Abstract
Purpose
Dendritic cells (DCs) are the most potent antigen-presenting cells that play a major role in initiating the antitumor immune response in different types of cancer. However, the prognostic significance of the accumulation of these cells in human early breast tumors is not totally clear. The aim of this study is to evaluate the prognostic relevance of CD1a( +) and CD83( +) dendritic cells in early breast cancer patients.
Methods
We conducted immunohistochemical assays to determine the number of stromal CD1a( +) and CD83( +) DCs in primary tumors from early invasive ductal breast cancer patients, and analyzed their association with clinico-pathological characteristics.
Results
Patients with high CD1a( +) DC number had lower risk of bone metastatic occurrence, as well as, longer disease-free survival (DFS), bone metastasis-free survival (BMFS) and overall survival (OS). Moreover, CD1a( +) DC number was an independent prognostic factor for BMFS and OS. In contrast, we found that patients with high number of CD83( +) DCs had lower risk of mix (bone and visceral)-metastatic occurrence. Likewise, these patients presented better prognosis with longer DFS, mix-MFS and OS. Furthermore, CD83( +) DC number was an independent prognostic factor for DFS and OS.
Conclusion
The quantification of the stromal infiltration of DCs expressing CD1a or CD83 in early invasive breast cancer patients serves to indicate the prognostic risk of developing metastasis in a specific site.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most common tumor type observed in women worldwide and the second leading cause of cancer death in the world with a mortality rate of 18 cases per 100,000 women in Argentina [1]. Despite advances in developing breast cancer therapies, the identification of new prognostic markers to be used in the clinic-pathological routine is needed for personalized treatment.
It is known that breast cancer pathogenesis depends on different factors [2]. The components of tumor microenvironment are implicated in promoting the “hallmarks” of cancer cells, as well as their proliferation and survival [3,4,5]. Particularly the immune system is an active component of the breast tumor microenvironment, interacting with tumor and non-tumor cells involved in the immunological response [6].
Dendritic cells (DCs) infiltrate most cancer types, like breast cancer, and serve a protective role in antitumor immunity through the expression of co-stimulatory molecules and inflammatory cytokines and by promoting the activation of T cells [7, 8]. DCs also promote immunosuppression by secreting anti-inflammatory cytokines [9,10,11,12], or by expressing negative immunological checkpoint molecules, which inhibit T cell activation [13]. Within the tumor, infiltrating DCs are heterogeneous in regard to maturation, differentiation and state of activation [14], which are controlled and regulated by a variety of microenvironmental signals, including cytokines and other surface molecules expressed on neighboring cells [15, 16].
CD1 has considerable structural homology with both major histocompatibility complex (MHC) class I and class II molecules, and is involved in T cell activation. In contrast to MHC, CD1a appears to present predominantly non-peptide molecules originating from lipids and glycolipids [8, 17,18,19,20,21]. Although CD1a is usually known as a marker for immature DCs, in vitro systems show that it is also expressed in mature DCs [22, 23]. Considering the lipid alterations existing during tumor development, CD1a could play an important antitumor role [18, 24]. Previous studies show that the tumor infiltration of CD1a( +) DCs is associated with a favorable prognosis in many types of cancer [23, 25,26,27]. However, its implication in breast tumor progression remains uncertain.
CD83 has been identified to be expressed on mature and activated DCs [28]. These cells have the unique ability for antigen cross-presentation for helper and cytotoxic T lymphocytes in secondary lymphoid organs [29, 30]. Tze LE et al. demonstrated that the transmembrane-domain of CD83 is both necessary and sufficient to stabilize MHC class II and CD86 surface expression on bone marrow DCs [31]. Thus, CD83 in DCs could also critically influences the outcome of T cell stimulation. However, the precise biologic function of CD83 on DCs remains the subject of controversial discussion. Interestingly, some studies show that the expression of the CD83 marker in DCs could predict the survival of patients with breast cancer [19, 32].
Therefore, the aim of this present work was to study and clarify the prognostic relevance of the number of CD1a( +) and CD83( +) DCs in early invasive ductal breast carcinoma (I/II stage).
Materials and methods
Patient sample selection
We conducted a retrospective study including consecutive patients (age range 35–85 years) with confirmed breast cancer histological diagnosis who had undergone surgery at the Hospital Italiano, Buenos Aires, Argentina. The patients were women with early invasive ductal breast carcinoma (I/II stage), according to the International Union Against Cancer TNM classification system [33], with a minimum of 5-year follow-up after surgery. The cases were diagnosed between 2001 and 2012. The study started with 122 samples. Eight patients were subsequently excluded due to prior neoadjuvant therapies, lack of tissue, and/or another primary tumor development, leaving 110 and 112 cases to study the expression of CD1a and CD83, respectively. All patients were treated according to the recommendations of the European Society for Medical Oncology [34]. In our particular case, 84.0% of the patients (n = 112) were Luminal-like, being 69.6% (n = 78) of them Luminal A [estrogen receptor (ER) + , progesterone receptor (PR) + , epidermal growth factor receptor (HER2/neu) -] and 14.4% (n = 16) Luminal B (ER ± , PR ± , HER2/neu +) and received hormone therapy and/or chemotherapy treatment. Moreover, 8.0% (n = 9) of the patients were Basal-like (ER -, PR -, HER2/neu -) and received chemotherapy. The remaining 8.0% (n = 9) of the patients had overexpression of HER2/neu and were treated with trastuzumab and chemotherapy. The Ethic Committees of the Instituto de Biología y Medicina Experimental (IBYME) and the Hospital Italiano approved this study; and informed consent was obtained from patients or their relatives (IBYME’ approval: CE 051/June 2015 and approvement of Hospital Italiano: nº1972/April 2013). This work was performed in accordance with the principles of the Helsinki Declaration. Patients’ medical records and the anonymity of the data were insured using a numeral code.
Classical prognostic markers were categorized according to cutoffs used in the protocols of the Hospital Italiano [35] including (a) age < 50 or ≥ 50 years; (b) tumor size ≤ 2 or > 2 cm; (c) histological grade according to the Scarff–Bloom–Richardson grading system [36]; which is expressed as differentiated (G1), intermediate (G2), and poor (G3); (d) expression of ER and PR and HER2/neu was defined as negative or positive according to Wernicke M et al. [35]; (e) presence of regional metastatic lymph nodes was recorded as negative (negative nodes in axillary dissection or sentinel lymph node) or positive (including micrometastasis) (Table 1). Outcome data also included local relapse, metastatic occurrence, bone metastatic occurrence, visceral metastatic occurrence, mix (bone + visceral) metastatic occurrence, disease-free survival (DFS), local relapse-free survival, metastasis-free survival (MFS), bone metastasis-free survival (BMFS), visceral metastasis-free survival (VMFS), mix metastasis-free survival (mix-MFS), and overall survival (OS). DFS, MFS, BMFS, VMFS and mix-MFS were defined as the interval from the date of surgery to the first observation of tumor occurrence (metastatic event and/or local relapse) or last follow-up. The interval from the date of surgery until death or last follow-up was defined as OS [37].
The site of breast cancer metastasis and the number of patients per site of metastasis were the following: bone metastasis [costal arches (n = 3), lumbar spine (n = 2), sternal body (n = 2), sacrum (n = 1) and multiple bone sites (n = 2)], visceral metastasis [hepatic (n = 4), pleural (n = 3) and pulmonary (n = 3)] and mix-metastases [sacrum–hepatic–pulmonary (n = 1), cost arch–hepatic (n = 1), spine–hepatic–pulmonary (n = 3), cost arch–hepatic–gastric (n = 1) and finally iliac bone–hepatic (n = 1)] (Table 1).
Tissue processing
Breast tissues were processed as described by Martinez LM et al. [37].
Analysis of intratumoral stroma CD1a and CD83 DCs
Immunohistochemistry technique was used to determine the numbers of CD1a and CD83 DCs as we described in a previous work using 0.01 M citrate buffer, pH 6, (anhydrous sodium citrate, #7171, Anedra, Buenos Aires, Argentina) as antigen-retrieval [37]. Briefly, the sections were incubated overnight at 4 °C in a humidified chamber with primary antibodies anti-human CD1a (1/50, rabbit IgG, EP3622, Cell Marque, Rocklin, CA, USA) or CD83 (1/40, mouse IgG1, Ab49324, Abcam, Cambridge, UK). We revealed the presence of these DCs with a peroxidase-based immunohistochemistry staining method (K0690, Dako, Santa Clara, CA, USA) and a 3,3′-diaminobenzidine tetrahydrochloride substrate system (K3468, Dako, Santa Clara, CA, USA) was used as the chromogen. Hematoxylin (#121, Biopur, Rosario, Santa Fe, Argentina) was employed for counterstaining, and the slides mounted for viewing using Canada Balsam (#141, Biopur, Rosario, Santa Fe, Argentina). Negative controls were performed with an irrelevant antibody as an isotype control: mouse IgG1 isotype (X0931, Dako, Santa Clara, CA, USA) and normal rabbit immunoglobulins (X0936, Dako, Santa Clara, CA, USA), according to the concentration of the primary antibodies.
Cells displaying membranous staining, nuclear counterstaining and typical DC morphology were counted in the tumor stroma. DC density was quantified as the mean number of intratumoral stroma CD1a( +) or CD83( +) cells of 5 representative optical field areas per tissue section (X400 magnification). The reading of the slides was estimated independently by two pathologists. There was 87.5% agreement in immunohistochemical evaluation between the two observers (Kappa value = 0.840).
Statistical analysis
The statistical analysis of the associations between the number of intratumoral stroma CD1a or CD83 DCs and clinico-pathological characteristics, as well as the determination of the optimal cutoff value, was made as previously described by Martinez LM et al. [37]. The cutoff value was used to classify the number of DCs as negative/low or high. To determine the optimal cutoff value, the first quartile (Q1), median, and the third quartile (Q3) values were used for the binomial classification of samples. Then we individually tested the association between categorized intratumoral stroma number of DCs and OS of patients in univariate analysis. The cutoff value with the lowest p value was chosen. The optimal cutoff values for intratumoral stroma DCs were as follows: CD1a = 2.80 (median) and CD83 = 0.00 (Q1). In the case of the CD83 cutoff value, any CD83( +) DC number was considered above the cutoff.
We used Fisher’s exact test to evaluate the association of intratumoral stroma DCs with classical prognostic markers as well as local relapse, metastatic occurrence, bone metastatic occurrence, visceral metastatic occurrence and mix-metastatic occurrence. The relation between the number of DCs and metastatic occurrence (bone, visceral and mix) was displayed as a heat map prepared using Excel (Fig. 1). Univariate analyses of DFS, local relapse-free survival, MFS, BMFS, VMFS, mix-MFS and OS were evaluated using the Kaplan–Meier method, and the differences were evaluated using the log-rank (Mantel–Cox) test [34]. The application of the Cox proportional hazards model to the multivariate survival analysis used backward stepwise selection (likelihood ratio) incorporating only the significant variables in the univariate analysis. Finally, it is important to highlight that Cox regression (Cox proportional hazards regression) model for survival-time (time-to-event) demonstrated that the total number of events included in this study was sufficient to strengthen our results. Signification level was set at 0.05. Statistical analysis was performed by an expert statistician using SPSS software (version 18.00, Chicago, Illinois).
Results
Association of the number of intratumoral stroma CD1a and CD83 DCs with patients’ clinico-pathological characteristics
Our data demonstrated that CD1a( +) and CD83( +) DC numbers were associated with tumor size (p = 0.0049 and p = 0.0316, respectively. Table 2). Patients with tumor size ≤ 2 cm had a high number of CD1a and CD83 DCs (Table 2).
In addition, patients with high numbers of CD1a( +) DCs had a significantly lower risk of metastatic and bone metastatic occurrence than patients with low number, p = 0.0067 and 0.0348, respectively (Table 2, Fig. 1 heat map). Patients with high number of CD83( +) DCs had a significantly lower risk of metastatic and mix-metastatic occurrence than patients with low number, p = 0.0038 and 0.0223, respectively (Table 2, Fig. 1 heat map).
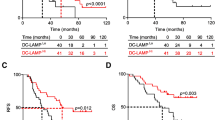
Furthermore, we observed an association of the high number of CD1a( +) DCs with longer DFS, MFS, BMFS and OS (p = 0.0111, 0.0016, 0.0109 and 0.0245, respectively. Table 3 and Fig. 2). The values of DFS, MFS, BMFS and OS of the patients with high versus low number of CD1a( +) were (months as mean ± SE) as follows: DFS = 172.96 ± 8.80 vs. 124.95 ± 9.43, MFS = 177.04 ± 8.82 vs. 120.47 ± 10.81, BMFS = 196.41 ± 3.55 vs. 152.18 ± 8.25 and OS = 184.62 ± 6.58 vs. 143.24 ± 7.82, respectively.
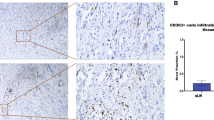
Association of CD1a( +) dendritic cells number with disease-free survival, metastasis-free survival, bone metastasis-free survival and overall survival in early invasive ductal breast cancer patients. A Kaplan–Meier curve (Univariate analysis) shows data for tumor samples with high and negative/low of CD1a( +) dendritic cells number. B Photographs show a representative immunohistochemistry staining for CD1a( +) dendritic cells of primary tumor tissue from a breast cancer patient. The arrows show positive staining of evaluated CD1a( +). Inset shows positive expression of CD1a dendritic cell. No staining was observed in the tissue when we incubated with normal rabbit IgGs as a negative control. Nuclei were counterstained with hematoxylin (purple). Original magnification: 400 × . The scale bar represents 25 and 10 μm in the inset
High number of CD83( +) DCs was associated with longer DFS, MFS, mix-MFS and OS (p = 0.0018, 0.0016, 0.0099 and 0.0028, respectively. Table 3, Fig. 3). The values of DFS, MFS, mix-MFS and OS of the patients with high number of CD83( +) DCs were as follows (months as mean ± SE): 155. 65 ± 6.60, 154.97 ± 7.01, 173.59 ± 3.31 and 165.34 ± 5.01, respectively. While, the values of DFS, MFS, mix-MFS and OS of the patients with low/negative CD83( +) DC number were: 133.74 ± 11.63, 138.49 ± 11.22, 176.05 ± 8.38 and 153.02 ± 10.17, respectively.
Association of CD83( +) dendritic cells number with disease-free survival, metastasis-free survival, mix metastasis-free survival and overall survival in early invasive ductal breast cancer patients. A Kaplan–Meier curve (Univariate analysis) shows data for tumor samples with high and negative/low CD83( +) dendritic cells number. B Photographs show a representative immunohistochemistry staining for CD83( +) dendritic cells of primary tumor tissues from breast cancer patients. The arrows show positive staining of evaluated CD83( +). Inset shows positive expression of CD83 dendritic cell. No staining was observed in the tissue when we incubated with normal mouse IgG1 as a negative isotype control. Nuclei were counterstained with hematoxylin (purple). Original magnification: 400 × . The scale bar represents 25 and 10 μm in the inset
Association of classical prognostic factors with tumor progression
Age was associated with visceral metastatic occurrence in these patients (p = 0.0037, Table 4). Furthermore, the age was associated with DFS, MFS and VMFS (p = 0.0406, 0.0298 and 0.0008, respectively. Table 3). In addition, tumor size was associated with metastatic occurrence, as well as bone and mix-metastatic occurrence (p = 0.0002, 0.0219 and 0.0045, respectively. Table 4). Patients with tumors > 2 cm had a higher risk of developing metastasis, bone metastasis and mix metastasis. All these patients also had significantly lower values of DFS, MFS, BMFS, mix-MFS and OS compared with those patients with tumor size ≤ 2 cm (p = 0.0009, 0.0001, 0.0048, 0.0005 and 0.0004, respectively. Table 3). Moreover, there was an association between tumor differentiation grade and MFS (p = 0.0411. Table 3). Likewise, the patients with ER positive had a significantly lower risk of metastatic occurrence (p = 0.0443. Table 4). Even more, these patients presented higher values of DFS, MFS, VMFS and OS (p = 0.0255, 0.0152, 0.0469 and 0.0006, respectively. Table 3). Finally, the patients with PR positive had a significantly lower risk of developing visceral metastatic occurrence (p = 0.0356. Table 4). These patients showed higher values of VMFS and OS compared with PR negative (p = 0.0270 and 0.0047, respectively. Table 3).
Multivariate analysis
Intratumoral stroma CD1a( +) DC number was an independent prognostic factor for MFS, BMFS and OS (p = 0.0229, 0.0342 and 0.0222, respectively. Table 5). Also, CD83( +) DC number was an independent prognostic factor for DFS and OS (p = 0.0257 and 0.0371, respectively. Table 5). ER expression was an independent prognostic factor for OS (p = 0.0020), while tumor size was an independent prognostic factor for DFS and mix-MFS (p = 0.0285 and 0.0162, respectively. Table 5).
Discussion
Our study revealed that the number of CD1a( +) DCs into breast tumor tissue had a significant impact on the prognosis after surgery. Interestedly, our results showed that patients with a high number of intratumoral stroma CD1a( +) DCs had lower risk of metastatic occurrence, in particular in bone, as well as longer DFS, MFS, BMFS and OS. Moreover, the number of CD1a( +) DCs was an independent prognostic factor for MFS, BMFS and OS. All these results suggest that the pathological evaluation of CD1a( +) DCs in tumor samples could have clinical implications regarding the selection of specific therapies for patients with early invasive ductal breast cancer. Our findings are similar to those found in other types of cancer [18, 23, 27, 38, 39]. Although Coventry BJ et al. [38] found that tumor-infiltrating CD1a ( +) DCs did not correlate with OS at the 5-year time point following surgery, they found an association trend. In relation to this result, Coventry BJ and co-authors hypothesized that the small sample size could have been a possible limitation of the study (n = 48).
We also observed that a high number of intratumoral stroma CD83( +) DCs were associated with good prognosis. Importantly, the high number of CD83( +) DCs correlated with a decreased risk of metastatic occurrence, in particular mix-metastatic occurrence. Furthermore, these patients presented better prognosis with longer DFS, MFS, mix-MFS and OS. Moreover, the number of CD83( +) DCs was an independent prognostic factor for DFS and OS. These last results are in agreement with the observations of Iwamoto M et al. [19], who reported a significant association between the increasing number of CD83( +) DC infiltration and longer local relapse-free survival and OS.
Anti-tumor immune responses are often present in patients with cancers, but appear to be ineffective, with apparent local immunosuppression present in many cases [40]. Inhibition of DC function, perhaps by CD1a and CD83 down-regulation, is a possible mechanism for this immunosuppression. One of the possible causes of the decreased infiltration of CD1a( +) and CD83( +) DCs in breast tumor may be the presence of immunosuppressive factors, which would modify the events of differentiation, maturation and activation of DCs [19, 41, 42].
Sombroek C et al. [42] found that CD1a expression can be inhibited by tumor-derived factors, like IL-10, IL-6, and PGE2, from different cancer cell lines, like breast cancer. This event could not only interfere with DC detection (using CD1a as a marker) but also reduce antigen presentation via CD1a pathway. In previous work, using the same cohort of patients, we observed that IL-6 was differentially expressed between tumor and normal breast tissue [43]. We found an increase of IL-6 expression in both tumor cells and spindle-shaped stromal cells, not associated with the vasculature, compared to normal breast tissue [43]. In parallel, other investigators observed that IL-6 secreted by breast cancer cells can shift monocyte differentiation into macrophages at the expense of DCs, thereby skewing antigen presentation toward antigen degradation [44, 45]. Moreover, studies showed that this inflammatory cytokine is involved in promoting tumor proliferation, angiogenesis and vasculogenesis as well as osteoclastogenesis [46,47,48]. Finally, it is known that patients with bone metastasis have elevated serum levels of IL-6 and soluble IL-6R and they are associated with a poor clinical outcome [49]. Taking into account all these previous results of other authors and ours, we can infer that the presence of IL-6 could decrease the amount of CD1a( +) DC infiltration in primary tumors increasing the development of bone metastasis in early invasive breast cancer patients.
In the case of CD83( +) DCs, it is known that CD83 has been identified to be expressed on mature and activated DCs [19, 32]. Mature DCs may be of great importance in initiating the primary antitumor immune response [19]. In particular, the transmembrane-domain of CD83 is both necessary and sufficient to stabilize MHC class II and CD86 surface expression on bone marrow DCs [31]. Thus, CD83( +) DCs have the unique ability for antigen cross-presentation for helper and cytotoxic T lymphocytes in secondary lymphoid organs [29, 30]. It is known that generally the infiltration of DCs in tumors has been associated with better prognosis and less occurrence of metastases [19]. In relation, Iwamoto M et al. found that the number of CD83 ( +) DCs was associated with both local relapse-free survival and OS in patients with breast cancer [19]. However, the precise biologic function of CD83( +) DCs in the progression of breast cancer remains a subject of discussion. Therefore, future studies need to be done to understand why the high number of CD83( +) DCs correlated in particular with a decreased risk of mix-metastatic occurrence.
In summary, we demonstrated that a high number of intratumoral stroma CD1a( +) and CD83( +) DCs serve as prognostic markers of good patient outcome. Specifically, we found that high stromal infiltration of CD1a( +) DCs indicates a low risk of developing bone metastasis in early invasive breast cancer patients. Meanwhile, high stromal infiltration of CD83( +) DCs indicates a low risk of developing mix-metastasis (bone + visceral). These new findings could help in the selection of therapies for a subgroup of patients with a poor outcome. So, it may provide a rationale for further studies designed to combine immunotherapy with the capability to activate the host immune system (like antigen-pulsed DC second-line therapy) together with routine treatment, heralding the development of a more effective therapeutic strategy for breast carcinoma.
Availability of data and material
Not applicable.
References
International Agency for Research on Cancer. GLOBOCAN cancer fact sheet 2018. 2018. http://globocan.iarc.fr/factsheet/cancer.
Place AE, Jin Huh S, Polyak K. The microenvironment in breast cancer progression: biology and implications for treatment. Breast Cancer Res. 2011;13:227. https://doi.org/10.1186/bcr2912.
Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–22. https://doi.org/10.1016/j.ccr.2012.02.022.
Criscitiello C, Esposito A, Curigliano G. Tumor-stroma crosstalk: targeting stroma in breast cancer. Curr Opin Oncol. 2014;26:551–5. https://doi.org/10.1097/CCO.0000000000000122.
Cirri P, Chiarugi P. Cancer-associated-fibroblasts and tumour cells: a diabolic liaison driving cancer progression. Cancer Metastasis Rev. 2012;31:195–208. https://doi.org/10.1007/s10555-011-9340-x.
Sadeghalvad M, Mohammadi-Motlagh HR, Rezaei N. Immune microenvironment in different molecular subtypes of ductal breast carcinoma. Breast Cancer Res Treat. 2020;185:261–79. https://doi.org/10.1007/s10549-020-05954-2.
Dunn GP, Bruce AT, Ikeda H, et al. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–8. https://doi.org/10.1038/ni1102-991.
Bell D, Chomarat P, Broyles D, et al. In breast carcinoma tissue, immature dendritic cells reside within the tumor, whereas mature dendritic cells are located in peritumoral areas. J Exp Med. 1999;190:1417–26. https://doi.org/10.1084/jem.190.10.1417.
DeNardo DG, Coussens LM. Inflammation and breast cancer. Balancing immune response: crosstalk between adaptive and innate immune cells during breast cancer progression. Breast Cancer Res. 2007;9:212. https://doi.org/10.1186/bcr1746
Ghiringhelli F, Ménard C, Terme M, et al. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-β-dependent manner. J Exp Med. 2005;202:1075–85. https://doi.org/10.1084/jem.20051511.
Faget J, Bendriss-Vermare N, Gobert M, et al. ICOS-ligand expression on plasmacytoid dendritic cells supports breast cancer progression by promoting the accumulation of immunosuppressive CD4 + T cells. Can Res. 2012;72:6130–41. https://doi.org/10.1158/0008-5472.CAN-12-2409.
Michea P, Noël F, Zakine E, et al. Adjustment of dendritic cells to the breast-cancer microenvironment is subset specific. Nat Immunol. 2018;19:885–97. https://doi.org/10.1038/s41590-018-0145-8.
Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–61. https://doi.org/10.1016/j.ccell.2015.03.001.
Becker Y. Anticancer role of dendritic cells (DC) in human and experimental cancers—a review. Anticancer Res. 1992;12:511–20.
Banchereau J, Steinman R. Dendritic cells and the control of immunity. Exp Hematol. 1998;392:245–52. https://doi.org/10.1038/32588.
Tazi A, Bouchonnet F, Grandsaigne M, et al. Evidence that granulocyte macrophage-colony-stimulating factor regulates the distribution and differentiated state of dendritic cells/Langerhans cells in human lung and lung cancers. J Clin Investig. 1993;91:566–76. https://doi.org/10.1172/JCI116236.
Coventry B. CD1a positive putative tumour infiltrating dendritic cells in human breast cancer. Anticancer Res. 1999;19:3183–7.
Coventry B, Heinzel S. CD1a in human cancers: a new role for an old molecule. Trends Immunol. 2004;25:242–8. https://doi.org/10.1016/j.it.2004.03.002.
Iwamoto M, Shinohara H, Miyamoto A, et al. Prognostic value of tumor-infiltrating dendritic cells expressing CD83 in human breast carcinomas. Int J Cancer. 2003;104:92–7. https://doi.org/10.1002/ijc.10915.
Mori L, De Libero G. Presentation of lipid antigens to T cells. Immunol Lett. 2008;117:1–8. https://doi.org/10.1016/j.imlet.2007.11.027.
Salio M, Silk JD, Cerundolo V. Recent advances in processing and presentation of CD1 bound lipid antigens. Curr Opin Immunol. 2010;22:81–8. https://doi.org/10.1016/j.coi.2009.12.008.
Porcelli SA, Modlin RL. The CD1 system: antigen-presenting molecules for T cell recognition of lipids and glycolipids. Annu Rev Immunol. 1999;17:297–329. https://doi.org/10.1146/annurev.immunol.17.1.297.
Kai K, Tanaka T, Ide T, et al. Immunohistochemical analysis of the aggregation of CD1a-positive dendritic cells in resected specimens and its association with surgical outcomes for patients with gallbladder cancer. Translational Oncology. 2021;14: 100923. https://doi.org/10.1016/j.tranon.2020.100923.
Golmoghaddam H, Pezeshki AM, Ghaderi A, et al. CD1a and CD1d genes polymorphisms in breast, colorectal and lung cancers. Pathology and Oncology Research. 2011;17:669–75. https://doi.org/10.1007/s12253-011-9367-x.
Ni YH, Zhang X, Xin, Lu Z yi, , et al. Tumor-Infiltrating CD1a+ DCs and CD8+/FoxP3+ Ratios Served as Predictors for Clinical Outcomes in Tongue Squamous Cell Carcinoma Patients. Pathol Oncol Res. 2020;26:1687–95. https://doi.org/10.1007/s12253-019-00701-5.
Hilly O, Rath-Wolfson L, Koren R, et al. CD1a-positive dendritic cell density predicts disease-free survival in papillary thyroid carcinoma. Pathol Res Pract. 2015;211:652–6. https://doi.org/10.1016/j.prp.2015.05.009.
Eisenthal A, Polyvkin N, BramanteSchreiber L, et al. Expression of dendritic cells in ovarian tumors correlates with clinical outcome in patients with ovarian cancer. Hum Pathol. 2001;32:803–7. https://doi.org/10.1053/hupa.2001.26455.
Grosche L, Knippertz I, König C, et al. The CD83 Molecule—an important immune checkpoint. Front Immunol. 2020;11:721. https://doi.org/10.3389/fimmu.2020.00721.
Smyth MJ, Crowe NY, Hayakawa Y, et al. NKT cells—conductors of tumor immunity? Curr Opin Immunol. 2002;14:165–71. https://doi.org/10.1016/S0952-7915(02)00316-3.
Prechtel AT, Steinkasserer A. CD83: An update on functions and prospects of the maturation marker of dendritic cells. Arch Dermatol Res. 2007;299:59–69. https://doi.org/10.1007/s00403-007-0743-z.
Tze LE, Horikawa K, Domaschenz H, et al. CD83 increases MHC II and CD86 on dendritic cells by opposing IL-10—Driven MARCH1-mediated ubiquitination and degradation. J Exp Med. 2011;208:149–65. https://doi.org/10.1084/jem.20092203.
Coventry B, Lee P, Gibbs D, et al. Dendritic cell density and activation status in human breast cancer—CD1a, CMRF-44, CMRF-56 and CD-83 expression. Br J Cancer. 2002;86:546–51. https://doi.org/10.1038/sj.bjc.6600132.
Brierley JD, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumours. 8th ed. Wiley-Blackwell; 2017.
Senkus E, Kyriakides S, Penault-Llorca F, et al. Primary breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26:8–30. https://doi.org/10.1093/annonc/mdv298.
Wernicke M, Roitman P, Manfre D, et al. Breast cancer and the stromal factor. The “prometastatic healing process” hypothesis. Medicina. 2011;71:15–21.
Bloom HJG, Richardson WW. Histological Grading and Prognosis in Breast Cancer. Br J Cancer. 1957;11:359–77. https://doi.org/10.1038/bjc.1957.43.
Martinez LM, Labovsky V, De Lujan CM, et al. CD105 expression on CD34-negative spindle-shaped stromal cells of primary tumor is an unfavorable prognostic marker in early breast cancer patients. PLoS ONE. 2015;10:e0121421. https://doi.org/10.1371/journal.pone.0121421.
Coventry B, Morton J. CD1a-positive infiltrating-dendritic cell density and 5-year survival from human breast cancer. Br J Cancer. 2003;89:533–8. https://doi.org/10.1038/sj.bjc.6601114.
Goldman SA, Baker E, Weyant RJ, et al. Peritumoral CD1a-positive dendritic cells are associated with improved survival in patients with tongue carcinoma. Arch Otolaryngol Head Neck Surg. 1998;124:641–6. https://doi.org/10.1001/archotol.124.6.641.
Coventry B, Weeks S, Heckford S, et al. Lack of IL-2 cytokine expression despite Il-2 messenger RNA transcription in tumor-infiltrating lymphocytes in primary human breast carcinoma: selective expression of early activation markers. J Immunol. 1996;156:3486–92.
Lin A, Schildknecht A, Nguyen LT, et al. Dendritic cells integrate signals from the tumor microenvironment to modulate immunity and tumor growth. Immunol Lett. 2010;127:77–84. https://doi.org/10.1016/j.imlet.2009.09.003.
Sombroek CC, Stam AGM, Masterson AJ, et al. Prostanoids play a major role in the primary tumor-induced inhibition of dendritic cell differentiation. J Immunol. 2002;168:4333–43. https://doi.org/10.4049/jimmunol.168.9.4333.
Labovsky V, Martinez LM, Calcagno M de L, et al. Interleukin-6 receptor in spindle-shaped stromal cells, a prognostic determinant of early breast cancer. Tumor Biol. 2016;37:13377–84. https://doi.org/10.1007/s13277-016-5268-7.
Chomarat P, Banchereau J, Davoust J, et al. IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat Immunol. 2000;1:510–4. https://doi.org/10.1038/82763.
Delamarre L, Pack M, Chang H, et al. Differential lysosomal proteolysis in antigen-presenting cells determines antigen fate. Science. 2005;307:1630–4. https://doi.org/10.1126/science.1108003.
Ara T, DeClerck YA. Interleukin-6 in bone metastasis and cancer progression. Eur J Cancer. 2010;46:1223–31. https://doi.org/10.1016/j.ejca.2010.02.026.
Axmann R, Böhm C, Krönke G, et al. Inhibition of interleukin-6 receptor directly blocks osteoclast formation in vitro and in vivo. Arthritis Rheum. 2009;60:2747–56. https://doi.org/10.1002/art.24781.
Kudo O, Sabokbar A, Pocock A, et al. Interleukin-6 and interleukin-11 support human osteoclast formation by a RANKL-independent mechanism. Bone. 2003;32:1–7. https://doi.org/10.1016/S8756-3282(02)00915-8.
Kovacs E. Investigation of interleukin-6 (IL-6), soluble IL-6 receptor (sIL-6R) and soluble gp130 (sgp130) in sera of cancer patients. Biomed Pharmacother. 2001;55:391–6. https://doi.org/10.1016/S0753-3322(01)00079-8.
Acknowledgements
This research was supported by grants from the PIP300 (2014-2016) from the National Council of Scientific and Technical Research (CONICET), Argentina; René Barón Foundation (2016-2020), Argentina and Williams Foundation (2016-2020), Argentina.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Conceptualization: Norma lejandra Chasseing, María Belén Giorello, and Vivian Labovsky. Metodhology: María Belén Giorello, Matas Ayelén, Marenco Pablo, Davies Kevin Mauro, Borzone Francisco Raúl, García-Rivello Hernán, and Wernicke Alejandra. Formal analysis and investigation: María Belén Giorello, Vivian Labovsky, and Calcagno María de Luján. Writing—original draft preparation: María Belén Giorello, Norma Alejandra Chasseing, Vivian Labovsky, and Leandro Marcelo Martinez. Writing—review and editing: Norma Alejandra Chasseing, Vivian Labovsky, and Leandro Marcelo Martinez. Supervision: Norma Alejandra Chasseing, Vivian Labovsky, and Leandro Marcelo Martinez.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare there are no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Giorello, M.B., Matas, A., Marenco, P. et al. CD1a- and CD83-positive dendritic cells as prognostic markers of metastasis development in early breast cancer patients. Breast Cancer 28, 1328–1339 (2021). https://doi.org/10.1007/s12282-021-01270-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-021-01270-9