Abstract
Daily feeding of drinking water containing lead acetate (160 mg/l) or 10% alcohol by volume or a combination of both to rats for a month produced certain deleterious effects through oxidative stress. Both heavy metal lead and alcohol are capable of doing such damages. The deleterious alterations observed were in the parameters of blood, serum and tissues, viz; Hb, Pb, proteins, lipids, lipid per oxidation, Vitamins C and E levels and enzyme activities of AST, ALT, and catalase. Simultaneous feeding of either of the two antioxidants garlic oil (GO) and vitamin E at equal doses of 100 mg/kg/day, to the rats counteracted the deleterious effects of the above two chemicals significantly. The maximum damage was brought about by feeding of drinking water containing both lead acetate and alcohol. The protective effects of GO and Vitamin E were not significantly different. The mechanism of actions of the Vitamin E and GO is probably due to their efficiency as detoxifying agents and antioxidants, to scavenging free radicals as well as an independent action of GO on the removal of lead salt as lead sulfide.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lead is a ubiquitous environmental and industrial pollutant that has been detected in almost all phases of biological systems. Lead has been one of the most important non-essential toxic heavy metals with wide applications for making pipes, paints, enamels, glazes etc. The persistence of lead in the animals and humans with associated health risk is a topic of current debate and concern [1] Lead is known to induce a broad range of physiological, biochemical and behavioral dysfunctions in laboratory animals and humans [2] including central and peripheral nervous system [3]. The general population may get exposed to lead due to food, water and air pollution. It was observed that animals co-exposed to lead and ethanol were more susceptible to neurotoxic and hepatotoxic effects of lead [4, 5]. Harishekar observed the deleterious effects of lead salt in the drinking water of rats [6]. A Bulgarian preparation ‘Satlal’ from garlic has been used in curing the occupational lead poisoning among the factory workers as reported by Pet Kov et al. [7]. The beneficial effects of garlic oil (GO) in alcohol, CCl4 or isoproterenol feeding were previously reported by our teams [8–10].
Therefore a study was designed to conduct an investigation on the toxic effects of lead acetate using a concentration of it in drinking water far below that was used by Pinon-lataillade G [11] and others (3 g/l). It was found that the LD50 of lead acetate for rat is 1 g/kg body weight as reported by Alderto furtado Rahde (http://www.inchem.org/documents/pims/chemical/inorglea.htm). In our experiment each rat consumed only 8–10 ml of drinking water i.e. each consumed lead acetate at a dose of 1.6 mg/200 g body weight i.e. 8 mg/kg body weight. We have used this level of leadacetate concentration in the drinking water, so that it may produce some toxic damaging effects on tissues such as liver.
Materials and Methods
Chemicals used in this study were of analytical grade supplied by British Drug House, E-Merck and Sigma Chemicals. GO was prepared as reported earlier [9] and Vitamin E was purchased from Sigma Chemicals. Prior permission was obtained for the experiment from the institutional ethical committee (114/ac/07/CPCSEA). Female Sprague–Dawley albino rats selected from our colony of rats and weighing around 150 g were divided into ten groups of six in each and they were maintained under identical conditions on a laboratory rat feed supplied by Brook Bond Lipton India Ltd. Bangalore with a supply of tap water ad libitum. 12 h light/dark cycle was maintained. During the experiment, groups other than normal were supplied tap water containing lead acetate or alcohol or a mixture of both at fixed doses [6] as shown below for drinking ad libitum for a month. GO or Vitamin E fixed at equal doses and dissolved in 0.5 ml ground nut oil were fed to similar test groups for the same period by a stomach tube as shown below to study their effects. All remaining groups including normal were orally fed the same amount of ground nut oil also. The groups of rats and other details are given below.
-
Group 1: Normal group. Maintained on standard rat feed and tap water ad libitum
-
Group 2: Lead acetate group. Maintained on standard rat feed and lead acetate containing water (160 mg/l) ad libitum.
-
Group 3: Lead acetate as in group 2 + GO. Maintained as in group 2 and treated orally by gastric intubation with GO (100 mg/kg/day) dissolved in ground nut oil.
-
Group 4: Lead acetate as in group 2 + Vitamin E. Maintained as in group 2 and treated orally as above with Vitamin E (100 mg/kg/day) dissolved in ground nut oil.
-
Group 5: Alcohol Group. Maintained on rat feed and drinking water containing alcohol (10%v/v) ad libitum
-
Group 6: Alcohol as in group 5 + GO. Maintained as in group 5 and treated orally with GO (100 mg/kg/day) dissolved in ground nut oil.
-
Group 7: Alcohol as in group 5 + Vitamin E. Maintained as in group 5 and treated orally with Vitamin E (100 mg/kg/day) dissolved in ground nut oil.
-
Group 8: Lead acetate + alcohol group. Maintained on standard rat feed and drinking water containing both lead acetate (160 mg/l) and alcohol (10%v/v) ad libitum.
-
Group 9: Lead acetate and alcohol as in group 8 + GO Maintained as in group 8 and orally treated as above with GO as in group 3
-
Group 10: Lead acetate and alcohol as in group 8 + Vit. E. Maintained as in group 8 and orally treated as above with Vit. E as in group 4.
The daily intake of food and drinking water as well as water containing lead acetate or alcohol or both were recorded.
After 1 month of the above mentioned feeding or treatment, body weights of rats were recorded and all the rats were sacrificed in the morning. Their blood, liver, heart, kidneys and adipose tissue were collected for the determination of various parameters viz; proteins [12]. Lipids viz. total cholesterol [13] LDL cholesterol [14], HDL cholesterol [15] and TAG [16], Hb [17], BLL [18] (only in the first 4 groups are given). Certain enzymes, Viz; AST and ALT [19] and catalase [20] Vit. C [21], Vit. E [22] and lipid per oxidation in terms of TBARS [23], as the case may be in serum, liver, heart and kidney and tri acyl glycerol (TAG) in adipose tissue by standard methods. Histopathology of liver tissues was carried out as per the procedures followed before [9]. Statistical analysis of the results was carried out using one way ANOVA followed by calculation of significance at 5% level using t-values for separating out significant treatment effects. P < 0.05 was considered statistically significant.
Results
Food Consumption
Normal rats consumed food at an average weight of 11 g/rat/day. Lead acetate and alcohol groups and their combination group consumed the least amount of food in the range of 4–5 g/rat/day. Lead acetate + alcohol + Vitamin E, Lead acetate + alcohol + GO and Alcohol + Vitamin E groups consumed an average amount of food in the range of 6–7 g/rat/day. Lead acetate + GO, Lead acetate + Vitamin E and Alcohol + GO groups consumed food in the range of 8–9 g/rat/day ad libitum. Here toxin fed groups consumed lesser amounts of food and GO and Vitamin E treated groups improved their food consumption.
Water and Water Containing Lead Acetate/Alcohol Consumption
When normal group consumed an average of 8 ml tap water/rat/day ad libitum, lead acetate, alcohol and lead acetate + alcohol groups consumed the lead acetate and alcohol containing water only to the least i.e. around 4 ml/rat/day. Lead acetate + Vitamin E and alcohol + Vitamin E groups consumed the same around 7 ml/rat/day.
Lead acetate + GO, Lead acetate + alcohol + GO, Lead acetate + alcohol + Vitamin E groups consumed the same around 8.5 ml/rat/day ad libitum. However Alcohol + GO group drank the same above normal amount, i.e. 10 ml/rat/day. Here we can note that the consumption of water was the least for alcohol, lead acetate and their combination groups. However on treatment with GO and Vitamin E the consumption of water containing toxin or toxins, progressively increased for the concerned groups probably due to an increase in their appetite. The increase or decrease of water consumption runs parallel with the amount of food consumption approximately i.e. 4 ml water for 4–5 g food and 7–10 ml water for 6–10 g food intake.
Percentage Body Weight Changes
The percentage body weight (bw) increase for the normal group of rats was 25% for a month. However for lead acetate + Vitamin E, alcohol + GO and lead acetate + alcohol + GO it was 6.5–7% only. For lead acetate + alcohol, lead acetate + alcohol + Vitamin E and alcohol + Vitamin E groups the bw increase was in the range of 8–9%. For lead acetate and lead acetate + GO, it was in the range of 12–14% and for alcohol group it was a maximum of 30%. Here consumption of drinking water containing only alcohol which is a fat generating substance increased the bw of rats to the maximum. When lead acetate was also a component of the drinking water, the increase in the b.w of the rats progressively decreased. Similarly treatment with GO or Vitamin E, which are good hypolipidemic agents, also decreased the bw of the concerned groups of rats. Decreases in the adipose tissue fat of these groups are recorded later in this paper (Table 7).
Another significant observation of the study was that although with treatment the appetite for food and drinking water which contained toxic substances also increased, the adverse effect of the latter was counteracted by the antioxidant and hypolipidemic effects of GO and Vitamin E and thereby the bw of the concerned groups recorded only intermediate values compared to the other groups.
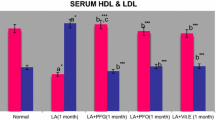
Results on serum and tissue protein level are given in Table 1. Concentration of serum protein significantly reduced on feeding lead acetate, alcohol or their combination. Liver protein was reduced to half the value in group 5 and to 40% in group 8 and on treatment with GO these values were ameliorated significantly in all groups. However Vitamin E was effective only in group 4. Results on Lipid parameters are given in Tables 2, 3 and 4. As given in Table 2 total cholesterol, LDL chol. and triglycerides increased significantly by feeding lead acetate/alcohol or their combination. Lead acetate and alcohol combination showed a comparatively high hyperlipidemic effect. On the contrary HDL cholesterol decreased significantly by each of the toxic substances and their combination. A similar pattern of lipid profile was observed in the liver and heart tissues of toxin-affected groups.
The variations in liver and heart lipid profile are shown Tables 3 and 4 and the adverse effects of the toxins were significant. Treatment with GO or Vitamin E ameliorated the damaging effects of the toxins on the tissues and serum significantly. The results on Hb and serum enzyme changes on feeding the toxins and on treatment are given in Table 5. Hb level decreased significantly in all toxin affected groups and on treatment with GO and Vit.E, both ameliorated significantly the Hb level in all groups, however the former was more effective than the latter. Serum aspartate transaminase (AST) and alanine transaminase (ALT) activities increased significantly in toxin-affected rats separately or in combination. Treatment with GO or Vitamin E ameliorated these adverse effects significantly. On the contrary serum catalase activity decreased significantly in the toxin affected groups and the maximum decrease of catalase activity was in lead acetate + alcohol fed group. Treatment with GO. and Vitamin E ameliorated these conditions. Results on lipid per oxidation in terms of TBARS (Thiobarbituric acid reacting substances) in serum, liver, heart, and kidney and blood level of lead in the first 4 groups are as follows in terms of micro gram/ml viz; 0.8, 1.5, 0.8 and 0.9, respectively, and the result show that GO and Vitamin E significantly decreased the BLL to near normal levels and Vitamins C and E levels in the serums and triacyl glycerols (TAG) in adipose tissue are given in Tables 6 and 7, respectively. The increase in serum lipid per oxidation in terms of TBARS was 57% in lead acetate fed group, 60% in alcohol fed group and 80% in lead acetate + alcohol fed group over the normal value respectively. On treatment with GO these values were brought down to normal and with Vitamin E to near normal level only i.e. 15–20% above the normal. More or less similar variations of lipid per oxidations in liver, heart, and kidney of the corresponding groups are observed and the ameliorations by treatment with GO and Vitamin E are significant in all the groups. Values of BLL in groups 1–4 are given in Table 7. BLL in group 2 increased significantly over the normal and other groups. The same decreased significantly on treatment with GO and Vitamin E in groups 3 and 4.
The Vitamin E levels in the serum of the above toxin-affected groups were lowered by 67, 60 and 76% of the normal, respectively. On treatment with GO, Vitamin E level in serum was raised to 86, 87 and 85% of the normal, respectively in the corresponding groups and these effects were significant too. Similarly the Vitamin C levels in the serum of the above toxin-affected groups were also lowered by 35, 42 and 56% of the normal, respectively. These values on treatment with GO were raised to 96, 92 and 72% of the normal, respectively in the corresponding groups. On treatment with Vitamin E, its serum levels in the corresponding groups were raised to a lower level only, i.e. 90, 85 and 65% of the normal, respectively in the corresponding groups and that of Vit. C to 88, 85 and 65% of the normal, respectively in the serum, and all these effects of GO and vitamin E are significant too (P < 0.05). TAG levels in adipose tissues of the toxin fed groups increased by 1.5 times and on treatment with GO or Vitamin E these elevated values were lowered to near normal level significantly and the increase or decrease of these values very well correspond with the increases in body weight of each group.
Histopathological changes of the liver of groups 1–3 can be observed in the Fig. 1. Treatment with GO or Vitamin E protected the structural integrity of liver to a great extent in all toxin affected rats as per the expert who observed all the sample diagrams. While lead acetate feeding produced congestion and necrosis of the liver tissue, treatment with GO or Vitamin E improved the condition of the tissues to a state of lesser damage i.e. focal necrosis only. When alcohol feeding damaged the liver with marginal necrosis only, alcohol + lead acetate feeding produced extensive necrosis. Treatment with GO and Vit E protected the liver of alcohol fed rats to a condition of very small necrosis. In the combined toxin affected groups treatment with GO or Vitamin E also protected the liver to a condition of lesser damage i.e. focal necrosis only.
Treatment with GO or Vitamin E protected the tissues viz; liver, heart and kidneys to a great extent from lipid per oxidation and related alterations in proteins and lipid levels (all values for kidney are not given due to shortage of space) in toxin affected rats. In general both GO and Vitamin E protected the rats to a great extent from the damages of the toxins used in the study. In most of the cases GO produced a better effect than Vitamin E.
Discussion
The results show that a majority of parameters in serum and tissue altered to a greater extent by feeding lead salt + alcohol to rats than by lead acetate or alcohol alone. The feeding of toxic substances to the rats for a month actually altered their normal levels of lipids, hemoglobin, BLL and proteins and also the activities of enzymes, particularly those we have investigated viz, ALT, AST and Catalase. As a whole the general damages to tissues were reflected by an increased rate of lipid per oxidation. These alterations were brought out in the body mainly through less consumption of food, oxidative stress and hepatotoxic actions of lead salt and alcohol and their metabolites e.g. free radicals, as liver is the main organ that handles and regulates the major pathways of metabolism. Lipid per oxidation in serum, liver, heart and kidney and alterations of marker enzymes such as ALT, AST and catalase in serum are the suitable indices for the derangement of metabolism induced by the above toxins. These data together with the histopathological examination of the liver clearly showed that the toxins actually damaged the liver, but improved its condition by the treatment with GO and Vitamin E. Ethanol due to its ability to diffuse across biological membranes, may affect the absorption of various exogenous compounds and thus potentiate the toxic effects of several toxicants, including heavy metals such as lead [5]. The damaging effects of lead salt may be due to its action on SH group enzymes [24–26] e.g. catalase as for any other heavy metal such as Cd or Hg. Lead toxicity leads to hemolysis and deamination of proteins. Lau [27] showed an experiment in which addition of lead, copper or mercury salt solutions to blood quickly produced hemolysis. However when he added these solutions to blood in presence of a preparation of garlic extract (kyolic) the hemolysis was prevented. The explanation is that garlic sulfides in the extract removed the heavy metals as their sulfides and thus prevented hemolysis. The absorption of lead salt from the intestine of rats might have been reduced by the feeding of GO as its organic sulfide could remove the metal as lead sulfide (PbS). Pet Kov’s preparation of garlic [7] also possessed such properties as to reduce lead toxicity.
The damaging effects of alcohol may be mainly due to its high rate of free radical production [28] and reducing equivalents. High ratio of the reducing equivalent (NADH + H+/NAD+) may decrease the oxidation of fatty acids [29] and increase the deposit of saturated FAs and cholesterol in blood vessels and tissues. Further high NADH level can enhance Fe3+ → Fe2+ conversion which in turn can increase reactive oxygen species (ROS) through Fenton reaction. Free radicals such as O •−2 and OH• attack all types of molecules that contain hydrogen, especially lipid, proteins and nucleic acids. Such reactions may lead to mutations in DNA and damages to receptors of various hormones like insulin and lipid components like LDL cholesterol. Free radicals may also damage –SH group proteins, enzymes etc. and oxidize glutathione. Oxidative stress on heart may lead to cardiovascular diseases (CVD), on liver to hepatotoxicity, on brain to strokes, dementia and Alzheimer’s disease and on eye to cataract. Alcohol and lead toxicity may lead to diseases of various nature including increases in fatty acids [29], ROS [30, 31], blood pressure [32] and hepatotoxicity/liver cirrhosis [33] etc. Further studies by Bailey et al. [34] suggested that the chronic ethanol related increase in hepatocyte ROS may be linked to depressed activity of H2O2 scavenging enzyme glutathione peroxidase. Davies [35] and later Berlett and Stadtman [36] found that proteins may be readily oxidized by various ROS through direct oxidative attack on specific aminoacid residues and that this result in the generation of carbonyl groups and subsequent destruction of protein. Lead in vivo and in vitro readily inhibits porphobilinogen synthase in erythrocytes that regulates Hb synthesis [6].
Chronic exposure to lead salts has been shown in animals by others to its accumulation in organs with maximum concentration in kidneys [37]. The toxic effects of lead were manifested by a decrease in erythrocyte count and haemoglobin level in peripheral blood [6]. These facts give an answer to our findings that why Hb level decreased in the rats exposed to lead salt/alcohol etc.
Jiun and Hsien [38] reported that high concentration of lead in blood leads to lipid peroxidation (LPO) and our findings on LPO may be related to this effect of lead in lead salt fed rats. Lead is reported to have an inhibitory action on the membrane bound enzymes such as Na+K+ATPase, Calcium ATPase and Magnesium ATPase in various vital organs and lead may alter the metabolism of HDL cholesterol [6]. These reports justify the present findings on lead toxicity in our experiment.
Lead induced oxidative stress has been explained by others in the following ways also.
-
1.
The inhibition of delta amino levulinicacid dehydratase by lead as reported by Farant and Wigfield [39] leads to accumulation of this acid which is a potential endogenous source of free radicals [40].
-
2.
Direct interaction of lead [41] with biological membranes induces lipid peroxidation in the presence of Fe2+.
-
3.
Lead induced decreases in free radical scavenging enzymes and glutathione, further contribute to a rise in free radicals [25].
The last reaction is due to high affinity of lead for –SH group or metal co-factor in these enzymes and molecules. In our study a fall observed in the activity of an –SH group enzyme catalase in lead exposed group may be viewed on this account. Pande et al. [42] attributed lead induced oxidative stress to the toxicity of its salts. Studies by Gurer and Ercal [43] confirmed the possible involvement of ROS in lead induced toxicity. According to Lawton and Donaldson [44] lead induced arachidonic acid elongation might be responsible for the enhanced lipid peroxidation in the membrane, which may be reflected in serum also. The fall in BLL parallels with the amelioration of damages brought about by GO and Vitamin E in concerned groups.
In our results we further found that the oxidative stress and related derangements of serum and tissue parameters induced by lead acetate, alcohol and their combination have been significantly counteracted by the antioxidant rich GO (enriched with diallyl disulfides, diallyl polysulfides and their oxides like ajoene) and Vitamin E. This Vitamin as well as Vitamin C are very good antioxidants. Many proved the antioxidant properties of Vitamin E [45, 46] as well as of Vitamin C [47]. They observed that administration of Vitamins C and E significantly inhibited lipid peroxidation of liver, brain, kidney etc. and increased the catalase levels of tissues in lead/Aluminium-exposed rats. Therefore our findings are justified which exhibit the counteracting antioxidant effects of GO comparable with Vitamin E. Here we may note that both Vitamins C and E counteracted the deleterious effects of lead toxicity only through their antioxidant action. But GO counteracts the lead toxicity both through binding with lead ion and also carrying out antioxidant actions. Vitamins C and E as well as organic sulfides of GO can scavange the OH• radical and regenerate the vitamins and sulfides by a process of recycling as reported by others [44, 49].
Moreover free radical removal by GO had definitely spared some Vitamins C and E from their time to time destruction by such free radicals as is evident from the results. The overall corrections to the oxidative damages and BLL brought about by GO are slightly better than that by Vitamin E in the management of lipid profile, protein and enzymes. The oxidants produced by the toxicity of lead salt and alcoholic metabolites consumed a lot of antioxidant vitamins and this was reflected in the vitamin levels of serum and also liver, heart and kidney tissues reported by other students in their project (all tables are not given due to the bulkiness of results).
Recently the antioxidant effects of garlic extract [50, 51] and Vitamin E [45, 52] have been reported by others also. A recent Nigerian study [53] showed that auto-mechanics forms a quite vulnerable group to lead accumulation in their blood during a period of 1–5 years. Now a days everybody is exposed to too much lead and other heavy metals due to polluted air in roads packed with vehicles and filled with heavy cigarette smokers.. Therefore daily consumption of GO/Vit. E or garlic paste in the form of capsules or as food supplement may prevent the excessive production of free radicals that oxidize LDL cholesterol [54] so as to protect our body from the hazards of oxidative stress and other consequences due to the above mentioned environmental pollutants.
References
Juberg DR, Klieman CF, Simons CK. Position paper of the American council on sciences and Health. Lead and human health. Ecotoxicol Environ Safety. 1997;38:162–80.
Goyer RA. Results of lead research. Prenatal exposure and neurological consequences. Environ Health Perspect. 1996;104:1050–4.
Bressler J, Kim KA, Chakraborti T, Goldstein G. Mechanism of lead neurotoxicity. Neurochem Res. 1999;24:595–600.
Flora SJS, Tandon SK. Effect of combined exposure to lead and ethanol on some biochemical indices in the rat. Biochem Pharmacol. 1987;36:537–41.
Flora SJS, Dube SN. Modulatory effects of ethanol ingestion on the toxicology of heavy metals. Ind J Pharmacol. 1994;26:240–8.
Harishekar MB. Studies on alcohol-lead interactive hepatotoxicity. Ph.D.Thesis (submitted), Rajiv Gandhi University of Health Sciences Bangalore, Karnataka. 2003. pp. 77–97.
Pet Kov V, Stoev V, Bakalov D, Petev L. The Bulgarian drug satlal as a remedy for lead intoxication in industrial conditions. Higiena Truda i Profesionaline Zabolevania. 1965;9(4):42.
Aminu B, Augusti KT, Joseph PK. Hypolipidemic effects of onion oil and garlic oil in ethanol fed rats. Ind J Biochem Biophys. 1984;21:211–3.
Augusti KT, Anuradha, Prabha SP. Nutraceutical effects of garlic oil, its non polar fraction and a ficus flavonoid as compared to Vitamin E in CC14 induced liver damage in rats. Ind J Exp Biol. 2005;43:437–44.
Augusti KT, Suneesh K, Krishnankutty BK. Prophylactic and curative effects of garlic oil as compared to α tocopherol against isoproterenol induced damages in rats In: Proceedings of the National Seminar on the Evaluation of Nutraceuticals that prevent diseases held at Thalassery Campus of Kannur University on 29th Nov–2nd Dec 2006. pp. 84–96.
Pinon-Lataillade G, Thoreux-Manlay A, Coffigny H. Effect of ingestion and inhalation of lead on the reproductive system and fertility of adult male rats and their progeny. Hum Exp Toxicol. 1993;12:165–72.
Jayaramman J editor. Modified Biuret method. In: Laboratory Manual in Biochemistry. New Delhi: Wiley Eastern Limited; 1981. p. 78.
Chiamori N, Henry R. Total cholesterol. Zlatkis (modified) method. In: Frankel S, Reitman S, Sonnenwirth AC, editors. Gradwohl’s Clinical Laboratory Methods and Diagnosis. Saint Louis: The C.V. Mosby Company; 1963. p. 257–8.
Friedewalt WT, Levy RT, Frederickson DS. Estimation of concentration of LDL in plasma with out use of preparative ultracentrifuge. Clin Chem. 1972;18:499–502.
Warnick RG, Albers JA. A comprehensive evaluation of the heparin-manganese precipitating procedure for estimating high density lipoprotein cholesterol. J Lipid Res. 1978;19:65–79.
Van Handel E, Zilversmit DB. Serum triglycerides micromethod. In: Frankel S, Reitman S, Sonnenwirth AC, editors. Gradwohl’s Clinical Laboratory Methods and Diagnosis. Saint Louis: The CV Mosby Company; 1963. p. 263–4.
Chaudhry K editor. Haemoglobin estimation by cyamethaemoglobin method. In: Biochemical Techniques. New Delhi: Jaypee Brothers Medical publishers; 1989. pp 83–85.
Tandon SK, Singh S, Prasad S. Influence of garlic on the disposition & toxicity of Lead & cadmium in the rat. Pharmaceu Biol. 2001;39:450–4.
Bergmeyer H, Bernet E, Colorimetric method for aspartatate and alanine aminotransferases. In: Varley H, Gowenlock AH, Bell M editor. Practical Clinical Biochemistry. Vol I, 5th ed. London: William Heinemann Medical Books Ltd.; 1980. p. 741742.
Maehly AC, Chance B. The assay of catalase and peroxidase. In: Glick D, editor. Methods Biochemical Analysis, vol. 1. New York: Interscience; 1954. p. 357–60.
Terada M, Watanabe Y, Kunitomo M, Hayashi E. Differential rapid analysis of AA and AAS by diphenyl hydrazine method. Anal Biochem. 1978;84:604–6.
Catiganin GL. An HPLC method for the simultaneous determination of retinol and α tocopherol in plasma or serum. Methods Enzymol. 1986;123:214–9.
Niehaus WG Jr, Samuelson B. Formation of malondialdehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur J Biochem. 1968;6:126–30.
Chiba M, Shinohara A, Matsushita K, Watanaba H, Inaba Y. Indices of lead exposure in blood and urine of lead-exposed workers and concentrations of major and trace elements and activities of SOD, GSH-Px and catalase in their blood. J Exp Med. 1999;178:49–62.
Sandhir R, Julka D, Gill KD. Lipoperoxidative damage on lead treatment in rat brain and its implications on membrane bound enzymes. Pharmacol Toxicol. 1994;74:66–71.
Sandhir R, Gill KD. Effects of lead on lipid peroxidation in liver of rats. Biol Trace Elem Res. 1995;48:91–7.
Lau BHS. Allergies, pollution, and today’s lifestyle. In: Garlic for Health. Wilmot, WI: Lotus Light Publications; 1988. p. 29–35.
Bailey SM, Cunningham CC. Acute and chronic ethanol increases reactive oxygen species generation and decreases viability in fresh, isolated rat hepatocytes. Hepatology. 1998;28:1318–26.
Blomstrand R, Kager L, Lantto O. Status on studies on the ethanol induced decrease of fatty acid oxidation in rats & human liver slices. Life Sci. 1973;13:113–23.
Ding Y, Gonick HC, Vaziri ND. Lead promotes hydroxyl radical generation and lipid peroxidation in cultured aortic endothetial cells. Am J Hypertens. 2001;13:552–5.
Ding Y, Gonick HC, Vaziri ND, Llang KW. Lead induced hypertension III increased hydroxyl radical production. Am J Hypertens. 2001;14:169–73.
Ellwood PC, Yarnell JWG, Oldham PD. Blood pressure and blood lead surveys in Wales. Am J Exp Demiol. 1988;127:942–5.
Barrio E, Rodriguez I, Tome S, Gude F, Quintela F. Liver disease in heavy drinkers with & without alcohol withdrawal syndrome. Alcohol Clin Exp Res. 2003;28(1):131–6.
Baily SM, Patel VB, Young TA. Chronic ethanol consumption alters the glutathione/glutathione peroxidase-1 system and protein oxidation status in rat liver. Alcohol Clin Exp Res. 2001;25:726–33.
Davies KJA. Protein damage and degeneration by oxygen radicals. J Biochem. 1987;262:9895–902.
Berlett BS, Stadtman ER. Protein oxidation in aging disease and oxidative stress. J Biol Chem. 1997;272(33):2031–6.
Humphreys DJ. Effects of exposure to excessive quantities of lead on animals. Br Vet J. 1991;147:18–30.
Juin YS, Hsien LT. Lipid peroxidation in workers exposed to lead. Arch Environ Health. 1994;49:256–9.
Farant JP, Wigfield DC. Biomonitoring of lead exposure with ALAD activity ratios. Int Arch Occup Environ Health. 1982;51:15–24.
Hermes-Lima M. How do Ca2+ and 5-aminolevulinic acid derived oxyradical promote injury to isolated mitochondria. Free Radic Biol Med. 1995;19:381–90.
Adonaylo VN, Otieza PI. Pb2+ promotes lipid peroxidation and alteration in membrane physical properties. Toxicology. 1999;132:19–32.
Pande M, Mehta A, Pant BP, Flora SJS. Combined administration of a chelating agent and an antioxidant in the prevention and treatment of acute lead intoxication in rats. Environ Toxicol Pharmacol. 2001;9:173–84.
Gurer H, Ercal N. Can antioxidant be beneficial in the treatment of lead poisoning? Free Radic Biol Med. 2000;29:927–45.
Lawton L, Donaldson WE. Lead induced tissue fatty acid alterations and lipid peroxidation. Biol Terace Elem Res. 1991;28:83–97.
Fatma M, El-Demerdash FM. Antioxidant effect of Vitamin E and selenium on lipid peroxidation, enzyme activities and biochemical parameters in rats exposed to aluminium. J Trace Elem Med Biol. 2004;18:113–22.
Buettner GR. The packing order of free radicals and antioxidants. Lipid peoxidation α-tocopherol and ascorbate. Arch Biochem Biophys. 1993;300:535–43.
Patra RC, Swarup D, Dwivedi SK. Antioxidant effects of α-tocopherol, ascorbic acid and L-methionine on lead induced oxidative stress to the liver, kidney and brain in rats. Toxicology. 2001;162:81–8.
May JM, Qu ZC, Mendiratta S. Protection and recycling of alpha tocopherol in human erythrocytes and intracellular ascorbic acid. Arch Biochem Biophys. 1998;349:281–9.
Klanns-Dieter A. Sulfur content of free radicals. In: Nygaard OF, Simic MG, editors. Radioprotectors and Anticarcinogenes. New York: Academic Press; 1983. p. 23–42.
El-Demerdash FM, Yousef MI, Abou El-Naga NI. Biochemical study on the hypoglycemic effects of onion and garlic in alloxan—induced diabetic rats. Food and Chem Technol. 2005;43(1):57–63.
Nuutila AM, Pimia RP, Arni M, Caldentey KMO. Comparison of antioxidant activities of onion and garlic extracts by inhibition of lipid peroxidation and radical scavenging activity. Food Chem. 2003;81(4):485–93.
Onyeze GO. Effect of Vitamin E on mono sodium glutamate induced hepato toxicity, oxidative stress in rats. Ind J Biochem Biophys. 2006;43:20–4.
Babalola OO, Ojo LO, Aderemi MO. Lead levels in some biological samples of auto-mechanic in Abeokutta, Nigeria. Ind J Biochem Biophys. 2005;42:401–3.
Lau BHS. Suppression of LDL oxidation by garlic. J Nutr. 2001;131:958S–88S.
Acknowledgments
The authors acknowledge with thanks Sree Mookambika Institute of Medical Sciences and the M.G. University authorities for some financial support for the work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sajitha, G.R., Jose, R., Andrews, A. et al. Garlic Oil and Vitamin E Prevent the Adverse Effects of Lead Acetate and Ethanol Separately as well as in Combination in the Drinking Water of Rats. Indian J Clin Biochem 25, 280–288 (2010). https://doi.org/10.1007/s12291-010-0042-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12291-010-0042-x