Abstract
Autologous stem cell transplantation (ASCT) is considered as standard of care in patients with multiple myeloma (MM) patients aged 65 years or younger. We analyzed data of 94 patients of plasma cell dyscrasias who underwent 95 autologous transplants at our institute from October 2003 to Aug 2016. Other than 76 patients of newly diagnosed multiple myeloma, we also transplanted two patients of POEMS syndrome, two patients of plasma cell leukemia, three patients of concurrent light chain deposition disease, three patients of multifocal plasmacytomas, and eight patients of isolated light chain myeloma. One patient underwent transplant twice. The median age of patients was 53 years (range 21–65). The average interval between diagnosis and transplant was 10.51 ± 5.42 months. The predominant stage in the study cohort was ISS-III. IgG kappa was the commonest subtype of plasma cell dyscrasia (27.9%) followed by IgG lambda (16.27%). Renal involvement was seen in 25% patients at the time of transplantation. Following chemotherapy, 42% patients were in CR, 39% in VGPR, 5% had PR and 14% had progressive disease at the time of transplantation. All patients were conditioned with melphalan (dose 120–200 mg/m2) except for one who received an additional bortezomib for his second transplant. The mean time to neutrophil and platelet engraftment was 11.09 ± 1.82 and 12.69 ± 4.55 days respectively. Mucositis was noted in all patients (grade 3 in 37.5% patients). The median PFS (biochemical) was 55.8% and PFS (clinical) was 76.7% at 6.5 years. Thirteen percent of the transplanted patients succumbed to their illness of which three patients died within 30 days of transplant. Median OS was 76.7% at 6.5 years. ASCT is a feasible option for MM in India and the results are comparable.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Autologous stem cell transplantation (ASCT) is the standard of care for younger multiple myeloma (MM) patients (defined variably as age less than 65–75 years) [1, 2]. A number of nonrandomized [3, 4], randomized [5, 6], and population-based studies [7] and meta-analyses [8, 9] have suggested that this approach is associated with improved response rates, and event-free survival (EFS) compared with conventional chemotherapy. Autologous stem cell transplantation can also be used to manage patients with progressive or relapsed disease as a form of salvage therapy.
The transplant experience in the real world is much different from the west. The transplant conversion ratio of newly diagnosed multiple myeloma (NDMM) is extremely low secondary to multiple factors such as social factors, universal availability of expertise and costs involved playing a major role [10]. The transplant outcomes are also different owing to the high background infection rate, poor hygiene standards of the patients/caregivers and the educational status of the patients for the diseases [11, 12]. There is a paucity of information on the outcome of ASCT in MM in resource constraint setting. By reporting our transplant experience, we aim to highlight the challenges in the real world and contribute to the Indian database.
Patients and Methods
Patients
The stem cell transplantation program was started in our institute in October 2003. A total of 94 MM patients underwent ASCT for MM till 30 Sep 2016 (Fig. 1). Of these 76 patients had complete data who were included in the outcome study analysis and 18 patients were excluded from outcome analysis due to irretrievable data.
Transplant Protocol
Pre-transplant Evaluation
Initial evaluation included history, physical examination, staging according to International Staging System (ISS). Details of previous treatment were recorded. Investigations including hemogram, differential count, renal and liver function tests, bone marrow examination, skeletal survey, serum and urine electrophoresis, immunofixation studies, serum β-2 microglobulin and immunoglobulin levels (quantitative) were done in all patients. Written informed consent was obtained. During follow-up, patients were seen in the ‘Transplant Clinic’ initially monthly, then bi-to tri-monthly for 3 years, then every 6 months thereafter. Follow-up information is available for all patients. Transplant cost was met by the individuals, government support, medical insurance, and charitable organizations.
All patients were evaluated as per the Institutional protocol for the suitability or fitness for the transplantation (Pre-transplantation Protocol as per Supplement 1). The hematopoietic cell transplantation comorbidity index (HCT-CI) score was calculated for the patients prior to the transplantation.
Stem Cells
As a source of stem cells, granulocyte colony-stimulating factor (G-CSF) mobilized peripheral blood stem cells were harvested. One to two leukapheresis were done using Cobe Spectra apheresis machine. Mononuclear cell (MNC) count was determined by automated cell counter and differential count by manual slide method. The CD34 enumeration was done using fluorescence 4 conjugated anti-CD34 and analyzed using a fluorescence-activated cell sorter scan flow cytometer to yield absolute CD34 counts. Stem cells were kept at 4 °C. Stem cells were transfused intravenously 24 h after intravenous high dose melphalan.
Conditioning Regimen
The myeloablative regimen consisted of melphalan in doses ranging from 120 to 200 mg/m2(doses adjusted according to renal function) slow intravenous (I.V.) push on day minus 1 (D-1) followed by forced alkaline diuresis. In addition, one patient received additional Bortezomib as the conditioning regimen during second transplant. Doses were adjusted according to renal function at the time of transplantation. Autologous blood stem cells were reinfused on day 0 through a central venous catheter preceded by pheniramine maleate 25 mg I.V.
Supportive Care and Monitoring
All patients received growth factors G-CSF 5 µg/kg daily subcutaneously on day + 6 onwards until engraftment. Patients were admitted to an isolation room (HEPA filtered with positive pressure and laminar airflow) and reverse barrier nursing was practiced. All patients received antimicrobial prophylaxis-levofloxacin, fluconazole, and acyclovir. Neutrophil engraftment was defined as the absolute neutrophil count (ANC > 500) for three consecutive days, of which the first day was considered the day of engraftment. The data for platelet engraftment was considered the post-transplantation day of platelet achievement of 20 × 109/L for 3 days without component support. We also collected the data for platelet achievement of 50 × 109/L without any component support. Packed red blood cells and platelet transfusions were administered to maintain a hemoglobin level above 8 g/dL and a platelet count above 10 × 109/L. All the blood products transfused during the post-transplant period were irradiated with 25 Gy. Patients received broad-spectrum antibiotics for fever as an institutional protocol; either amphotericin B or other antifungals were added if patients had a persistent fever after 4–5 days of intravenous antimicrobial therapy. Biochemical and clinical PFS has been defined as the time from transplant to the biochemical and clinical relapse respectively. Biochemical and clinical relapse have been defined in accordance with IMWG 2016 guidelines based on the retrospective data available [13].
Statistical Analysis
Analysis has been done as intent to treat analysis. Descriptive statistics (parametric variables: mean and SD; nonparametric variables: median and range) were calculated for all variables. Overall survival was defined as the time from date of transplant until death or date of censor. Survival curves were plotted according to a method of Kaplan and Meier. The analysis was carried out using JMP (version 13, SW) and SPSS version 16 statistical software. The data were censored on September 30, 2016.
Results
Patient Characters
The median age of the population was 53 years (range 28–65). Study group constituted of 70 (73.7%) males and 25 (26.3%) females.
Disease Characters
Among 94 patients of plasma cell dyscrasias who underwent 95 autologous transplants, other than 76 patients of NDMM, we also transplanted two patients of POEMS syndrome, two patients of plasma cell leukemia, three patients of concurrent light chain deposition disease (LCDD), three patients of multifocal plasmacytomas, and eight patients with isolated light chain myeloma. One patient received two transplants approximately 2.5 years apart for progressive disease. IgG kappa was the commonest subtype of plasma cell dyscrasia (27.9%) followed by IgG lambda (16.27%), IgA kappa (13.95%), IgA lambda (9.3%), pure kappa (11.63%), and isolated lambda (4.65%). For 16.27% of these patient’s reports were not available. Most of the patients in our study cohort had higher risk disease by ISS staging (ISS-3: 51%, ISS-2: 22% and ISS-1: 27%). The median ISS stage at the time of diagnosis for these patients was ISS-3.
Treatment Details
All patients underwent induction therapy either with two drugs’ or three drugs’ regimen. Eight patients also received radiotherapy. The median interval from disease onset to transplant was 9 months (range 3–33 months). Patients received a median of six chemotherapy cycles as induction therapy (range 2–19). Patients received a median of one chemo regimens as part of induction regimen (range 1–3), the commonest being CyBorDex (also known as VCD, 37.4%). Other regimens used were VTD (25.4%), VRD (17.5%) and miscellaneous regimens such as TD or combination of regimens (19.7%).
Pre-transplant Evaluation
Following chemotherapy, 42% patients were in CR, 39% in VGPR, 5% had PR and 14% had progressive disease at the time of transplantation. Renal involvement was seen in 25% patients at the time of transplantation. The performance status prior to transplant was ECOG-0, 1 2 in 19, 67, and 14% patients respectively. The hematological and biochemical parameters of the study population undergoing ASCT are tabulated in Table 1.
Transplantation Details
All these patients received Inj. G-CSF 10 µg/kg for a median duration of 5 days (range 4–8 days). Of these, 9 (11.8%) patients received Inj. Plerixafor (0.24 mg/kg) of which one patient received a double dose. Among the study population, 51.2% patients underwent single harvest and 48.8% patients underwent two harvests to achieve the minimum CD34 dose (2 × 106/kg), except in one patient wherein the ASCT at a dose of 1.28 × 106/kg was done as the second harvest couldn’t be done. Peripheral blood CD34 monitoring on the day prior to harvest was available in only 36.8% (n-28) of the patients (80% of the patients after 2011). The mean harvest volume was 348 ± 105 mL. The median harvest WBC count was 308.1 × 109/L (range 123–415.56). The median MNC/Kg count in the stem cell harvest was 5.67 × 108/kg (range 1.42–28.32). The median CD34/Kg count in the stem cell harvest was 5.1 × 106/kg (range 1.28–16.23).
Engraftment Characteristics
The mean duration of G-CSF post stem cell infusion was 5 days (range 4–8 days). Engraftment occurred in all but one patient who later succumbed to infection. Neutrophil engraftment occurred at an average of 11.09 ± 1.82 days (range 9–18 days). Platelet reconstitution with counts above 20 × 109/L was achieved at an average of 12.69 ± 4.55 days (range 7–39 days) and above 50 × 109/L was achieved at an average of 19.05 ± 5.08 days (range 9–41 days).
Outcomes
Toxicity Episodes
Regimen-related toxicity was seen in all patients. The most common toxicity was mucositis necessitating the use of total parenteral nutrition 31.25% (grade 1 mucositis—100%, grade 2 in 81.25% and grade 3 in 37.5% patients). The average duration of TPN use was 6.025 days. Mucositis was followed by diarrhea (≥ CTCAE grade 2 in 83.3%) and vomiting (≥ CTCAE grade 2 in 70.83%) as other regimen related toxicities.
Febrile Episodes
Febrile neutropenia was observed in 74.41% of patients requiring poly-antimicrobial therapy and lasted for an average of 7.125 days. Blood culture was positive in 11 patients and urine culture in one patient. Gram-negative bacilli (E. Coli, Klebsiella sp.) and Methicillin Resistant S. aureus were the most common organisms isolated. 58% of the febrile patients received antifungals for a median duration of 4 days (range 3–21 days).
Hospital Stay
The median duration of peri-transplant hospital stay (including a pre-transplant stay for evaluation and post-transplant period) was 29 days (range 15–52 days), whereas the median duration of hospital stay post-transplant was 18 days (range 7–45 days) (Fig. 2a, b).
Transplant-Related Mortality During the Post-transplant 30 Days (TRM-30)
Three patients died during first 30 days and all during the neutropenic period including one patient with engraftment failure. Two deaths were related to infectious complications and one due to melphalan related mucositis.
Relapses
A total of 32% had a biochemical relapse and 19% had a clinical relapse. The median duration at which patients developed biochemical relapse was 404 days (range 7–2479 days). The median duration at which patients developed clinical relapse was 511.5 days (range 7–3168 days) (Fig. 2c, d).
Survival
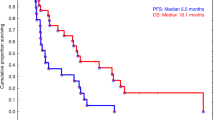
In our complete cohort, till the time of censoring, 13% patients succumbed to the illness. The median duration of survival from the date of transplantation is 599.5 days (range 7–4156 days) (Fig. 2e). The median survival at 6.5 years is 76.7% with median clinical and biochemical PFS being 76.7 and 55.8% respectively (Fig. 3).
Discussion
Transplantation remains the backbone in the management of the MM even in the era of novel agents. Transplantation in Indian settings not only has OS and PFS benefit but also improves the quality of life [14]. Despite its proven benefit, it was performed in only a 0.056% fraction of all the registered MM patients at this institute. These figures are no different from other institutions across the country [15,16,17,18,19] (Table 2). The major contributory factors for not going ahead with the ASCT include patient related factors (such as financial implications, comorbidities, older age, non-achievement of complete remission/VGPR) and medical care related factors (such as lack of the 2nd line drugs and active pharmaceutical based clinical trials in the country, non-availability of transplant beds, lack of outpatient transplantation facilities, doctors understanding of the necessity of transplantation) [17, 20,21,22]. The cost of the transplant in our cohort varied from 715$ to 4895$ (for the expendables and non-expendables purchased by the patient including the drug and medical supplies, this cost doesn’t include the infrastructure and man power costs) which is much lower than that ever reported from India [10]. The high variability in the cost is attributed to the wide range in hospitalization days among patients due to infections for whom expensive antimicrobials and antifungals were used. For better resource utilization, institutes have successfully tried ASCT in non-HEPA filter rooms and harvesting on D4 of GCSF mobilization [22, 23].
The median age of MM patients undergoing a transplant in our study was 53 years. The median age for MM patients undergoing ASCT from other centers from India was 52 [24], 50 [25], 49 [26] and 54 years [27]. The distribution of patients with monoclonal gammopathies is same as at other institutes. The majority of our patients were in ISS stage III at diagnosis, same as what was reported by Shah et al. [25], and Bagal et al. [26].
We used VCD as the commonest induction regimens, whereas studies from India have also studied other induction regimens such as VAD and VD with good results [28, 29]. The median duration of induction therapy/time to transplantation/number of chemotherapy cycles is higher in our case series as compared to the average duration of the induction therapy in the west and is secondary to multiple socio-economic factors other than the medical reasons. Prolonged induction can have a bearing on the stem cell mobilization as has been proven in multiple studies from India [30,31,32].
Approximately 50% of our patients required 2 harvests. The median duration of neutrophils and platelet engraftment was 11 and 12 days respectively which is in consonance with international transplant data [33]. The mean duration of post-transplant hospital stay is 18 days similar to that by Kumar et al. [24]. 74.41% of our study population developed febrile neutropenia which is higher than the international data on MM APBSCT [33].
In our study cohort, 32% of patients developed biochemical relapse and 19% had a clinical relapse. At a follow-up of 6.5 years, the median OS was 76.7%, biochemical PFS was 55.8% and Clinical PFS was 76.7%. In a report by Kumar et al. at a median follow-up of 7 years, median OS and EFS is 85.5 and 41 months, respectively. Estimated OS and EFS at 5 years was 62 ± 0.04% and 41 ± 0.04%, respectively [24]. In a report by Bagal et al. [26] the estimated probability of OS and PFS from transplant by Kaplan–Meier method at 3 years was 92 and 62% respectively.
Conclusions
Autologous stem cell transplantation (ASCT) in multiple myeloma is a low-risk procedure and should be offered to all eligible patients in developing countries.
References
Kyle RA, Rajkumar SV (2008) Multiple myeloma. Blood 111(6):2962–2972
Kumar L, Verma R, Radhakrishnan VR (2010) Recent advances in the management of multiple myeloma. Nat Med J India 23(4):210–218
Selby PJ, McElwain TJ, Nandi AC, Perren TJ, Powles RL, Tillyer CR et al (1987) Multiple myeloma treated with high dose intravenous melphalan. Br J Haematol 66(1):55–62
Desikan R, Barlogie B, Sawyer J, Ayers D, Tricot G, Badros A et al (2000) Results of high-dose therapy for 1000 patients with multiple myeloma: durable complete remissions and superior survival in the absence of chromosome 13 abnormalities. Blood 95(12):4008–4010
Attal M, Harousseau J-L, Stoppa A-M, Sotto J-J, Fuzibet J-G, Rossi J-F et al (1996) A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. N Engl J Med 335(2):91–97
Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K et al (2003) High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med 348(19):1875–1883
Lenhoff S, Hjorth M, Holmberg E, Turesson I, Westin J, Nielsen JL et al (2000) Impact on survival of high-dose therapy with autologous stem cell support in patients younger than 60 years with newly diagnosed multiple myeloma: a population-based study. Blood 95(1):7–11
Koreth J, Cutler CS, Djulbegovic B, Behl R, Schlossman RL, Munshi NC et al (2007) High-dose therapy with single autologous transplantation versus chemotherapy for newly diagnosed multiple myeloma: a systematic review and meta-analysis of randomized controlled trials. Biol Blood Marrow Transpl 13(2):183–196
Lévy V, Katsahian S, Fermand JP, Mary JY, Chevret S (2005) A meta-analysis on data from 575 patients with multiple myeloma randomly assigned to either high-dose therapy or conventional therapy. Medicine 84(4):250–259
Sharma SK, Choudhary D, Gupta N, Dhamija M, Khandelwal V, Kharya G et al (2014) Cost of hematopoietic stem cell transplantation in India. Mediterr J Hematol Infect Dis 6(1):e2014046
Kumar L, Ghosh J, Ganessan P, Gupta A, Hariprasad R, Kochupillai V (2009) High-dose chemotherapy with autologous stem cell transplantation for multiple myeloma: what predicts the outcome? Experience from a developing country. Bone Marrow Transpl 43(6):481–489
Nath UK, Chakrabarti P, Ray SS, Chaudhuri U (2010) Starting autologous transplantation in a non-hepa filtered unit in a medical college with severe resource constraints. Indian J Hematol Blood Transfus 26(4):172
Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P et al (2016) International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol 17(8):e328–e346
Ashok P, Mani K, Sadashivudu G, Srinivas ML (2015) Study of quality of life in multiple myeloma patients after autologous stem cell transplantation. Indian J Hematol Blood Transf 1:S67–S68
Gupta S, Kumar L, Raju GM, Kochupillai V, Shukla DK (2000) Autologous bone marrow/stem cell transplantation: initial experience at a north Indian referral centre. Natl Med J India 13(2):61–66
Gupta A, Kumar L, Dabkara D, Gupta D, Sharma O, Sreeniwas V (2009) Multiple myeloma: autologous stem cell transplantation versus conventional chemotherapy—a retrospective age and stage matched analysis. J Clin Oncol 1:7041
Lu J, Hou J, Liu K-Y, Parmar S, De La Fuente A, Andersson B et al (2016) Asia-Pacific Hematology Consortium Report on approach to multiple myeloma. Survey results from the 6th international hematologic malignancies conference: Bridging the Gap 2015, Beijing, China. Leuk Lymphoma 57(7):1534–1538
Prakash Yadav S, Kalra M, Sachdeva A, Dinand V, Parashar N, Kohli S et al (2009) The experience of hematopoietic stem cell transplantation from an emerging centre in north India. In: Blood conference: 51st annual meeting of the American society of hematology, ASH New Orleans, LA United States Conference Start, vol 114, no. 22
Sagar TG, Rathinam K, Trivadi G, Rejiv R, Prasanth G (2015) Outcomes in multiple myeloma post high dose chemotherapy and autologous stem cell transplantation-single institute experience. Indian J Hematol Blood Transf 1:S65
Kumar L, Malik PS, Prakash G, Prabu R, Radhakrishnan V, Katyal S et al (2011) Autologous hematopoietic stem cell transplantation-what determines the outcome: an experience from North India. Ann Hematol 90(11):1317–1328
Kumar L (2013) Autologous stem cell transplant for multiple myeloma. Indian J Hematol Blood Transf 29(4):243
Dolai TK, Kumar M, Kumar Mandal P, Saha S, Bagchi B, Panigrahi A et al (2013) Auto-SCT for myeloma patients in non-HEPA filtered room: initial experience of a tertiary care center. Leuk Lymphoma 54:26
Kaul E, Kothari S, Sharma SK, Dhamija M, Kharya G, Khandelwal V et al (2015) Day 4 peripheral blood stem cell collection in myeloma patients is feasible and cost-effective in a majority of patients. Clin Lymphoma Myeloma Leuk 15:e169–e170
Kumar L, Cyriac SL, Tejomurtula TV, Bahl A, Biswas B, Sahoo RK et al (2013) Autologous stem cell transplantation for multiple myeloma: identification of prognostic factors. Clin Lymphoma Myeloma Leuk 13(1):32–41
Shah CA, Karanwal A, Desai M, Pandya M, Shah R (2015) Hematopoietic stem-cell transplantation in the developing world: experience from a center in Western India. J Oncol 2015:710543
Bagal B, Jain H, Dangi U, Sengar M, Kannan S, Rangarajan V et al (2014) Pretransplant PET positivity predicts early relapse with poor outcome in patients of multiple myeloma undergoing autologous stem cell transplantation. Blood 124(21):3997
Mukhopadhyay A, Gupta P, Basak J, Chakraborty A, Bhattacharyya D, Mukhopadhyay S et al (2012) Stem cell transplant: an experience from eastern India. Indian J Med Paediatr Oncol 33(4):203–209
Aggarwal S, Bhalla A, Khatri SL, Anand Simar S, Bhargava M, Saran R (2011) Bortezomib plus dexamethasone as induction treatment followed by autologous peripheral blood stem cell transplantation in patients with multiple myeloma: a study from india. In: Blood conference: 53rd annual meeting of the American society of hematology, ASH, vol 118, no. 21
Bagal BP, Khattry N, Dongre A, Kanan S, Menon H, Sengar M et al (2012) Outcomes of autologous stem cell transplant (ASCT) in multiple myeloma from a tertiary cancer center in India. J Clin Oncol Conf 30(15 SUPPL. 1):e17003
Kumar L, Iqbal N, Mookerjee A, Verma RK, Sharma OD, Batra A et al (2014) Complete response after autologous stem cell transplant in multiple myeloma. Cancer Med 3(4):939–946
Boya RR, Bakhshi S, Gogia A, Sahoo RK, Kumar L (2015) Prediction of poor mobilization of Peripheral Blood Stem Cells (PBSC) with G-CSF alone, in multiple myeloma (MM) patients undergoing autologous transplantation: a prospective study. Clin Lymphoma Myeloma Leuk 15:e171–e172
Reddy R, Bakhshi S, Sharma A, Sahoo R, Gogia A, Malik PS et al (2015) Poor mobilization of peipheral blood stem cells (PBSC) in patients undergoing autologous stem cell transplantation (ASCT): a prospective study. J Clin Oncol Conf 33(15 SUPPL. 1):e18013
Munker R, Shi R, Nair B, Devarakonda S, Cotelingam JD, McLarty J et al (2015) The Shreveport myeloma experience: survival, risk factors and other malignancies in the age of stem cell transplantation. Acta Haematol 135(3):146–155
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All Authors declare that they have no conflict of interest.
Human and Animal Rights
This research is involving human participants.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Malhotra, P., Yanamandra, U., Khadwal, A. et al. Autologous Stem Cell Transplantation for Multiple Myeloma: Single Centre Experience from North India. Indian J Hematol Blood Transfus 34, 261–267 (2018). https://doi.org/10.1007/s12288-017-0876-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12288-017-0876-y