Abstract
Background
Electronic Health (eHealth) may have a positive effect on healthcare, such as patient education and decreasing the costs of healthcare services. Evidence suggests that such interventions can also improve physical activity (PA) of patients. This systematic review aimed to investigate the effects of PA interventions provided through eHealth on breast cancer patients.
Methods
This study was conducted through a search in electronic databases up to July 2018. PubMed, EMBASE, Cochrane Central Register of Controlled Trials, Web of Science, Scopus, Science Direct, and Google Scholar databases were searched without time limitation.
Results
In total, 2187 articles were retrieved and finally 16 articles remained. Five were pre/post and 11 were randomized trial studies. Different platforms were used in these studies including web-based, mobile-based, both web-and-mobile-based and email. In total, these articles comprise 2304 breast cancer patients with the mean age of 51 years and 50% were conducted in the USA. Four studies measured PA using wearable devices such as accelerometers and pedometers. All studies reported an increase in PA level at least in one of moderate or vigorous PA, although not all these results were significant.
Conclusion
The results show that eHealth interventions can improve the level of PA in breast cancer patients. Although there are numerous eHealth interventions focusing on PA in cancer patients, there is still an essential need for eHealth interventions to be tailored for breast cancer patients specifically. Clinical trials with appropriate methodology, enough intervention time and follow-up are needed to make evidence-based results more generalizable.
Trial Registration
PROSPERO CRD42018092422; https://www.crd.york.ac.uk/PROSPERO/.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The number of new cases of cancer diagnosed annually in the world is quickly increasing from 14.1 million in 2012 to an estimated value of over 20 million by 2030 [1]. From among these, breast cancer (BC) is one of the most prevalent cancers in women worldwide [2]. Despite a good prognosis and advanced treatments, BC survivors suffer from many negative consequences following the initial treatment of cancer, including mental, physical, and family and financial problems [3]. Shoulder morbidity is one of the most important consequences of treatment which may be accompanied by pain, reduced shoulder range of motion (ROM), and lymphedema [4]. These shoulder morbidities, especially the reduced ROM of the shoulder and arms in the long term, can significantly reduce the quality of life (QOL) [5,6,7], decrease upper limb function, and reduce the patients’ return to workability [4]. Thus, these issues must be diagnosed and treated on time. Various types of physical therapies can treat these functional problems [4, 8,9,10]. Following the surgery, exercise therapy must be combined with daily care so that patients at risk of increased shoulder problems would face fewer complications [6, 11]. The majority of these complementary therapies are neglected due to limited resources, especially in low- and moderate-income countries [6]. The important point is that these interventions can not only play a role in the physical aspects of the overall QOL of patients with BC but also create a positive trend in the improvement of cancer site-specific QOL domains (in this case, breast and arms) [12].
The standard level of PA determined by the American College of Sports Medicine (ACSM) is a moderate-intensity exercise for more than a minimum of 150 min per week [13]. Unfortunately, the level of PA is low among BC patients [14]. On the other hand, BC survivors are at risk of overweight due to decreased PA [15]. Moreover, overweight is associated with a high risk of mortality. Therefore, PA can play a significant role in decreasing the risk of mortality following the diagnosis of BC [16].
Electronic Health (eHealth) is a new field between medical informatics, public health, and business, delivering information and health services via the Internet and related technologies [17]. Currently, eHealth can play a significant role in the improvement of self-care, communication between patient and healthcare team, and access to health information [18]. An increasing volume of evidence has proven the positive effects of eHealth in supporting patient-centered care [19,20,21,22].
Moreover, mobile Health (mHealth) is considered as an important part of eHealth [23]. mHealth is a wide term for describing the use of mobile technologies for the health care delivery [24]. mHealth has the potential for improving access to and enhancing the quality of healthcare [25], decreasing healthcare costs [26], supporting self-management for chronic diseases, reducing patients’ visit to healthcare centers, and enhancing the capability of providing individual, regional, and on-demand services [27, 28].
The analysis of BC apps showed that patient education is the first topic covered by mobile apps, followed by behavioral change and mental supports [29]. It has been shown that patients with BC and healthcare professionals have a positive attitude towards the use of mobile apps [30, 31]. mHealth tools can provide information with regard to BC education, self-examination, patient follow-up, lifestyle change, and PA [32,33,34,35,36,37,38]. Low level of PA has motivated the treatment team to discover new ways for optimizing the level of PA in patients. Today, with the popularity of information and communications technology (ICT), the use of electronic health (eHealth) approaches seems feasible for enhancing PA [39].
eHealth interventions may be an effective strategy for improving PA and provide a better QOL for patients with BC. The present systematic review aimed to find and evaluate studies related to PA designed for BC patients implemented through eHealth.
Materials and methods
Protocol
This systematic review was conducted based on PRISMA [40] guideline, which is described below. The present study is registered on PROSPERO (https://www.crd.york.ac.uk/PROSPERO/; ID: CRD42018092422).
Inclusion criteria
Participants in these studies were women aging above 18 who had received treatment, including surgery, radiotherapy, or chemotherapy for BC. Women of any race, ethnicity, employment status, occupational status, and role were included.
eHealth interventions had to be primarily focused on PA in BC patients. Interventions had to be designed with the aim of improving health-related behaviors (e.g., Increasing PA) or changing the lifestyle (e.g., weight loss activities through PA). More specifically, the primary outcomes in this systematic review had to directly or indirectly measure PA, whether through a change in the level of PA or physical functions, the time lapsed during PA, compliance with PA recommendations, and the consumed energy.
The employed technologies included mobile tools which were capable of establishing cellular and wireless communication. The following portable tools were acceptable in this study: mobile phones (including smartphones, Android, or IOS phones), personal digital assistants, tablets, and portable light laptops. The main focus was on smartphone apps, but other formats such as web-based interventions were also acceptable. Studies from any continent, country, or healthcare center, regardless of geographical borders, were acceptable. This method allowed us to collect comprehensive data from various sources in different countries.
Exclusion criteria
Exclusion criteria were as follows: studies which were merely a description of various phases of software development, with no specified outcome for the participants; studies which solely evaluated software usability; all studies on women at a high risk of BC, not patients with BC; studies published after June 2018; letters to the editor, review studies, and protocols; studies whose method was not clearly described; studies in languages other than English; and duplicate studies in which the same research was conducted using the same method with similar results.
Information sources and search strategy
This study was conducted through a search in electronic databases in July 2018. PubMed, EMBASE, Cochrane Central Register of Controlled Trials, IEEE, Web of Science, Scopus, Science Direct, and Google Scholar databases were searched without time limitation.
Search strategy consists of three main categories including (1) the condition “breast cancer”, (2) technology “eHealth”, and (3) “physical activity” and their synonym keywords in each category.
The search was conducted in English. The search strategy was modified and revised for all databases by a Health Information Management and Medical Informatics specialist. A manual search was also performed for retrieving grey literature and the bibliographies of relevant articles.
The full text of articles was extracted and evaluated. This study included randomized controlled trials (RCTs) and non-randomized studies. Non-randomized studies included case–control, cohort, cross-sectional and pre/post studies in which eHealth was the primary intervention used for BC patients.
Data extraction and quality assessment
Two authors (SD, FA) independently reviewed the full text and extracted all critical data from included studies including author, country of study, study design, sample size, retention rate, population studied, the age of participants, study duration, intervention type, intervention content, inclusion/exclusion criteria and outcomes measured. Any discrepancies were resolved through discussion with a third author.
Risk of bias in each study
The Cochrane Collaboration’s tool for assessing risk of bias was used to evaluate methodological quality of included studies [41]. Non-randomized studies were assessed for risk of bias using the RoBANS tool [42]. The RoBANS tool contains six domains including the selection of participants, confounding variables, measurement of intervention (exposure), blinding of outcome assessment, incomplete outcome data and selective outcome reporting.
Results
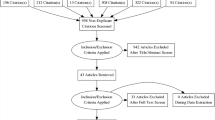
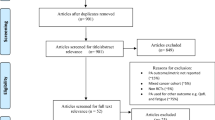
Upon searching electronic databases, 2187 citations were retrieved. Using manual search and finding the references of articles, 15 articles were added. After removing duplicates, 1390 articles remained which were evaluated based on the title and then abstract. Then, 151 records were selected for full-text evaluation. All evaluations were performed by two Medical Informatics Specialists. In case of disagreements, the opinions of a third specialist were used. Finally, 16 articles remained for final evaluation, all having an acceptable level of quality. The PRISMA flowchart is depicted in Fig. 1.
Finally, 16 articles were included [43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58]. In total, these articles comprise 2304 patients with the mean age of 51 years (sample size and mean age are presented in Table 1). All studies were conducted on patients with BC, of which six studies also included participants with other types of cancer [43, 45, 46, 48, 53, 55]. General characteristics of these studies are presented in Table 1.
Clinical characteristics
Eight studies were RCTs [43, 45,46,47,48, 50, 55, 57], three were randomized trial without control group [44, 56, 58], and five were pre/post studies [49, 51,52,53,54]. The characteristics of these studies are presented in Table 2.
In terms of journals, 63% (12 articles) belonged to Q1 journals (i.e., the top 25% in their specialized domain), 6% (1 article) belonged to a Q2 journal (i.e., Top 50%), and this factor was not calculated for the rest yet.
All the studies were written from 2012 to 2018, with the majority being published in 2016 (5 articles).
PA [43, 45, 47, 49, 51,52,53, 55, 56, 58] and related variables, including high- and moderate-intensity PA [44, 46, 48, 54, 57], physical strength and its related factors [57], and PA pattern [46] were considered in the articles. Other study variables (indirect outcomes) were QOL [45, 49, 51, 53, 58], health-related QOL [44, 50], fatigue [43, 45, 50, 53,54,55], consumption of vegetables [43, 48, 50, 51, 54], diet quality [50, 57], weight loss [52, 54], anxiety [50, 53], depression [43, 50, 53], insomnia [43, 53], mood [55], promotion of exercise [50], motivational readiness [50], self-efficacy [54, 56], acceptability [46, 54, 56], cardiovascular fitness [52, 57], physical activity readiness [47], psychosocial construct [52], and patient activation [49, 54].
Physical activity measurement
The included studies had assessed PA using various questionnaires, e.g., [49, 54, 58] using the International Physical Activity Questionnaire (IPAQ) which assesses the frequency (day per week) and duration (minute) of PA over the past 7 days in the following domains: job-related physical activity, transportation physical activity, housework, and recreation and leisure–time physical activity [59]. This tool provides an international measure for PA which has undergone numerous reliability and validity examinations. IPAQ covers all domains of moderate and vigorous PA in daily life as well as work-related PA items [54].
Some studies had only measured the duration of PA, e.g., [51]. In this study, the data of PA were collected using the log data of the Lose It! mobile app. Participants were encouraged to reach the level of the standard guideline of PA proposed by American College of Sports (ACM) [60] which included moderate-intensity cardiorespiratory exercises (150 min per week) and vigorous activity (over 40 min per week) in addition to resistance exercises for each set of major muscle groups.
Moreover, Lee et al. [50] measured the changes in the level of exercise over a 12-week intervention using data (type, duration, and intensity of exercise) entered by patients in a web-based app over the intervention time.
Some studies utilized the Physical Activity Readiness Questionnaire as the inclusion criteria for patient recruitment [47, 52].
In References [47, 55], the 7-day physical activity recall (PAR) [61] was administered. Participants reported hours spent in sleep, moderate activity, hard activity, and very hard activity. PAR was used in previous studies on cancer survivors [62, 63] and validated on multiple populations, including cancer-free youth [64].
Godin Leisure–Time Exercise Questionnaire (GLTEQ) [65, 66] has been used by Short [56]. The adapted version of GLETQ includes six items measuring the participants’ mild, moderate, or strenuous PA in a typical week in the past month, as well as three items on resistance-training which ask the participant to report the frequency (times per week) and volume (number of completed exercises in each session and the number of completed repeats for each exercise) of resistance exercises in the past month. This questionnaire was completed online by the participants. Forbes [45], Chapman [44], Puszkiewicz [53], and Bantum [43] used an old version of this questionnaire in addition to another one which served their purpose [53].
Sturgeon [57] utilized the Modifiable Activity Questionnaire. This questionnaire evaluates current physical activity at work and leisure time, as well as maximum levels of inactivity due to disability. This questionnaire is designed to be easily modified so that it can provide the maximum capability of evaluating PA in different populations [67].
The noted studies have used self-report questionnaires for determining changes in PA. However, some other studies measured the level of PA using wearable devices such as uniaxial accelerometers and pedometers as well as multi-sensor systems [46, 52, 54, 58]. Pope [52] recorded the level of PA using a mobile app with the help of auxiliary tools such as an accelerometer and Actigraph GT3X, thus minimizing the rate of self-report.
Level of physical activity
From a clinical viewpoint, all studies reported an increase in PA at least in one of the domains of moderate or vigorous PA. The majority of studies (10/16) reported positive effects on PA (p < 0.05). Results are presented in Table 3.
Although these differences were not significant in some studies. For instance, the study by McCarroll found no significant change in the PA pattern using the Lose It! app, although positive changes were observed in the other variables of this study, including weight loss and waist circumference [51]. Another example is a 12-week aerobic and resistance exercise program through Smart After-Care app reporting a significant improvement in the physical function, PA, and QOL of both groups compared to the baseline, while no significant between-group difference was observed [58]. Similarly, Forbes reports that, although total minutes of PA increased in the intervention group, these changes were not significant [45]. Moreover, Rabin achieved a moderate effect size for PA using a web-based 12-week intervention. Of course, this study was not specified to BC patients [55]. Some studies also used descriptive statistics and did not examine statistical significance [52, 54].
Eliminated studies included qualitative studies, feasibility studies, usability studies and case studies with a small sample size. Numerous studies were eliminated at the beginning of the process because new studies were limited to the provision of protocols and the preliminary steps of app development. Many of these studies, e.g., the one by Harder, described the steps of designing and developing a system using patient-centered approaches [68], whereas many others were qualitative and only sought the opinion of patients and the treatment team. Of the studies which were candidates for inclusion, those which failed to measure PA [69,70,71,72,73], did not specify the type of cancer [74], or measured functional activity and capacity [75], isometric and muscular strength [76] were removed from this study (Appendix 1).
Technological characteristics
The technology used in included studies comprised web-based [43,44,45, 48,49,50, 55,56,57], mobile-based (apps) [46, 52,53,54, 58], web-and-mobile-based apps [46, 51]as well as email [47]. The mobile apps used were Lose it! [51], Smart After Care [58], GAINFitness [53], MapMyFitness [52], and Fitbit [46, 54].
The main features of these apps were exercise and diet recording [51]; exercise with a pedometer [58]; diet and training plans [52]; individualized exercise [53]; daily recording of the number of steps, distance passed, and minutes of exercise; and sleep pattern [46]. None of these apps were developed specifically for BC patients. Of these apps, four failed to provide a strong positive evidence supporting the improvement of PA using mobile phones [51, 52, 54, 58]. In web-based technology, however, this value was 2 vs. 9 articles [45, 55].
Patient satisfaction
In the case of the noted apps, only one study had evaluated user satisfaction. Mean Likert scale of total user satisfaction was 4.27 out of 5 (85%) [58]. In the case of websites, three studies had assessed user satisfaction [45, 49, 55]. Kuijpers [49] reported a user satisfaction of 76% (3.8 out of 5), Rabin [55] 71%, and Forbes [45] 73%.
Risk of bias within studies
The Cochrane Collaboration’s tool [41] was used for assessing risk of bias in RCTs. The overall risk of bias in RCTs was assessed as low except in Hatchett et al. [47] and Rabin et al. [55] studies that rated as ‘unclear risk of bias’ (Table 4). Five non-randomized studies were assessed for risk of bias using the RoBANS tool [42]. The overall risk of bias in the studies by Kuijpers et al. [49], McCarroll et al. [51] and Pope et al. [52] was assessed as low, while the studies by Puszkiewicz et al. [53], Quintiliani et al. [54] were rated as high (Table 5). There was proper randomization sequence generation in the majority of the studies, while allocation concealment was unclear and performance bias was high.
Most of the studies had adequate sample sizes and only four studies had a small sample size < 20 [52,53,54,55]. However, having big sample size did not prevent study from other bias like retention bias as shown in the Short et al.’s [56] study. Self-reporting PA against direct measuring is another source of bias in most articles. In the five pre–post studies, the risk of bias increased due to the lack of control group.
Discussion
Overview
This systematic review provided a comprehensive evaluation of the effects of eHealth interventions on PA in women with BC. Results revealed that all the mentioned interventions can increase the level of PA, and the majority of studies (10/16) reported statistically significant positive effects on PA level.
In this study, the most commonly used technology was web-based interventions (nine articles), followed by mobile-based (five articles), and web- and mobile-based technology (two articles). Email was also used as an older form of technology in one article. Two studies provided apps with both mobile- and web-based versions [46, 51]. Apps which can be used on more than one device, e.g., Lose It! [51] and Fitbit [46], can provide more flexibility for patients.
All included studies reported an increase in PA, indicating the effectiveness of interventions for patients with BC, although significant differences were not observed in some studies [45, 51, 52, 54, 55, 58]. Some studies reported within-group differences compared to the baseline [58] which may be due to selection bias because those who had a PA less than the average range may reflect more effects in clinical results [45]. Moreover, those who have a more tendency for participation in these studies probably had more PA prior to these interventions.
Another important point is that the duration of intervention may have affected the stabilization of the results. For instance, the shortest period of intervention was 1 month which reported positive results only for weight loss. If the interventions had been longer with more follow-ups, the results of PA could have become significant [51]. In the present study, the longest duration of intervention was 12 months [48, 57]. The fact that interventions were not specified for patients with BC may be another important point affecting the significance of results. For instance, the study by Rabin obtained a moderate effect size for PA [55]. Studies which prescribe PA in a general manner may be inappropriate for patients with BC because the special condition of these patients and their requirements after treatment differ from those of other cancers.
Many e-Health studies evaluated the mental aspects of QOL in BC patients. The presence of a large number of studies on this topic demonstrates that studies on mental aspects have overtaken those on physical aspects. In addition, numerous studies investigated mental factors in addition to physical ones, depicting the mutual effect of mental and physical factors.
The use of novel devices and wearable technologies for recording PA is one way to directly and reliably measure PA. The most common devices for this purpose include uniaxial accelerometers and pedometers and multi-sensor systems which can transfer the received information to other devices, such as mobile phones or websites [77]. In this study, 25% of articles had employed this method for measuring PA [46, 52, 54, 58]. It is recommended that, in future, researchers use this method of measurement and minimize patient self-report methods.
The weak points of these studies were non-specificity of PA for patients with BC, small sample size, lacking a strong design (RCTs), and lack of a theoretical framework.
Breast cancer mobile apps
PA apps are now highly popular among users; from every five users, one has installed at least one PA app on his/her mobile phone [78]. Despite a large number of PA apps available for download, few apps specifically focus on the improvement of PA in cancer patients [79]. Despite the increase in the use of mobile apps for health purposes [80] and the possible potential for monitoring the QOL of cancer patients, providing treatment strategies, and improving patients’ conditions [81], the present study indicated that four out of five mobile apps failed to prove statistically the effectiveness of interventions for BC patients.
Designing eHealth interventions based on breast cancer patients need
BC treatment may lead to upper-limb dysfunction (ULD). Symptoms of ULD include pain, numbness, reduced shoulder ROM, reduced strength, joint limitations, axillary web syndrome, and lymphedema due to injury to the axillary lymphatic system [82,83,84,85,86]. To solve and manage these issues, tailored exercises and rehabilitation programs for these patients are required. Although no app specialized for BC patients was found in the present study, the use of self-management theories, frameworks, and models for designing apps specialized for patients with cancer can significantly help with this matter. The main challenge is ensuring that these apps effectively focus on cancer, during design, test, and use [87, 88]. There are protocols from RCTs which indicate that strong studies on this topic are being conducted and, in the near future, one can refer high-quality evidence-based results [38, 89,90,91,92,93].
Usability consideration
Cancer patients suffer from cognitive problems over the course of the disease. Therefore, issues related to usability and accessibility are points which must be taken into consideration in the design and development of systems, especially for the elderly [81]. Nowadays, a wide range of apps is available, determining which one is appropriate for BC patients and what is the best method of using them is difficult, and may confused patients in selecting the appropriate app [94]. A usability factor examined here was user satisfaction. In the present study, four articles provided information on the level of patient satisfaction. Overall, patient satisfaction with interventions ranged from 71 to 85%. These statistics show the relatively high satisfaction of participants with eHealth interventions, indicating that researchers care about patient preferences and design their products based on the principles of patient-centered design. The user interface designed for patients must be specialized to them and meet their needs. Many studies had considered this point and designed apps based on the guidelines provided by the National Cancer Institute or other sources.
Limitations
A limitation of the present study is that PA measurement differed across studies and, therefore, it was not possible to calculate the final level of effect on PA. The language of the search was limited to English, so there may have been other articles which were not included.
Conclusion and recommendations
The reviewed articles can reflect the first scientific efforts for increasing PA in BC patients. Results showed that the use of eHealth tools is effective in promoting PA in BC patients and can be used as a supportive opportunity for these patients. Still, studies on this topic must expand and increase in number.
The present study indicated that there are some issues in this regard, including inappropriate methodology, short duration of intervention and follow-up, inhomogeneity in studies, and patients’ self-report of their PA in some studies instead of directly measuring PA.
It is recommended that future studies perform clinical trials with appropriate methodology, enough intervention time and follow-up. Furthermore, physical interventions specialized for BC patients must be designed and implemented in various stages of treatment. It is also suggested that the type of PA measurement be changed from patient self-report to direct measurement using new technologies.
References
Bray F. Transitions in human development and the global cancer burden. New York: Springer; 2014. p. 54–68.
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108.
Post KE, Flanagan J. Web based survivorship interventions for women with breast cancer: an integrative review. Eur J Oncol Nurs. 2016;25:90–9.
Mafu TS, September AV, Shamley D. The potential role of angiogenesis in the development of shoulder pain, shoulder dysfunction, and lymphedema after breast cancer treatment. Cancer Manag Res. 2018;10:81.
Fenlon D, Powers C, Simmonds P, Clough J, Addington-Hall J. The JACS prospective cohort study of newly diagnosed women with breast cancer investigating joint and muscle pain, aches, and stiffness: pain and quality of life after primary surgery and before adjuvant treatment. BMC Cancer. 2014;14(1):467.
Nesvold I-L, Reinertsen KV, Fosså SD, Dahl AA. The relation between arm/shoulder problems and quality of life in breast cancer survivors: a cross-sectional and longitudinal study. J Cancer Surv. 2011;5(1):62–72.
Jeong HJ, Sim Y-J, Hwang KH, Kim GC. Causes of shoulder pain in women with breast cancer-related lymphedema: a pilot study. Yonsei Med J. 2011;52(4):661–7.
Galantino ML, Stout NL. Exercise interventions for upper limb dysfunction due to breast cancer treatment. Phys Ther. 2013;93(10):1291–7.
Loh SY, Musa AN. Methods to improve rehabilitation of patients following breast cancer surgery: a review of systematic reviews. Breast Cancer (Dove Med Press). 2015;7:81.
Tatham B, Smith J, Cheifetz O, Gillespie J, Snowden K, Temesy J, et al. The efficacy of exercise therapy in reducing shoulder pain related to breast cancer: a systematic review. Physiother Can. 2013;65(4):321–30.
McNeely ML, Campbell K, Ospina M, Rowe BH, Dabbs K, Klassen TP, et al. Exercise interventions for upper-limb dysfunction due to breast cancer treatment. Cochrane Database Syst Rev. 2010;6:CD005211.
Zeng Y, Huang M, Cheng AS, Zhou Y, So WK. Meta-analysis of the effects of exercise intervention on quality of life in breast cancer survivors. Breast Cancer. 2014;21(3):262–74.
Haskell WL, Lee I-M, Pate RR, Powell KE, Blair SN, Franklin BA, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116(9):1081.
Smith SG, Chagpar AB. Adherence to physical activity guidelines in breast cancer survivors. Am Surg. 2010;76(9):962–5.
Demark-Wahnefried W, Peterson BL, Winer EP, Marks L, Aziz N, Marcom PK, et al. Changes in weight, body composition, and factors influencing energy balance among premenopausal breast cancer patients receiving adjuvant chemotherapy. J Clin Oncol. 2001;19(9):2381–9.
Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293(20):2479–86.
Eysenbach G. What is e-health? J Med Internet Res. 2001;3(2):e20.
Moghadasi H, Asadi F, Hosseini A, Hossein ZE. A model for measuring e-health status around the world. Pajoohande. 2012;16(7):347–57.
Finkelstein J, O'connor G, Friedmann R. Development and implementation of the home asthma telemonitoring (HAT) system to facilitate asthma self-care. Stud Health Technol Inf. 2001;84(Pt 1):810–4.
Hoffman AJ, Brintnall RA, Brown JK, von Eye A, Jones LW, Alderink G, et al. Virtual reality bringing a new reality to postthoracotomy lung cancer patients via a home-based exercise intervention targeting fatigue while undergoing adjuvant treatment. Cancer Nurs. 2014;37(1):23–33.
Smith L, Weinert C. Telecommunication support for rural women with diabetes. Diabetes Educ. 2000;26(4):645–55.
Wantland DJ, Portillo CJ, Holzemer WL, Slaughter R, McGhee EM. The effectiveness of web-based vs. non-web-based interventions: a meta-analysis of behavioral change outcomes. J Med Internet Res. 2004;6(4):e40.
Tokosi TO, Fortuin J, Douglas TS. The impact of mHealth interventions on breast cancer awareness and screening: systematic review protocol. JMIR Res Protocol. 2017;6(12):e246.
Darlow S, Wen K-Y. Development testing of mobile health interventions for cancer patient self-management: a review. Health Inf J. 2016;22(3):633–50.
Karami S, Asadi F, Emami H. Thematic categorization of mobile health software packages and their priority from the perspective of Iranian physicians. J Health Biomed Inf. 2017;4(3):216–21.
Aranda-Jan CB, Mohutsiwa-Dibe N, Loukanova S. Systematic review on what works, what does not work and why of implementation of mobile health (mHealth) projects in Africa. BMC Public Health. 2014;14(1):188.
Free C, Phillips G, Galli L, Watson L, Felix L, Edwards P, et al. The effectiveness of mobile-health technology-based health behaviour change or disease management interventions for health care consumers: a systematic review. PLoS Med. 2013;10(1):e1001362.
Kumar S, Nilsen WJ, Abernethy A, Atienza A, Patrick K, Pavel M, et al. Mobile health technology evaluation: the mHealth evidence workshop. Am J Prev Med. 2013;45(2):228–36.
Ginossar T, Shah SFA, West AJ, Bentley JM, Caburnay CA, Kreuter MW, et al. Content, usability, and utilization of plain language in breast cancer mobile phone apps: a systematic analysis. JMIR mhealth uhealth. 2017;5(3):e20.
Bravo C, O'Donoghue C, Kaplan CP, Luce J, Ozanne E. Can mHealth improve risk assessment in underserved populations? Acceptability of a breast health questionnaire app in ethnically diverse, older, low-income women. J Health Dispar Res Pract. 2014;7(4):6. http://europepmc.org/abstract/MED/25705576.
Maguire R, McCann L, Miller M, Kearney N. Nurse's perceptions and experiences of using of a mobile-phone-based Advanced Symptom Management System (ASyMS©) to monitor and manage chemotherapy-related toxicity. Eur J Oncol Nurs. 2008;12(4):380–6.
Egbring M, Far E, Roos M, Dietrich M, Brauchbar M, Kullak-Ublick GA, et al. A mobile app to stabilize daily functional activity of breast cancer patients in collaboration with the physician: a randomized controlled clinical trial. J Med Internet Res. 2016;18(9):e238.
Mobasheri MH, Johnston M, King D, Leff D, Thiruchelvam P, Darzi A. Smartphone breast applications—what's the evidence? Breast (Edinburgh, Scotland). 2014;23(5):683–9.
Smith SA, Whitehead MS, Sheats JQ, Fontenot B, Alema-Mensah E, Ansa B. Formative research to develop a lifestyle application (app) for African American breast cancer survivors. J Ga Public Health Assoc. 2016;6(1):50–9.
Haque MM, Kawsar F, Adibuzzaman M, Ahamed SI, Love R, Dowla R, et al., editors. Findings of e-ESAS: a mobile based symptom monitoring system for breast cancer patients in rural Bangladesh. Conference on human factors in computing systems—proceedings; 2012.
Wheelock A, Bock M, Mihalis E, Hwang J, Shepard LN, Rugo H, et al. Incorporation of web-based symptom reporting and management in follow-up (FU) care for early-stage breast cancer. Support Care Cancer. 2012;20:S269–S270270.
Berg S, Gielissen M, Custers J, Graaf W, Ottevanger P, Prins J. BREATH: web-based self-management for psychological adjustment after primary breast cancer-results of a multicenter randomized controlled trial. J Clin Oncol. 2015;33(25):2763–71. https://doi.org/10.1200/JCO.2013.54.9386.
Zhu J, Ebert L, Liu X, Chan S-C. A mobile application of breast cancer e-support program versus routine care in the treatment of Chinese women with breast cancer undergoing chemotherapy: study protocol for a randomized controlled trial. BMC Cancer. 2017. https://doi.org/10.1186/s12885-017-3276-7.
Vandelanotte C, Müller AM, Short CE, Hingle M, Nathan N, Williams SL, et al. Past, present, and future of eHealth and mHealth research to improve physical activity and dietary behaviors. J Nutr Educ Behav. 2016;48(3):219–28 (e211).
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9.
Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions 5.1.0. Cochrane Collab. 2011;2011:33–49.
Park J, Lee Y, Seo H, Jang B, Son H, Kim S, et al., editors. Risk of bias assessment tool for non-randomized studies (RoBANS): development and validation of a new instrument. 19th Cochrane Colloquium; 2011.
Bantum EO, Albright CL, White KK, Berenberg JL, Layi G, Ritter PL, et al. Surviving and thriving with cancer using a web-based health behavior change intervention: randomized controlled trial. J Med Internet Res. 2014;16(2):e54.
Chapman J, Fletcher C, Flight I, Wilson C. Pilot randomized trial of a volitional help sheet-based tool to increase leisure time physical activity in breast cancer survivors. Br J Health Psychol. 2018;23(3):723–40.
Forbes CC, Blanchard CM, Mummery KK, Courneya KS. Feasibility and preliminary efficacy of an online intervention to increase physical activity in Nova Scotian cancer survivors: a randomized controlled trial. JMIR Cancer. 2015;1(2):e12.
Hartman SJ, Nelson SH, Weiner LS. Patterns of fitbit use and activity levels throughout a physical activity intervention: exploratory analysis from a randomized controlled trial. J Med Internet Res. 2018;20(2):e29.
Hatchett A, Hallam J, Ford M. Evaluation of a social cognitive theory-based email intervention designed to influence the physical activity of survivors of breast cancer. Psycho-oncology. 2013;22(4):829–36. https://doi.org/10.1002/pon.3082.
Kanera I, Willems R, Bolman C, Mesters I, Verboon P, Lechner L. Long-term effects of a web-based cancer aftercare intervention on moderate physical activity and vegetable consumption among early cancer survivors: a randomized controlled trial. Int J Behav Nutr Phys Act. 2017. https://doi.org/10.1186/s12966-017-0474-2.
Kuijpers W, Groen WG, Oldenburg HS, Wouters MW, Aaronson NK, van Harten WH. eHealth for breast cancer survivors: use, feasibility and impact of an interactive portal. JMIR Cancer. 2016;2(1):e3.
Lee M, Yun Y, Park H, Lee E, Jung K, Noh D. A Web-based self-management exercise and diet intervention for breast cancer survivors: pilot randomized controlled trial. Int J Nurs Stud. 2014;51(12):1557–67. https://doi.org/10.1016/j.ijnurstu.2014.04.012.
McCarroll ML, Armbruster S, Pohle-Krauza RJ, Lyzen AM, Min S, Nash DW, et al. Feasibility of a lifestyle intervention for overweight/obese endometrial and breast cancer survivors using an interactive mobile application. Gynecol Oncol. 2015;137(3):508–15.
Pope Z, Lee JE, Zeng N, Lee HY, Gao Z. Feasibility of smartphone application and social media intervention on breast cancer survivors' health outcomes. Transl Behav Med. 2019;9(1):11–22.
Puszkiewicz P, Roberts AL, Smith L, Wardle J, Fisher A. Assessment of cancer survivors' experiences of using a publicly available physical activity mobile application. JMIR Cancer. 2016;2(1):e7.
Quintiliani LM, Mann DM, Puputti M, Quinn E, Bowen DJ. Pilot and feasibility test of a mobile health-supported behavioral counseling intervention for weight management among breast cancer survivors. JMIR Cancer. 2016;2(1):e4.
Rabin C, Dunsiger S, Ness K, Marcus B. Internet-based physical activity intervention targeting young adult cancer survivors. J Adolesc Young Adult Oncol. 2012;1(4):188–94. https://doi.org/10.1089/jayao.2011.0040.
Short C, Rebar A, James E, Duncan M, Courneya K, Plotnikoff R, et al. How do different delivery schedules of tailored web-based physical activity advice for breast cancer survivors influence intervention use and efficacy? J Cancer Surviv. 2017;11(1):80–91.
Sturgeon K, Dean L, Heroux M, Kane J, Bauer T, Palmer E, et al. Commercially available lifestyle modification program: randomized controlled trial addressing heart and bone health in BRCA1/2+ breast cancer survivors after risk-reducing salpingo-oophorectomy. J Cancer Surviv. 2017;11(2):246–55. https://doi.org/10.1007/s11764-016-0582-z.
Uhm K, Yoo J, Chung S, Lee J, Lee I, Kim J, et al. Effects of exercise intervention in breast cancer patients: is mobile health (mHealth) with pedometer more effective than conventional program using brochure? Breast Cancer Res Treat. 2016. https://doi.org/10.1007/s10549-016-4065-8.
Craig CL, Marshall AL, Sjorstrom M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–95.
Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee I-M, et al. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334–599.
Blair SN, Haskell WL, Ho P, Paffenbarger JR, Ralph S, Vranizan KM, Farquhar JW, et al. Assessment of habitual physical activity by a sevenday recall in a community survey and controlled experiments. Am J Epidemiol. 1985;122(5):794–804.
Pinto BM, Frierson GM, Rabin C, Trunzo JJ, Marcus BH. Home-based physical activity intervention for breast cancer patients. J Clin Oncol. 2005;23(15):3577–87.
Rabin C, Pinto B, Dunsiger S, Nash J, Trask P. Exercise and relaxation intervention for breast cancer survivors: feasibility, acceptability and effects. Psycho-Oncology. 2009;18(3):258–66.
Dishman RK, Steinhardt M. Reliability and concurrent validity for a 7-day re-call of physical activity in college students. Med Sci Sports Exerc. 1988;20(1):14–25.
Shephard R. Godin leisure-time exercise questionnaire. Med Sci Sports Exerc. 1997;29(suppl 6):S36–S3838.
Godin G, Shephard R. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. 1985;10(3):141–6.
Momenan AA, Delshad M, Sarbazi N, Rezaei GN, Ghanbarian A, Azizi F. Reliability and validity of the Modifiable Activity Questionnaire (MAQ) in an Iranian urban adult population. Arch Iran Med. 2012;15(5):279–82.
Harder H, Holroyd P, Burkinshaw L, Watten P, Zammit C, Harris PR, et al. A user-centred approach to developing bWell, a mobile app for arm and shoulder exercises after breast cancer treatment. J Cancer Surviv. 2017;11(6):732–42.
Everts FZB, van der Marije L, de Meezenbroek EJ. Web-based individual mindfulness-based cognitive therapy for cancer-related fatigue—a pilot study. Internet Interv. 2015;2(2):200–13.
Camargo C, Cavalheiro G, Cardoso A, Lamounier E, Andrade AO, Mendes I, et al editors. Virtual rehabilitation in women with post breast cancer—a case study. Virtual Rehabilitation (ICVR), 2013 International Conference on; 2013: IEEE.
Lynch SM, Stricker CT, Brown JC, Berardi JM, Vaughn D, Domchek S, et al. Evaluation of a web-based weight loss intervention in overweight cancer survivors aged 50 years and younger. Obes Sci Pract. 2017;3(1):83–94.
Soto-Perez-De-Celis E, Kim H, Rojo-Castillo MP, Sun CL, Chavarri-Guerra Y, Navarrete-Reyes AP, et al. A pilot study of an accelerometer-equipped smartphone to monitor older adults with cancer receiving chemotherapy in Mexico. J Geriatr Oncol. 2018;9(2):145–51.
Valle C, Deal A, Tate D. Preventing weight gain in African American breast cancer survivors using smart scales and activity trackers: a randomized controlled pilot study. J Cancer Surviv. 2017;11(1):133–48. https://doi.org/10.1007/s11764-016-0571-2.
Hong YA, Goldberg D, Ory MG, Towne SD Jr, Forjuoh SN, Kellstedt D, et al. Efficacy of a mobile-enabled web app (iCanFit) in promoting physical activity among older cancer survivors: a pilot study. JMIR Cancer. 2015;1(1):e7.
Galiano-Castillo N, Arroyo-Morales M, Lozano-Lozano M, Fernandez-Lao C, Martin-Martin L, Del-Moral-Avila R, et al. Effect of an Internet-based telehealth system on functional capacity and cognition in breast cancer survivors: a secondary analysis of a randomized controlled trial. Support Care Cancer Off J Multinatl Assoc Support Care Cancer. 2017;25(11):3551–9.
Galiano-Castillo N, Cantarero-Villanueva I, Fernandez-Lao C, Ariza-Garcia A, Diaz-Rodriguez L, Del-Moral-Avila R, et al. Telehealth system: a randomized controlled trial evaluating the impact of an internet-based exercise intervention on quality of life, pain, muscle strength, and fatigue in breast cancer survivors. Cancer. 2016;122(20):3166–74.
Casey B, Coote S, Donnelly A. Objective physical activity measurement in people with multiple sclerosis: a review of the literature. Disabil Rehabil Assist. 2018;13(2):124–31.
Fox S, Duggan M. Health online 2013. Washington: Pew Internet & American Life Project; 2013.
Pandey A, Hasan S, Dubey D, Sarangi S. Smartphone apps as a source of cancer information: changing trends in health information-seeking behavior. J Cancer Educ. 2013;28(1):138–42.
Dorsey ER, McConnell MV, Shaw SY, Trister AD, Friend SH. The use of smartphones for health research. Acad Med. 2017;92(2):157–60.
Rincon E, Monteiro-Guerra F, Rivera-Romero O, Dorronzoro-Zubiete E, Sanchez-Bocanegra CL, Gabarron E. Mobile phone apps for quality of life and well-being assessment in breast and prostate cancer patients: systematic review. JMIR Mhealth Uhealth. 2017;5(12):e187.
Harrington S, Padua D, Battaglini C, Michener LA. Upper extremity strength and range of motion and their relationship to function in breast cancer survivors. Physiother Theory Pract. 2013;29(7):513–20.
Johansen S, Fosså K, Nesvold IL, Malinen E, Fosså SD. Arm and shoulder morbidity following surgery and radiotherapy for breast cancer. Acta Oncol. 2014;53(4):521–9.
Merchant C, Chapman T, Kilbreath S, Refshauge K, Krupa K. Decreased muscle strength following management of breast cancer. Disabil Rehabil. 2008;30(15):1098–105.
Monleon S, Ferrer M, Tejero M, Pont A, Piqueras M, Belmonte R. Shoulder strength changes one year after axillary lymph node dissection or sentinel lymph node biopsy in patients with breast cancer. Arch Phys Med Rehabil. 2016;97(6):953–63.
Verbelen H, Gebruers N, Eeckhout F-M, Verlinden K, Tjalma W. Shoulder and arm morbidity in sentinel node-negative breast cancer patients: a systematic review. Breast Cancer Res Treat. 2014;144(1):21–31.
Bender JL, Yue RYK, To MJ, Deacken L, Jadad AR. A lot of action, but not in the right direction: systematic review and content analysis of smartphone applications for the prevention, detection, and management of cancer. J Med Internet Res. 2013;15(12):e287.
Collado-Borrell R, Escudero-Vilaplana V, Ribed-Sánchez A, Ibáñez-García S, Herranz-Alonso A, Sanjurjo-Sáez M. Smartphone applications for cancer patients; what we know about them. Acad Med. 2016;40(1):25–35.
Jiemin Z, Ebert L, Xiangyu L, Chan SW-C. A mobile application of breast cancer e-support program versus routine Care in the treatment of Chinese women with breast cancer undergoing chemotherapy: study protocol for a randomized controlled trial. BMC Cancer. 2017;17:1–9.
Langius-Eklöf A, Crafoord MT, Christiansen M, Fjell M, Sundberg K. Effects of an interactive mHealth innovation for early detection of patient-reported symptom distress with focus on participatory care: protocol for a study based on prospective, randomised, controlled trials in patients with prostate and breast cancer. BMC Cancer. 2017;17(1):466.
Lyons E, Baranowski T, Basen-Engquist K, Lewis Z, Swartz M, Jennings K, et al. Testing the effects of narrative and play on physical activity among breast cancer survivors using mobile apps: study protocol for a randomized controlled trial. BMC Cancer. 2016;16:202. https://doi.org/10.1186/s12885-016-2244-y.
Maguire R, Fox P, McCann L, Miaskowski C, Kotronoulas G, Miller M, et al. The eSMART study protocol: a randomised controlled trial to evaluate electronic symptom management using the advanced symptom management system (ASyMS) remote technology for patients with cancer. BMJ Open. 2017;7(5):e015016. https://doi.org/10.1136/bmjopen-2016-015016.
Ritvo P, Obadia M, Santa Mina D, Alibhai S, Sabiston C, Oh P, et al. Smartphone-enabled health coaching intervention (iMOVE) to promote long-term maintenance of physical activity in breast cancer survivors: protocol for a feasibility pilot randomized controlled trial. JMIR Res Protoc. 2017;6(8):e165.
van Velsen L, Beaujean DJ, van Gemert-Pijnen JE. Why mobile health app overload drives us crazy, and how to restore the sanity. BMC Med Inform Decis Making. 2013;13(1):23.
Acknowledgements
This study was part of a PhD project conducted at Shahid Beheshti University of Medical Sciences.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Dorri, S., Asadi, F., Olfatbakhsh, A. et al. A Systematic Review of Electronic Health (eHealth) interventions to improve physical activity in patients with breast cancer. Breast Cancer 27, 25–46 (2020). https://doi.org/10.1007/s12282-019-00982-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-019-00982-3