Abstract
Background
The optimal duration of endocrine therapy for patients with hormone receptor-positive (HR-positive) breast cancer is still unclear. This meta-analysis aims to determine the optimal duration of endocrine therapy with extended aromatase inhibitors (AI) for postmenopausal patients with HR-positive early breast cancer who have finished 5 years of endocrine therapy.
Methods
Eligible randomized controlled trials were classified into three categories according to the whole duration of endocrine therapy (10 years versus 5 years, 7–8 years versus 5 years, and 10 years versus 7–8 years). For each category, hazard ratio (HR) for disease-free survival (DFS) and overall survival (OS), and risk ratio (RR) for the incidence of adverse events were pooled.
Results
Altogether 9 RCTs enrolling a total of 22,313 postmenopausal women with HR-positive breast cancer were included. Pooled data showed an improvement in DFS when extending endocrine therapy from 5 to 7–8 years (HR = 0.79 [0.69, 0.91]), specifically among those who had been treated with only tamoxifen (HR = 0.40 [0.22, 0.73]) or sequential tamoxifen followed by AI (HR = 0.82 [0.71, 0.95]), with tumors that were node-positive (HR = 0.72 [0.56, 0.93]), estrogen receptor (ER) and progesterone receptor (PR) positive (HR = 0.61 [0.47, 0.78]), or ≥ 2 cm in size (HR = 0.72 [0.51, 0.98]). However, no improvement in DFS was obtained when extending from 7–8 to 10 years (HR = 0.98 [0.87, 1.11]). In addition, the extension of endocrine therapy was not associated with an improvement in OS, but was associated with an increased risk of bone fracture and osteopenia/osteoporosis.
Conclusion
Patients who have been treated with AI for 5 years, with tumors that are node-negative, ER+/PR− or ER–/PR+, and < 2 cm in size do not need to receive extended AI therapy. For those who have been treated with only tamoxifen or sequential tamoxifen followed by an AI for a total of 5 years, with tumors that are node-positive, ER+/PR+ or ≥ 2 cm in size, 2–3 years of extended AI is necessary and maybe enough.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the malignant tumor with the highest incidence among women. Studies have note that about 70% of breast cancers are estrogen receptor (ER) or progesterone receptor (PR) positive [1]. Endocrine therapy is the main choice for these patients to reduce recurrence and mortality [2].
In the 1970s, the clinical application of tamoxifen became a milestone in breast cancer endocrine therapy, which had been considered the optimal choice for patients with hormone receptor-positive (HR-positive) breast cancer for a long time [3]. In the 1990s, the advent of the third generation aromatase inhibitors (AI) brought breast cancer endocrine therapy into a new era. Clinical studies including ATAC, BIG 1–98, ABCSG-8 and ITA and the EBCTCG meta-analysis have proven the superiority of 5-year AI-containing therapies over 5-year tamoxifen among postmenopausal patients with HR-positive early breast cancer [4,5,6,7,8]. Consequently, international guidelines recommended the use of AI or sequential tamoxifen followed by an AI for a total of 5 years in postmenopausal patients with HR-positive early breast cancer [3].
Despite the success of 5-year adjuvant endocrine therapy, it is reported that women with HR-positive breast cancer have a prolonged risk of recurrence, which means that recurrences can still occur after 5-year adjuvant endocrine therapy. In recent years, many clinical trials have evaluated the efficacy of extended endocrine therapy and have drawn inconsistent conclusions. With extended endocrine therapy, improved disease-free survival (DFS) was demonstrated in studies including AERAS, MA17, GIM4 LEAD, but was not demonstrated in NSABP B-42, NSABP B-33, DATA, ABCSG-16 and IDEAL [9,10,11,12,13,14,15,16,17]. In the above-mentioned studies, the whole duration of endocrine therapy was not the same, with some extending from 5 to 7–8 years, and the others extending from 5 to 10 years.
The duration of adjuvant endocrine therapy for breast cancer is one of the hot topics in recent years. Although a previous meta-analysis published in 2020 have indicated the potential benefit of endocrine therapy with extended AI [18], there is no meta-analysis focusing on the optimal duration of endocrine therapy so far. Considering the economic burden, psychological burden, and side effects of extended therapy, the optimal duration of and the potential population who can benefit from endocrine therapy with extended AI are still under discussion [19, 20].
Here we will report a meta-analysis of randomized controlled trials (RCT) to explore the optimal duration of endocrine therapy with extended AI for postmenopausal patients with HR-positive early breast cancer who have finished 5 years of endocrine therapy.
Materials and methods
Literature search
On March 9, 2020, RCTs were searched in PubMed, EMBASE and Cochrane databases using the terms “aromatase inhibitor* OR aromatase inhibiting OR aromatase inhibitor OR anastrozole OR exemestane OR letrozole”, “extended OR prolonged OR continued OR duration”, “postmenopausal OR post-menopausal or post-menopause”, “breast neoplasm OR breast cancer” and “trial OR study”. In addition, RCTs were also searched among conference papers of the San Antonio Breast Cancer Symposium (SABCS) and the American Society of Clinical Oncology (ASCO). For studies no enough data were reported in published articles, the study authors were contacted.
Inclusion and exclusion criteria
Studies meeting all of the following criteria were included: (1) RCTs without obvious bias; (2) conducted among postmenopausal patients with HR-positive early breast cancer; (3) investigating the efficacy and safety of endocrine therapy with extended AI; (4) enrolling patients who have received prior endocrine therapy including tamoxifen, AI, or tamoxifen followed by AI; (5) can provide data comparing endocrine therapies administrated for a total of 10 years versus 5 years, 7–8 years versus 5 years, or 10 years versus 7–8 years. There were no restrictions on publication dates or languages.
Studies meeting any of the following criteria were excluded: (1) reviews, meta-analyses, letters, editorials, and animal experiments; (2) with obvious bias; (3) the extended therapies were not AI; (4) the duration of prior endocrine therapy had been much longer than 5 years.
Literature screening and data extraction
Articles retrieved from different sources were pooled and duplicates were removed. Then the titles and abstracts of all articles were reviewed, and those not meeting the inclusion criteria were removed. For articles that cannot be judged through titles and abstracts, full texts were reviewed.
For studies that were judged to have meet all the inclusion criteria, the following information were extracted: study code, study design, patient population, treatment before randomization, treatment after randomization, total duration of endocrine therapy, number of patients, median age, median follow-up, DFS, overall survival (OS), and adverse events.
The above-mentioned processes were conducted by two researchers independently. Disagreements were resolved through discussion or were referred to a third person.
Quality assessment
The Cochrane risk of bias tool embedded in Review Manager 5.2 was used to assess the risk of bias for included studies. For each study, low risk, unclear risk or high risk of bias was assigned to each domain of the tool, including selection bias, performance bias, detection bias, attrition bias, reporting bias and other potential bias.
Statistical analysis
This study was designed to explore the optimal duration of endocrine therapy with extended AI for postmenopausal patients with HR-positive early breast cancer who have finished 5 years of endocrine therapy (including tamoxifen, AI, or tamoxifen followed by AI). According to whole duration of endocrine therapy (the duration of prior endocrine therapy plus that of extended AI), all RCTs were classified into three categories: (1) comparing 10 years versus 5 years of endocrine therapy; (2) comparing 7–8 years versus 5 years of endocrine therapy; (3) comparing 10 years versus 7–8 years of endocrine therapy.
DFS was the endpoint of special interest in the study, and OS and the incidence of adverse events were evaluated as well. Pooled HR along with 95% confidence interval (CI) were calculated for DFS and OS. Pooled risk ratio (RR) along with 95% CI were calculated for the incidence of adverse events. In addition, subgroup analyses were performed for DFS based on the following stratification factors: the type of prior 5 years of endocrine therapy (5 years of tamoxifen, 5 years of AI, or 2–3 years of tamoxifen followed by 3–2 years of AI), node status (negative, positive), hormone receptors status (estrogen receptor [ER] positive and progesterone receptor [PR] positive, ER or PR positive) and tumor size (< 2 cm, ≥ 2 cm). The heterogeneity were evaluated by I2. The fixed-effect model would be used if I2 ≤ 50%, and the random-effect model would be used if I2 > 50%. All statistical analysis were conducted in Stata 15. All tests were 2-tailed, and P < 0.05 was considered as statistically significant.
Results
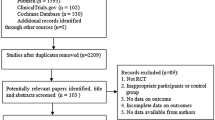
Altogether 1051 records were identified through searching in Pubmed, EMBASE and Cochrane Library, or from ASCO or SABCS conference papers. In addition, four additional records were obtained by contacting study authors. After removing the duplicates, 596 records were screened with titles and abstracts, among which 532 were excluded because of title or abstract not relevant. The remaining 64 records were assessed with full texts, among which 32 were excluded because of ineligibility: 8 records were not RCTs; 20 records did not investigate the safety and efficacy of extended AI therapy; 1 record cannot provide data comparing endocrine therapies administrated for 10 years versus 5 years, 7–8 years versus 5 years, or 10 years versus 7–8 years because of its intermittent extension design (the SOLE study) [21]; 1 record has obvious bias because of enrolling a larger proportion of patients with node-positive, > 2 cm tumors in the observation group as compared to the letrozole group (the LATER study) [22]; two records were related to one study in which patients had received 10 years of endocrine therapy before randomization (the MA 17R study) [23]. As to the remaining 32 articles, the results of 9 RCTs were reported, with some RCTs having been reported in more than 1 publications because of subgroup analysis or updated analysis. The PRISMA flow diagram is shown in Fig. 1.
The included studies were summarized in Table 1, and the risk of bias for included studies were shown in Fig. 2 [10,11,12,13,14,15,16,17, 24]. According to the total duration of endocrine therapy, the nine RCTs were classified into three categories as follows: (1) 10 years versus 5 years of endocrine therapy, including AERAS, MA17, NSABP B-42 and NSABP B-33; (2) 7–8 years versus 5 years of endocrine therapy, including ABCSG 6a, DATA and GIM4 LEAD; (3) 10 years versus 7–8 years of endocrine therapy, including ABCSG-16 and IDEAL.
Disease-free survival (DFS)
All 9 RCTs enrolling a total of 22,313 postmenopausal women with HR-positive breast cancer were available for the analysis of DFS. Figure 3 shows the forest plot of HR for DFS. Pooled data showed that extending endocrine therapy can improve DFS as compared to shorter treatment duration (HR = 0.77 [0.68, 0.89]; I2 = 66.4%, random effects model), especially when extending from 5 to 10 years (HR = 0.67 [0.52, 0.85]; I2 = 68.7%, random effects model) and from 5 to 7–8 years (HR = 0.79 [0.69, 0.91]; I2 = 0.0%, fixed effects model). However, statistical data shown that extending endocrine therapy from 7–8 to 10 years cannot significantly improve DFS (HR = 0.98 [0.87, 1.11]; I2 = 0.0%, fixed effects model).
Forest plot of the hazard ratio of DFS stratified by the whole duration of endocrine therapy. Pooled values were from random-effect analysis, except for the comparisons between 7–8 and 5 years and between 10 and 7–8 years, which were from fixed-effect analysis because of low heterogeneity. Y years, HR hazard ratio, CI confidence interval
Subgroups analyses of DFS
Table 2 shows the HR for DFS stratified by initial 5 years of therapy (5 years of tamoxifen, 5 years of AI, or 2–3 years of tamoxifen followed by 3–2 years of AI), node status (negative, positive), hormone receptors status (ER+ and PR+, ER+ or PR+) and tumor size (0–2 cm, ≥ 2 cm).
When extending endocrine therapy from 5 to 10 years, the benefit of extended AI in reducing recurrence could be observed in patients who had been treated only with previous 5 years of tamoxifen (HR = 0.61 [0.49, 0.76]) or 2–3 years of tamoxifen followed by 3–2 years of AI (HR = 0.74 [0.57, 0.97]), with tumors that were node-positive (HR = 0.63 [0.48, 0.83]), ER + /PR + (HR = 0.5 [0.42, 0.71]), or ≥ 2 cm in size (HR = 0.51 [0.35, 0.75]), but could not be observed in those who had received 5 years of AI, with tumors that were node-negative, ER+/PR− or ER−/PR+, and < 2 cm in size.
When extending endocrine therapy from 5 to 7–8 years, the benefits of extended AI in reducing recurrence could be observed in patients who had been treated only with previous 5 years of tamoxifen (HR = 0.40 [0.22, 0.73]) or 2–3 years of tamoxifen followed by 3–2 years of AI (HR = 0.82 [0.71, 0.95]), with tumors that were node-positive (HR = 0.72 [0.56, 0.93]), ER+/PR+ (HR = 0.61 [0.47, 0.78]), or ≥ 2 cm in size (HR = 0.72 [0.51,0.98]), but could not be observed in those who had received 5 years of AI, with tumors that were node-negative, ER+/PR− or ER−/PR+, and < 2 cm in size.
When extending endocrine therapy from 7–8 to 10 years, no statistically significant benefit of extended AI in reducing recurrence can be observed in the whole population or in any subgroup of patients, with the 95% CI of HR including one (P ≥ 0.05) for all analysis.
Overall survival (OS)
Except for GIM4 LEAD and NSABP B-33, all other 7 RCTs enrolling a total of 18,659 postmenopausal women with HR-positive breast cancer were available for the analysis of OS. Figure 4 shows the forest plots of HR for OS generated by the fixed effects model. Our analysis revealed that the extension of adjuvant endocrine therapy was not associated with an decreased risk of death from any cause (HR = 1.01 [0.90, 1.13]; I2 = 0.0%, fixed effects model), no matter extension from 5 to 10 years (HR = 1.05 [0.87, 1.27]; I2 = 20.4%, fixed effects model), from 5 to 7–8 years (HR = 0.90 [0.69, 1.17]; I2 = 0.0%, fixed effects model) or from 7–8 to 10 years (HR = 1.02 [0.86, 1.20]; I2 = 0.0%, fixed effects model).
Adverse events (AE)
All 9 RCTs have reported adverse events. Table 3 summarizes the most commonly reported adverse events reported in these studies. According to our analysis, the extension of adjuvant endocrine therapy from 5 to 10 years was associated with an increased risk of bone fracture (RR = 1.27 [1.05, 1.54]), osteopenia/osteoporosis (RR = 1.24 [1.10, 1.40]), bone pain (including arthralgia) (RR = 1.28 [1.11, 1.49]), joint stiffness (RR = 2.40 [1.68, 3.43]), myalgia (RR = 1.24 [1.10, 1.39]) and alopecia (RR = 1.42 [1.09, 1.85]). The extension of adjuvant endocrine therapy from 5 years to 7–8 years was associated with an increased risk of bone fracture (RR = 1.36 [1.01, 1.84]), osteopenia/osteoporosis (RR = 1.73 [1.22, 2.45]) and bone pain (including arthralgia) (RR = 1.34 [1.03, 1.73]). The extension of adjuvant endocrine therapy from 7–8 to 10 years was associated with an increased risk of bone fracture (RR = 1.57 [1.22, 2.02]) and osteopenia/osteoporosis (RR = 1.70 [1.28, 2.26]).
Discussion
Before 2010, patients with HR-positive breast cancer were generally recommended to receive endocrine therapy for five years. However, in recent years, studies have shown that HR-positive breast cancer has two peaks of recurrence during 2–3 years and 7–8 years after surgery, with more than 50% of recurrences occurring after discontinuing 5 years of adjuvant endocrine therapy [25, 26]. Therefore, the benefits and risks of extended adjuvant endocrine therapy has become a hot topic in this field. In recent years, the results of clinical studies on extended adjuvant endocrine therapy have been disclosed gradually. Studies including AERAS, MA17, GIM4 LEAD and ABCGS 6a shown that extended AI therapy can reduce recurrences, while studies such as NSABP B-42, NSABP B-33, DATA, ABCSG-16 and IDEAL failed to support this point [10,11,12,13,14,15,16,17, 24]. In the above-mentioned studies, the whole duration of endocrine therapy was not the same, with some extended to 7–8 years, and the others extended to 10 years. Our study is the first meta-analysis focusing on the optimal duration of endocrine therapy.
According to the results of DFS in this meta-analysis, extending endocrine therapy from 5 to 7–8 years or to 10 years could reduce disease recurrence significantly. To be more specifically, according to data presented in previous studies, distant recurrence (HR for 10 years vs 5 years: 0.51 [0.32, 0.84], 0.60 [0.43, 0.84] and 0.72 [0.53, 0.97] reported in study AERAS, MA17 and NSABP B-42, respectively; HR for 7–8 years vs 5 years: 0.53 [0.29, 0.96] reported in study ABCSG 6a) could be reduced significantly by AI extension [10, 12, 17, 24]. Due to data limitation, whether local recurrence and contralateral breast cancer can be reduced by AI extension needs further investigation.
As to the comparison between 10 and 7–8 years, we found that the DFS of extending to 10 years was not significantly better than that of extending to 7–8 years. Study SOLE, which is not included in this meta-analysis because of its intermittent extending design, indicated that the effect of extending letrozole by 5 years continuously was not significantly better than that of extending letrozole by 5 years intermittently, which can also support our findings to some extent [21]. However, numerically the HR for 10 years vs 5 years is better than that for 7–8 years vs 5 years (0.67 vs 0.79). Due to the limited number of studies comparing 10 years vs 7–8 years of endocrine therapy, we cannot rule out the possibility that there might be a potential benefit of extending endocrine therapy from 7–8 to 10 years. Therefore, although statistical data shown that 7–8 years of endocrine therapy might be enough, we hold the point that more studies are in need to compare the effect of 7–8 years to 10 years of treatment.
A meta-analysis published in 2018 demonstrated that extended AI after initial endocrine therapy didn’t significantly improve DFS [27]. Another meta-analysis published in 2020 revealed a significant improvement in DFS with extended AI after initial AI-containing therapy [18]. In our meta-analysis, there are four improvements: first, the above-mentioned two meta-analysis were designed to investigate whether extended AI can improve DFS, while the duration of AI extension was not distinguished between 2–3 and 5 years, therefore, our study is the first meta-analysis exploring the optimal duration of AI extension; second, HR, a more commonly used and more suitable statistics for survival analysis, was used in our analysis; third, more stringent inclusion criteria was applied in this study, according to which study LATER was excluded because of obvious bias, and study SOLE was excluded because of the intermittent extending design; fourth, a more detailed subgroup analysis was conducted in our study, which suggesting that 5 years of endocrine therapy is enough for a certain subgroup of patients, and 7–8 years of endocrine therapy might be enough for other patients.
From the perspective of the subgroup analysis, extending endocrine therapy from 5 to 7–8 years or to 10 years couldn’t reduce recurrence among patients who had been treated with AI for 5 years, with tumors that were node-negative, ER+/PR− or ER−/PR+, and < 2 cm in size. However, as to those who had been treated with only tamoxifen or sequential tamoxifen followed by an AI for a total of 5 years, with tumors that were node-positive, ER+/PR+ or ≥ 2 cm in size, extending endocrine therapy could reduce recurrence significantly. Patients with tumors that are node-positive, ER+/PR+ or ≥ 2 cm in size may face a higher risk of recurrence, and therefore obtain more benefit from extended therapy [26]. In recent years, many studies investigating the predicting value of biomarkers have been conducted [28,29,30]. In the future, tools that can accurately predict the risk of recurrence need to be developed to further facilitate the selection of patients who should receive extended endocrine therapy in clinical practice.
The results of OS in this meta-analysis indicated that extending endocrine therapy, whether to 7–8 years or to 10 years, did not bring survival benefit. That is to say, the benefit of extended endocrine therapy in reducing recurrences cannot be translated into survival benefit, which has been supported by all studies included in this meta-analysis. A proper reason is that the follow-up period is not long enough. For early-stage breast cancer, a disease with relatively high survival rate, endocrine therapy might have a carryover effect on overall survival which increases over time [4]. It is not surprising that it is difficult to observe OS benefits during a relatively short follow-up period of time.
Our meta-analysis shows that higher risk of bone fracture, osteoporosis and bone pain may be caused by the extension of endocrine therapy, which has been reported in many previous studies [31,32,33]. It means that the extending of endocrine therapy is not an easy choice for patients and there should be a balance between toxicities and benefits in clinical practice. For patients who need to receive extended endocrine therapy, bisphosphonates, cholecalciferol and calcium supplementation can be administrated to protect bone health.
The main limitation of this study is the number of studies included in the analysis. A total of 9 studies were included, but after being classified into three categories, only 2–4 studies were included in each category. Extending endocrine therapy from 7–8 to 10 years didn’t lead to significant improvement in DFS is an important finding that should be paid attention to by clinicians, but this finding is only supported by 2 studies. Considering the non-significant but numerically better HR for 10 years vs 5 years than that for 7–8 years vs 5 years, more studies comparing the effect of 7–8 to 10 years are in need to further confirm this finding. The second limitation is that the data of GIM4, ABCSG-16 and AERAS trials were from conference presentations instead of published papers. We included these trials because of the limited number of published studies. After the publication of more detailed data for these studies and other similar studies in the future, more published results should be included in future meta-analysis. Another limitation is that the definition of DFS are not the same for all included studies, which may cause heterogeneity. Specifically, the definition of DFS in ABCSG 6a and MA 17 does not include death, which was different from other studies. Considering the limited number of studies, we included these two studies in our analysis.
Even with the above-mentioned limitations, our data suggests that patients who have been treated with AI for 5 years, with tumors that are node-negative, ER+/PR− or ER−/PR+, and < 2 cm in size do not need to receive extended AI therapy. For those who have been treated with only tamoxifen or sequential tamoxifen followed by an AI for a total of 5 years, with tumors that are node-positive, ER+/PR+ or ≥ 2 cm in size, 2–3 years of extended AI is necessary and maybe enough. We hope that our research results can attract more attention, and stimulate more studies comparing the effect of 7–8 years versus 10 years of treatment, so that the optimum duration of endocrine treatment can be further clarified.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Yip CH, Rhodes A. Estrogen and progesterone receptors in breast cancer. Future Oncol. 2014;10(14):2293–301.
van Hellemond I, Geurts S, Tjan-Heijnen V. Current status of extended adjuvant endocrine therapy in early stage breast cancer. Curr Treat Options Oncol. 2018;19(5):26.
Burstein HJ, Prestrud AA, Seidenfeld J, Anderson H, Buchholz TA, Davidson NE, et al. American Society of Clinical Oncology clinical practice guideline: update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol. 2010;28(23):3784–96.
The Early Breast Cancer Trialists’ Collaborative Group. Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;386(10001):1341–52.
Goetz MP, Suman VJ, Hoskin TL, Gnant M, Filipits M, Safgren SL, et al. CYP2D6 metabolism and patient outcome in the Austrian Breast and Colorectal Cancer Study Group trial (ABCSG) 8. Clin Cancer Res. 2013;19(2):500–7.
Cuzick J, Sestak I, Baum M, Buzdar A, Howell A, Dowsett M, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol. 2010;11(12):1135–41.
Koeberle D, Thuerlimann B. Letrozole as upfront endocrine therapy for postmenopausal women with hormone-sensitive breast cancer: BIG 1–98. Breast Cancer Res Treat. 2007;105(Suppl 1):55–66.
Boccardo F, Rubagotti A, Guglielmini P, Fini A, Paladini G, Mesiti M, et al. Switching to anastrozole versus continued tamoxifen treatment of early breast cancer. Updated results of the Italian tamoxifen anastrozole (ITA) trial. Ann Oncol. 2006;17(Suppl 7):i10–4.
Mann BS, Johnson JR, Kelly R, Sridhara R, Williams G, Pazdur R. Letrozole in the extended adjuvant treatment of postmenopausal women with history of early-stage breast cancer who have completed 5 years of adjuvant tamoxifen. Clin Cancer Res. 2005;11(16):5671–7.
Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Piccart MJ, et al. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J Natl Cancer Inst. 2005;97(17):1262–71.
Mastro LD, Mansutti M, Bisagni G, Ponzone R, Durando A, Amaducci L. Benefit from letrozole as extended adjuvant therapy after sequential endocrine therapy: A randomized, phase III study of Gruppo Italiano Mammella (GIM). In: edito, ACSO 2019 2019.
Mamounas EP, Bandos H, Lembersky BC, Jeong JH, Geyer CJ, Rastogi P, et al. Use of letrozole after aromatase inhibitor-based therapy in postmenopausal breast cancer (NRG Oncology/NSABP B-42): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(1):88–99.
Mamounas EP, Jeong JH, Wickerham DL, Smith RE, Ganz PA, Land SR, et al. Benefit from exemestane as extended adjuvant therapy after 5 years of adjuvant tamoxifen: intention-to-treat analysis of the National Surgical Adjuvant Breast and Bowel Project B-33 trial. J Clin Oncol. 2008;26(12):1965–71.
Tjan-Heijnen V, van Hellemond I, Peer P, Swinkels A, Smorenburg CH, van der Sangen M, et al. Extended adjuvant aromatase inhibition after sequential endocrine therapy (DATA): a randomised, phase 3 trial. Lancet Oncol. 2017;18(11):1502–11.
Gnant M, Steger G, Greil R, Fitzal F, Mlineritsch B, Manfreda D, et al. A prospective randomized multi-center phase-III trial of additional 2 versus additional 5 years of anastrozole after initial 5 years of adjuvant endocrine therapy—results from 3484 postmenopausal women in the ABCSG-16 trial. In: edito, 2017 San Antonio Breast Cancer Symposium 2017.
Blok EJ, Kroep JR, Meershoek-Klein KE, Duijm-de CM, Putter H, van den Bosch J, et al. Optimal duration of extended adjuvant endocrine therapy for early breast cancer; results of the IDEAL Trial (BOOG 2006–05). J Natl Cancer Inst. 2018;110(1):40–8.
Ohtani S, Iijima K, Higaki K, Sato Y, Hozumi Y, Hasegawa Y, et al. A prospective randomized multi-center open-label phase III trial of extending aromatase-inhibitor adjuvant therapy to 10 years—results from 1697 postmenopausal women in the N-SAS BC 05 trial: Arimidex extended adjuvant randomized study (AERAS). In: edito, 2018 San Antonio Breast Cancer Symposium 2019.
Qian X, Li Z, Ruan G, Tu C, Ding W. Efficacy and toxicity of extended aromatase inhibitors after adjuvant aromatase inhibitors-containing therapy for hormone-receptor-positive breast cancer: a literature-based meta-analysis of randomized trials. Breast Cancer Res Treat. 2020;179(2):275–85.
Tanaka M, Itoh S, Takeuchi Y. Effectiveness of bisphosphonate combined with activated vitamin D in patients with aromatase inhibitor-induced osteoporosis after breast cancer operation. Osteoporos Sarcopenia. 2018;4(3):102–8.
Wiwanitkit S, Wiwanitkit V. Postmenopausal osteoporosis, breast cancer, and aromatase inhibitor. Rev Assoc Med Bras. 2013;59(3):217.
Colleoni M, Luo W, Karlsson P, Chirgwin J, Aebi S, Jerusalem G, et al. Extended adjuvant intermittent letrozole versus continuous letrozole in postmenopausal women with breast cancer (SOLE): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2018;19(1):127–38.
Zdenkowski N, Forbes JF, Boyle FM, Kannourakis G, Gill PG, Bayliss E, et al. Observation versus late reintroduction of letrozole as adjuvant endocrine therapy for hormone receptor-positive breast cancer (ANZ0501 LATER): an open-label randomised, controlled trial. Ann Oncol. 2016;27(5):806–12.
Goss PE, Ingle JN, Pritchard KI, Robert NJ, Muss H, Gralow J, et al. Extending aromatase-inhibitor adjuvant therapy to 10 years. N Engl J Med. 2016;375(3):209–19.
Jakesz R, Greil R, Gnant M, Schmid M, Kwasny W, Kubista E, et al. Extended adjuvant therapy with anastrozole among postmenopausal breast cancer patients: results from the randomized Austrian Breast and Colorectal Cancer Study Group Trial 6a. J Natl Cancer Inst. 2007;99(24):1845–53.
Ruhstaller T, Giobbie-Hurder A, Colleoni M, Jensen MB, Ejlertsen B, de Azambuja E, et al. Adjuvant letrozole and tamoxifen alone or sequentially for postmenopausal women with hormone receptor-positive breast cancer: long-term follow-up of the BIG 1–98 Trial. J Clin Oncol. 2019;37(2):105–14.
Richman J, Dowsett M. Beyond 5 years: enduring risk of recurrence in oestrogen receptor-positive breast cancer. Nat Rev Clin Oncol. 2019;16(5):296–311.
Clement Z, Kollias J, Bingham J, Whitfield R, Bochner M. Extended duration of adjuvant aromatase inhibitor in breast cancer: a meta-analysis of randomized controlled trials. Gland Surg. 2018;7(5):449–57.
Martin M, Brase JC, Ruiz A, Prat A, Kronenwett R, Calvo L, et al. Prognostic ability of EndoPredict compared to research-based versions of the PAM50 risk of recurrence (ROR) scores in node-positive, estrogen receptor-positive, and HER2-negative breast cancer. A GEICAM/9906 sub-study. Breast Cancer Res Treat. 2016;156(1):81–9.
Yazdi MF, Rafieian S, Gholi-Nataj M, Sheikhha MH, Nazari T, Neamatzadeh H. CYP2D6 genotype and risk of recurrence in tamoxifen treated breast cancer patients. Asian Pac J Cancer Prev. 2015;16(15):6783–7.
Dowsett M, Turner N. Estimating risk of recurrence for early breast cancer: integrating clinical and genomic risk. J Clin Oncol. 2019;37(9):689–92.
Yao S, Laurent CA, Roh JM, Lo J, Tang L, Hahn T, et al. Serum bone markers and risk of osteoporosis and fragility fractures in women who received endocrine therapy for breast cancer: a prospective study. Breast Cancer Res Treat. 2020;180(1):187–95.
Ramchand SK, Cheung YM, Yeo B, Grossmann M. The effects of adjuvant endocrine therapy on bone health in women with breast cancer. J Endocrinol. 2019;241(3):R111–24.
Grossmann M, Ramchand SK, Milat F, Vincent A, Lim E, Kotowicz MA, et al. Assessment and management of bone health in women with oestrogen receptor-positive breast cancer receiving endocrine therapy: position statement of the Endocrine Society of Australia, the Australian and New Zealand Bone and Mineral Society, the Australasian Menopause Society and the Clinical Oncology Society of Australia. Clin Endocrinol (Oxf). 2018;89(3):280–96.
Acknowledgements
The research was supported by National Key Research and Development Program (2016YFC0104805) and Chinese Academy of Medical Science Initiative for Innovative Medicine (2017-I2M-2-003). The funding bodies did not participate in the design of the study, the collection, analysis and interpretation of data, and the writing of the manuscript.
Author information
Authors and Affiliations
Contributions
The design of the study was put forwarded by ZLOY and QS, the collection, analysis and interpretation of data, and the writing of the manuscript were performed by JC and XHZ. YL and TZ participated in the collection of data. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Ethics approval and informed consent
This is a meta-analysis, and no study was conducted on individual participants. Therefore, neither ethical approval nor informed consent is required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Chen, J., Zhang, X., Lu, Y. et al. Optimal duration of endocrine therapy with extended aromatase inhibitors for postmenopausal patients with hormone receptor-positive breast cancer: a meta-analysis. Breast Cancer 28, 630–643 (2021). https://doi.org/10.1007/s12282-020-01196-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-020-01196-8