesterol, polyethylene glycol 2000 (PEG 2,000), and MTT solution (0.5 mg/ml) were supplied by Sigma Co. RPMI 1640 tissue culture was also purchased from Invitrogen Co. Ethanol and Isopropanol were provided from Merck Company. MCF-7 cell line was purchased from Pasteur Institute of Iran.
Preparation of Nanoliposomal and Pegylated Drug
Cholesterol and phosphatidylcholine and PEG 2,000 (ratio of 1:5:14) were dissolved in 100 ml of 98 % ethanol (400 rpm, room temperature), then, 1 mg of artemisinin was added and mixed by means of magnetic stirrer (300 rpm, 15 min, room temperature). Ethanol was then evaporated with Heidolph Co. Rotary evaporator and the reproduced gelose was dissolved in 12 ml physiological serum. Then, the formulations were sonicated for 5 min (Bandelin Sonorex Digitec, 60 Hz).
Size Measurement of Nanoliposomes
One mg of the formulation was dissolved in 10 ml of physiological serum and after the measuring of its absorption in 630 nm, the mean diameter of the nanoliposomes were determined by Zeta sizer (Nano ZS Malvern Instruments Ltd).
Encapsulation Efficiency
In order to measure the amount of entrapped drug, 1 mg of the formulation was centrifuged for 30 min at 4 °C and at 50,000 rpm. Then, optical density of the supernatant of each formulation was determined at 195 nm by means of spectrophotometer (UV 1601PC, Shimadzu Co.). Encapsulation efficiency was calculated by using Formula 1 [3].
Drug Release Study
The study of the pattern of drug release from pegylated nanoliposomes was carried out while equal volumes of both formulations was poured in a dialysis bag in 25 ml of PBS pH 7.4 placed on a magnetic stirrer (300 rpm, 48 h, 37 °C). Then, the drug released in PBS was measured at 195 nm in different time intervals within 48 h by spectrophotometry and the percentage of paclitaxel releasing of pegylated nanoliposomal formulation was obtained using standard curve.
Cytotoxicity Assay
Subsequent to MCF-7 cell line culture, 100 μl of the suspension containing 10,000 cells was put in the wells of a 96-well plate and incubated by CO2, 5 % at 37 °C. 24 h later, the supernatant of the cells was collected and different densities of nanoliposomal artemisinin product plus their controls and also pegylated nanoliposomal artemisinin and its controls, as well as standard artemisinin drug were added to the cells; then it was incubated by CO2, 5 % at 37 °C within 24 h. Then the supernatant was decanted and 100 μl of the MTT solution (0.5 mg/ml) was added and after 3 h of incubation the purple color (due to formazane formation) was obtained by dissolving 100 μl of isopropanol in living cells. Absorption at 570 nm was measured (Power Eave XS spectrophotometer) and IC50 was calculated by using Pharm Software.
Statistical Analysis
The results are expressed as mean ± standard deviation (SD, n = 3). The data was statistically analyzed by one-way analysis of variance using IBM Statistics SPSS software version 19, and significant difference was set at p < 0.05.
Results
The mean diameter of liposomes for pegylated nanoliposomal artemisinin was 455 nm obtained by means of Zeta sizer.
Encapsulation Efficiency
For the calculation of encapsulation efficiency (EE %) of nanoliposomal artemisinin regarding Formula 1, the amount of artemisinin that was not encapsulated was obtained, which was 91.62 ± 3.5 by considering the amount of primary drug percentage of encapsulation efficiency.
Release Study
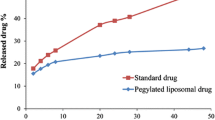
The amount of drug release in PBS buffer for both formulations was obtained for 2, 4, 6, 24 and 48-h periods by applying artemisinin standard curve was obtained 5.17 % reported Fig. 1.
Cytotoxicity Assay
Drug toxicity with different concentration was studied through MTT technique. Figure 2 shows the obtained results of IC50 for pegylated nanoliposomal and standard drug by means of Pharm Software.
Discussion
Pegylated nanoliposomal technology is a new trend for improving pharmacokinetic treatment proteins specifications. Pegylated nanoliposomes are used as carriers with joined non-covalently proteins, but with great characteristics in the external layer. This technology does not vary the amino acid sequence of a protein and does not cause the covalent attachment of stability factors, unlike other methods such as mutagenesis, direct pegylation or fusion to active protein [10]. Although pegylated drugs have disadvantage of unexpected clearance time which may result in accumulation of drug in liver, studies have shown that they stay stable in blood circulation longer than other drugs [11–13]. In other words, they have longer half time. Pegylated drugs are water soluble and they have low level of antigenicity and immunogenicity. Additionally they can be used for drug targeting [14]. In this study cytotoxicity impact of pegylated nonliposomal artemisinin formulation is studied.
After the liposomal production through reverse phase evaporation, the mean diameter of nanoparticles was measured, this confirms the nano dimension of liposomes [15]. In addition, as polyethylene glycol is so hydrophilic and absorbent, it penetrates into liposomal layers and compresses them together. Therefore, the produced nano particles have smaller diameter compared to liposomes. Results show that significant amount of artemisinin was surrounded by nanoliposomes. At the same time, it seems that drug emergence was slower and its stability was more, since polyethylene glycol covers nanoliposome containing artemisinin. Besides, polyethylene glycol changes insoluble drugs like artemisinin to soluble state, which leads to encapsulation increase.
Cytotoxicity effect of the produced formulation was analyzed by means of MTT method. The result shows pegylated nonliposomal formulation has less IC50 compared to standard drug, which shows the toxicity of the produced formulation is more than the standard type of the drug. As a conclusion, polyethylene glycol causes the stability to increase and drug release to decrease.
References
Yatuv R, Robinson M, Dayan-Tarshish I, Baru M. The use of pegylated liposomes in the development of drug delivery applications for the treatment of hemophilia. Int J Nanomed. 2010;5:581–91.
Dass CR, Choong PF. Carrier-mediated delivery of peptidic drugs for cancer therapy. Peptides. 2006;27:3020–8.
Xiang L, Yan Z, Wang G, Liu W, Tang K, Liao Z. Relative expression of genes involved in artemisinin biosynthesis and artemisinin accumulation in different tissues of artemisia annua. Zhongguo Zhong Yao Za Zhi. 2012;37:1169–73.
Latif N, Bachhawat BK. Liposomes in immunology. J Biosci. 1984;6:491–502.
Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–60.
Lai H, Singh NP. Oral artemisinin prevents and delays the development of 7,12-dimethylbenz[a]anthracene (DMBA)-induced breast cancer in the rat. Cancer Lett. 2006;231:43–8.
Guo J, Bourre L, Soden DM, O’Sullivan GC, O’Driscoll C. Can non-viral technologies knockdown the barriers to siRNA delivery and achieve the next generation of cancer therapeutics? Biotechnol Adv. 2011;29:402–17.
Maurer N, Fenske DB, Cullis PR. Developments in liposomal drug delivery systems. Expert Opin Biol Ther. 2001;1:923–47.
Buzea C, Pacheco II, Robbie K. Nanomaterials and nanoparticles: sources and toxicity. Biointerphases. 2007;2:17–71.
Gabizon A, Papahadjopoulos D. Liposome formulations with prolonged circulation time in blood and enhanced uptake by tumors. Proc Natl Acad Sci USA. 1988;85:6949–53.
Nakase I, Lai H, Singh NP, Sasaki T. Anticancer properties of artemisinin derivatives and their targeted delivery by transferrin conjugation. Int J Pharm. 2008;354:28–33.
Veronese FM. Peptide and protein PEGylation: a review of problems and solutions. Biomaterials. 2001;22:405–17.
Kawai F. Microbial degradation of polyethers. Appl Microbiol Biotechnol. 2002;58:30–8.
Ryan SM, Mantovani G, Wang X, Haddleton DM, Brayden DJ. Advances in PEGylation of important biotech molecules: delivery aspects. Expert Opin Drug Deliv. 2008;5:371–83.
Woodley JF. Liposomes for oral administration of drugs. Crit Rev Ther Drug Carrier Syst. 1985;2:1–18.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dadgar, N., Alavi, S.E., Esfahani, M.K.M. et al. Study of Toxicity Effect of Pegylated Nanoliposomal Artemisinin on Breast Cancer Cell Line. Ind J Clin Biochem 28, 410–412 (2013). https://doi.org/10.1007/s12291-013-0306-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12291-013-0306-3