Abstract
Development of innovative strategies for cancer treatment is a pressing public health issue. Despite recent advances, the mechanisms of cancer progression and the resistance to cancer treatment have not been fully elucidated. Sphingolipids, including ceramide and sphingoshin-1-phosphate, are bioactive mediators that regulate cancer cell death and survival through the dynamic balance of what has been termed the ‘sphingolipid rheostat’. Specifically, ceramide, which acts as the central hub of sphingolipid metabolism, is generated via three major pathways by many stressors, including anti-cancer treatments, environmental stresses, and cytokines. We have previously shown in breast cancer patients that elevated ceramide correlated with less aggressive cancer phenotypes, leading to a prognostic impact. Recent studies showed that ceramide have the possibility of becoming the reinforcing agent of cancer treatment as well as other roles such as nanoparticles and diagnostic biomarker. We review ceramide as one of the key molecules to investigate in overcoming resistance to current drug therapies and in becoming one of the newest cancer treatments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer is the second leading cause of death in the United States [1], with 1735,350 new cases and 609,640 deaths were estimated in the US in 2018. Similarly in Japan, 1013,600 new cases and 379,900 deaths are estimated in 2018. Cancer treatments, including chemotherapy and radiation, have improved in the last decade; however, treatment responses can be short term in many patients [2]. Recent progress in molecular research has identified that many factors are associated with cancer development, leading to new treatment strategies to target those factors involved in the cell cycle [3], cell survival [4], DNA repair [5], and tumor immunity [6]. One of the emerging areas of research include sphingolipids, where technical advances in accurate measurements of its levels provided new evidence that these lipids play important roles in cancer biology.
Sphingolipid mediators, such as ceramide [7] and sphingosine-1-phosphate (S1P) [8], are important signaling molecules that influence cancer cell fate. Their opposing signaling pathway has been coined “sphingolipid rheostat” [9, 10] (Fig. 1). The sphingolipid rheostat regulates sphingolipid metabolites, which in turn controls cell fate and subsequent cell death [9, 11]. Several reports have supported the role of the sphingolipid rheostat in cell fate determination, in cancer initiation and progression [12], and in drug sensitivity of cancer [13]. Recently, our group and others have found and reported the roles of sphingolipids in cancer progression not only in experimental models but also in human tissue and patients [14], which suggests that the field of sphingolipid research is advancing to the next phase, its clinical relevance.
The sphingolipid rheostat. The schema shows the important enzymes and organelles that regulate the levels of ceramide and sphingosine-1-phosphate (S1P). ABC ATP-binding cassette, CDase ceramidase, Cer ceramide, CerS ceramide synthases, ER endoplasmic reticulum, SphK sphingosine kinase, Spns2 spinster homologue 2, SPP S1P phosphatase, S1PR S1P receptor
This review will focus on ceramide as a sphingolipid mediator. We highlight the role of ceramide from basic research to the clinical application for cancer treatment.
Ceramide, a bioactive lipid mediator
Sphingolipids are comprised of a variety of structural lipids with a range of bioactive functions [15]. Ceramide is considered one of the central components of sphingolipid metabolism because ceramide is a precursor for all major sphingolipids. It has a wide range of functions, with processes involved in the cell cycle, differentiation, senescence, and apoptosis. Ceramide is produced through three distinct but interrelated mechanisms, which include the de novo pathway, the salvage pathway, and the sphingomyelin pathway [16] (Fig. 2). Ceramide functions as a “coordinator” of stress responses. Many inducers of stress result in ceramide accumulation, typically as a result of activation of either sphingomyelinase (SMases) or the de novo pathway, as well as through inhibition of ceramide clearance, mediated by SM synthase or ceramidases (CDases). Ceramide inducers include chemotherapeutic agents (paclitaxel, 5-fluorouracil (5-FU), cisplatin), environmental stresses (heat, ultraviolet radiation, hypoxia/reperfusion), and cytokines (tumor necrosis factor (TNF-α), fatty acid synthase (Fas), nerve growth factor) [15].
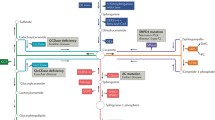
Pathways for ceramide metabolism. Ceramide synthesis has three main metabolic pathways: the de novo pathway, the salvage pathway, and sphingomyelin pathway. Ceramide is metabolized to ceramide-1-phosphate (C1P) and sphingosine-1-phosphate (S1P) through catabolic pathways. CDase ceramidase, CerS ceramide synthases, DES1 dihydroceramide desaturase 1, GBA glucocerebrosidase, GCS glucosylceramide synthase, SM sphingomyelin, SphK sphingosine kinase, SPP S1P phosphatase
Ceramide causes cell death by various mechanisms. Ceramide mediates cell death by enhancing the activity of proapoptotic membrane molecules, like CD95, and initiating apoptosis [17]. Ceramide also forms mitochondrial transmembrane channels regulated by the members of the Bcl-2 family of receptors, and these channels subsequently release apoptosis-inducing proteins [18].
Ceramide plays an important role in normal breast epithelial cells as well as in breast cancer cells. The mammary ducts are structurally composed of a basement membrane, a layer of myoepithelial cells, and luminal ductal cells. Ceramide negatively regulates Rb and CDK2 proteins, which act as cell proliferation inhibitors in the normal mammary epithelium. In invasive cancer, luminal cancer cells are demonstrated to highjack ceramide metabolism that results in the loss and/or degradation of the basement membrane, allowing cancer cells to migrate and invade the surrounding tissue [19]. Moreover, an abnormal production of ceramide increases S1P, which promotes activation of the PI3K/Akt and MAPK pathways through S1PRs, inducing cell proliferation. Much research has attempted to regulate ceramide to induce apoptosis of cancer cells through different pathways. In the next section, we discuss sphingolipid pathways.
Ceramide and sphingolipid pathway
Ceramide is generated from three essential pathways; (1) the de novo pathway, (2) the salvage pathway, and (3) the sphingomyelin pathway. Ceramide is then metabolized to other lipids, such as S1P and ceramide 1-phosphate (C1P) (Fig. 2). As shown in Fig. 2, ceramide metabolism is quite complex. Recent studies show that there are six distinct ceramide synthases [20], five CDases [21], and five SMases [22]. Additionally, ceramide metabolism is subject to local metabolism controlled by these enzymes, which have specific interaction with enzymes located in the mitochondria, plasma membrane, lysosomes, Golgi or endoplasmic reticulum [15]. Because of these diverse characteristics and interactions, ceramide has been shown to have a variety of cellular functions.
De novo pathway
De novo synthesis of sphingolipid begins with the condensation of palmitate and serine catalyzed by serine palmitoyl transferase, which leads to the formation of dihydrosphingosine (Fig. 2). Dihydrosphingosine is converted to dihydroceramide by six ceramide synthases. Ceramide is generated from dihydroceramide by dihydroceramide desaturase 1. These enzymatic activities occur not only in the endoplasmic reticulum but also in the lysosome and mitochondria, resulting in compartment-specific ceramide generation. Dihydroceramide is known to act as mediators of autophagy. Dihydroceramide desaturase 1 inhibitors activate autophagy via both dihydroceramide-dependent and -independent pathways [23]. Moreover, in hepatic steatosis models, dihydroceramide acts as a key metabolite that regulates autophagy and promotes fibrosis [24].
Salvage pathway
Ceramide can be a substrate to generate sphingosine, which is the product of sphingolipid catabolism. Sphingosine is salvaged through re-acylation, resulting in the generation of ceramide (Fig. 2). This “recycling” of sphingosine is termed the “salvage pathway”. The salvage pathway is not only subject to its own regulation, but it also modulates the formation of ceramide and subsequent ceramide-dependent cellular signals [16].
Ceramide is generated from glucosylceramide by glucosylceramidase in the salvage pathway via glucosylceramide synthase (GCS) [25]. Glucosylceramide, the core molecule of many glycosphingolipids, plays an important role in cancer cell proliferation, differentiation, and metastasis. GCS may be a prognostic indicator and potential target for the treatment of chemotherapy–refractory breast cancer [26]. Overexpression of GCS confers drug resistance in cancer cells, and suppression of GCS sensitizes cancers to chemotherapy in preclinical studies [27].
Sphingomyelin pathway
The sphingomyelin pathway is initiated by hydrolysis of the plasma membrane phospholipid sphingomyelin, leading to the generation of ceramide [28] (Fig. 2). The metabolism of sphingomyelin and ceramide involves the enzymes, SMase and SM synthase, and generates signals regulating cell proliferation and apoptosis. These effects can be dependent of cellular pH. For example, alkaline SMase activity preferentially decreases cellular activity in human colorectal carcinoma, whereas neutral SMase 2 results in decreased proliferation in MCF7 breast cancer cells [29].
Catabolic pathway
Ceramide is generated by ceramide synthases (CerS), of which six different enzymes have been identified (Fig. 2). Each CerS has a fatty acyl CoA chain of different length, resulting in a specific activity. For example, C16:0-ceramide generated by CerS1 suppresses tumor growth, while C18:0-ceramide generated by CerS5/6 protects tumor from apoptosis in human head and neck squamous cell carcinomas [30]. In human breast and colon cancer, long-chain and very long chain ceramide have opposite effects on cell growth. Sphingosine induces apoptosis and works as a reverse regulator of both the SAPK and ERK signaling pathways [31]. CerS expression may also be associated with enhanced cancer survival [32]. Elevated acid ceramidase (ACDase) expression reported in cancers has been associated with poorer cancer outcomes [33]. In prostate cancer cells, ACDase overexpression is related to increased proliferation and migration [34].
Sphingosine kinase, comprised of two major isoforms (SphK1 and SphK2), generates S1P inside the cells. S1P produced by SphK1 is secreted into the extracellular space by transporters, such as ATP-binding cassette (ABC) transporters or spinster homologue 2 (Spns2), and signals through its receptors on the outside of cells. This process is referred to as “inside-out” signaling [35, 36]. S1P can stimulate any of the five specific G protein-coupled receptors (S1PRs). S1P produced by SphK2 interacts with prohibitin 2 to regulate complex IV assembly and respiration [37]. Cancer cells are stimulated by S1P in an autocrine and/or paracrine manner. S1P also promotes metastasis, angiogenesis and change of immune function. Exogenous S1P upregulates matrix metalloproteinase 2 (MMP2) gene expressions, which is turn stimulate metastasis and angiogenesis [38]. Extracellular S1P can also influence fibroblasts and immune cells resulting in potent pro-cancer inflammatory effects [39].
S1P is irreversibly cleaved by S1P lyase or restored by S1P phosphatase [40]. The levels of these enzymes have a complex interaction with tumor aggressiveness. For example, in some cancers including lung and colon cancers, over-expression of S1P lyase predisposed cells to stress-induced apoptosis through the reduction of intracellular S1P [41]. On the contrary in prostate cancer, S1P lyase is downregulated [42]. Moreover, the S1P lyase gene may also be deleted in other human cancers [43].
Ceramide kinase
Ceramide can be directly phosphorylated by the enzyme ceramide kinase and derive C1P (Fig. 2). C1P works as second messenger of promoting cell survival and important mediator of the inflammatory response [44]. Thus, ceramide pathways and ceramide metabolites are very intricate. In the next section, we discuss how ceramide plays a role in cancer treatment including chemotherapy, radiation therapy and immunotherapy.
Ceramide and cancer treatment
Many interactions have been characterized between cancer treatments and sphingolipid metabolism. In this section, we review the data supporting the role of ceramide in cancer treatment.
Chemotherapy
Ceramide has been demonstrated to play a major role in cancer chemotherapy metabolism and efficacy. Ceramide is a regulator of the taxane-mediated spindle assembly checkpoint and taxane-induced cell death. Paclitaxel-treated MCF-7 cells activated acid sphingomyelinase (ASMase), leading to ceramide production that in turn results in a reduction in cell motility [45]. In addition, C6 ceramide enhances docetaxel-induced growth inhibition in breast cancer cells in vitro [46].
5-FU, an inhibitor of ribosomal RNA synthesis, functions through the inhibition of thymidylate synthase and commonly used in the treatment of colorectal cancer, gastric cancer, and breast cancer [47]. The addition of sphingomyelin increased the efficacy of 5-FU in human colonic xenografts [48]. Further, increase in SMase substrate also led to higher levels of apoptosis [49].
Platinum agents are used for many types of malignancies such as esophagus cancer [50], gastric cancer [51], and triple-negative breast cancer (TNBC) [52]. Cisplatin functions by forming DNA intrastrand crosslinks resulting in DNA damage. In glioma cells, the blockade of sphingomyelinases inhibits cisplatin-induced cell death [53]. Moreover, decrease of CerS2 led to increased sensitivity to cisplatin in HeLa cells through upregulation of C16 ceramide but downregulation of C24 ceramide, which suggested that C16 ceramide functions in apoptosis, while C24 induces survival [54]. Cisplatin activates ASMase and induces ceramide production, which leads to the redistribution of the death receptor CD95 in HT29 human colon cancer and induces cell death [55]. Thus, increasing ceramide by blocking ceramide catabolic enzyme or adding ceramide and precursor might increase the efficacy of chemotherapeutic drug treatment. In this way, ceramide is expected to act as the chemotherapy enhancer.
Furthermore, ceramide is now expected to be used against chemotherapeutic drug resistance. The efficiency of chemotherapeutic drugs can be reduced by mutations in a number of different cell cycle regulators, deregulation of various apoptotic pathways, or incomplete drug penetration such as the blood–brain barrier and defective vasculature formations [56]. These resistance mechanisms need be addressed to enhance the effect of chemotherapeutic agents and avoid cancer progression. Ceramide has been used in nanoparticles for that purpose. Nanoparticles, measuring up to 400 nm in size, have demonstrated efficacy for carrying, protecting, and delivering therapeutic molecules with diverse physiological properties [57]. For example, Chang et al. developed both photosensitizer- and anticancer drug-encapsulated hyaluronic acid–ceramide nanoparticles to increase the therapeutic efficacy of photodynamic therapy [58]. It has also been reported that nanoparticles containing a combination of C6 ceramide and paclitaxel inhibited the cancer cell proliferation in comparison with nanoparticles containing paclitaxel alone [59]. With emerging studies that ceramide can influence chemotherapy through nanoparticles, ceramide might have a more significant role in combination with chemotherapy.
The blood–brain barrier is a specific arrangement of blood vessels that separates the circulating blood from the brain and extracellular fluid in the central nervous system [60]. Because the blood–brain barrier can regulate the diffusion of water and biochemical molecules, the available chemotherapies for brain tumor are currently limited. FTY720, a structural analog of S1P, has been shown to cross the blood–brain barrier and could act directly on neural cells [61]. In contrast to S1P, there is no ceramide analog in clinical use. However, there are some reports describing the association between ceramide metabolites and the blood–brain barrier. C1P increases blood–brain barrier regulation via prostaglandin E2 signaling [62]. Acid ceramidase inhibitors (N-acylsphingosine amidohydrolase 1; ASAH1) could cross the blood–brain barrier and have possible activity in glioblastoma treatment [63].
Ceramide has been proposed to be a key regulatory molecule to fight breast cancer chemotherapy resistance. Ceramide has been reported to enhance docetaxel-induced growth inhibition and apoptosis in MCF-7 and MDA-231 cells [46]. Uridine diphosphoglucose ceramide glucosyltransferase (UGCG), one of the enzymes in the salvage pathway that degrade ceramide, is also involved in breast cancer chemotherapy resistance. The decrease of ceramide through activation of UGCG has been implicated in the resistance of breast cancer cells to chemotherapy [64].
Endocrine therapy
Tamoxifen is the most common hormonal therapy for both early and advanced estrogen receptor-positive breast cancers. Tamoxifen inhibits ceramide hydrolysis by the enzyme acid ceramidase, which leads to suppression of ceramide metabolism and formation of S1P [65]. The interaction between tamoxifen and ceramide also elicits decreases in mitochondrial membrane potential and subsequent increases in the release of mitochondrial proapoptotic proteins [66]. Moreover, nanoliposomal tamoxifen enhances nanoliposomal C6-ceramide cytotoxicity in cultured TNBC cells by induction of cell-cycle arrest in G(1) and G(2), caspase-dependent induction of DNA fragmentation, and enhanced mitochondrial and lysosomal membrane permeability. Taken together, ceramide may act to overcome chemoresistance through interactions with tamoxifen. The combination of tamoxifen and ceramide may also serve as a promising therapy for chemoresistance and TNBC irrespective of estrogen receptor status [67].
Radiation therapy
Radiation therapy is another standard local therapy for cancer. Delivered alone or in combination with chemotherapeutic agents, radiation is routinely used to treat many kinds of tumors, including brain cancer [68] and breast cancer [69]. When ionizing radiation is administered to living tissues, highly reactive particles are generated [70]. It is well known that tumor cell response to radiation depends on the microvascular damage [71]. Radiation therapy induces apoptosis through two ways associated with ceramide. The first is mediated through ASMase and the second is mediated through CerS. Lymphoblasts that lack ASMase expression are insensitive to radiation-induced apoptosis but can be sensitized by genetic transfection of ASMase [72]. The radiation sensitivity decided by CerS activation may lead to the efficiency of radiotherapy.
Immunotherapy
Cancer immunotherapy is increasingly becoming an integral part of the management of many cancers. Now, anti-programmed death (PD)-1 antibody is a new class of cancer immunotherapy that specifically hinders immune effector inhibition, reinvigorating and potentially expanding preexisting anticancer immune responses [73]. Thus, the blockade of immune checkpoints is the one of the most newly investigated approaches to activating therapeutic antitumor immunity.
Invariant natural killer T cells (iNKT) are T cells that can recognize glycolipid antigens, such as α-galactosylceramide (αGalCer). In vivo activation of iNKT cells with αGalCer results in robust cytokine production. Parekh et al. found that αGalCer-activated iNKT cells have rapidly upregulated expression of PD-1. PD-1:PD-L blockade prevents energy induction and enhances the anti-tumor activities of αGalCer-activated iNKT cells [74].
The sphingolipids and breast cancer progression
While sphingolipids have important roles in cancer treatment, there are still unknown mechanisms in cancer progression. Although many studies showed the important role of sphingolipids in breast cancer progression, the evidence in human breast cancer patients had been limited until recently. Our group has been studying the biology of sphingolipids, including ceramide and S1P in human breast cancer. We have shown that the levels of sphingolipids were higher in cancer and peri-tumoral tissue compared to normal breast tissue in human surgical specimens [75]. Since ceramide is generated from the three major pathways (i.e. the de novo pathway, the salvage pathway, and the sphingomyelin pathway), we hypothesized that all of these pathways were activated within tumor tissues. Consistent with this hypothesis, we found that both precursors and enzymes of all three ceramide biosynthesis pathways were activated to increase ceramide in breast cancer tissue [76]. High S1P associated significantly with lymph node metastasis [77], suggesting that S1P affects the tumor microenvironment, which leads to cancer metastasis [75]. Conversely, the levels of ceramide (as opposed to S1P) were highest within cancer and interstitial fluid specimens but lowest in normal breast. Taken together, these findings suggest that ceramide acts in an opposing manner to S1P [76].
We also showed that ceramide was associated with less aggressiveness, as measured by nuclear grade and Ki-67 [76]. Interestingly, higher levels of individual ceramide-related enzymes were found to associate with elevated ceramide expression and with worse survival. Further, breast cancer cells with high S1P to ceramide ratio demonstrated enhansed proliferation potency. Taken together, it is reasonable to hypothesize that the S1P converted from ceramide enhanse cancer aggressiveness through the complex pathways involved in the “sphingolipid rheostat” [76]. More data from the clinical setting are now needed to reveal “sphingolipid rheostat” based on in vitro and in vivo models to increase the understanding of the biology of cancer progression.
Future direction
Despite recent advances in the treatment of breast cancer, many patients with advanced disease remain incurable for many reasons, including the resistance to chemotherapy. Jing et al. studied the association between the ceramide metabolism and resistance to chemotherapy in a small cohort of patients with advanced breast cancer [64]. Interestingly, the level of ceramide was decreased in all patients, which indicates that ceramide downregulation is a common characteristic in the chemotherapy itself. This study suggests that ceramide works by not only promoting apoptosis but also sensitizing cancer cell response to chemotherapy. Recognizing that the identification of the molecular mechanisms behind resistance is essential to the development of targeted therapy, targeting ceramide metabolism has significant promise.
Although it is a limited and negative study, a phase II trial on ceramide treatment for cutaneous breast cancer patients was reported. One of 25 patients treated with a topical ceramide manifested a partial response without undesirable side effects [78]. Given that ceramide is known to have a moisturizing property and may penetrate the skin [79], ceramide may have a potential to be therapeutically utilized topically.
Recently, it was shown that ceramide plays important roles not only in cancer treatment but also in cancer diagnosis by functioning as a diagnostic biomarker. For example, it has been shown that serum levels of ceramides differ significantly between the patients with hepatocellular carcinoma (HCC) and patients with cirrhosis alone, despite the fact that majority of HCC are accompanied with cirrhosis [80]. This is an emerging evidence that ceramide has the potential to function as a diagnostic biomarker in liver cancer. However, further studies are needed to develop novel therapies for cancer patients based on ceramide pathways.
Conclusions
In this review, we discussed the roles of ceramide in cancer. As ceramide functions as the central hub of sphingolipid metabolism, ceramide has the possibility to be used for cancer treatment by using ceramide directly as anti-cancer agent, by regulating ceramide metabolism to suppress cancer, or even as a biomarker. Recent studies showed that ceramide promotes apoptosis and has the possibility of becoming a supplementary agent for cancer treatment. Ceramide has been shown to function within nanoparticles and as a diagnostic biomarker. These studies strongly suggested that sphingolipids, such as ceramide, have potential for becoming incorporated into innovative cancer treatments.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30.
Chen Z, Fillmore CM, Hammerman PS, Kim CF, Wong KK. Non-small-cell lung cancers: a heterogeneous set of diseases. Nat Rev Cancer. 2014;14:535–46.
Hartwell LH, Kastan MB. Cell cycle control and cancer. Science. 1994;266:1821–8.
Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science. 1999;286:1358–62.
Khanna KK, Jackson SP. DNA double-strand breaks: signaling, repair and the cancer connection. Nat Genet. 2001;27:247–54.
Sotomayor EM, Borrello I, Levitsky HI. Tolerance and cancer: a critical issue in tumor immunology. Crit Rev Oncog. 1996;7:433–56.
Hannun YA, Obeid LM. The Ceramide-centric universe of lipid-mediated cell regulation: stress encounters of the lipid kind. J Biol Chem. 2002;277:25847–50.
Spiegel S, Cuvillier O, Edsall L, Kohama T, Menzeleev R, Olivera A, et al. Roles of sphingosine-1-phosphate in cell growth, differentiation, and death. Biochemistry. 1998;63:69–73.
Newton J, Lima S, Maceyka M, Spiegel S. Revisiting the sphingolipid rheostat: evolving concepts in cancer therapy. Exp Cell Res. 2015;333:195–200.
Cuvillier O, Pirianov G, Kleuser B, Vanek PG, Coso OA, Gutkind S, et al. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature. 1996;381:800–3.
Obeid LM, Linardic CM, Karolak LA, Hannun YA. Programmed cell death induced by ceramide. Science. 1993;259:1769–71.
Nagahashi M, Ramachandran S, Kim EY, Allegood JC, Rashid OM, Yamada A, et al. Sphingosine-1-phosphate produced by sphingosine kinase 1 promotes breast cancer progression by stimulating angiogenesis and lymphangiogenesis. Cancer Res. 2012;72:726–35.
Salas A, Ponnusamy S, Senkal CE, Meyers-Needham M, Selvam SP, Saddoughi SA, et al. Sphingosine kinase-1 and sphingosine 1-phosphate receptor 2 mediate Bcr-Abl1 stability and drug resistance by modulation of protein phosphatase 2A. Blood. 2011;117:5941–52.
Young MM, Kester M, Wang HG. Sphingolipids: regulators of crosstalk between apoptosis and autophagy. J Lipid Res. 2013;54:5–19.
Hannun YA, Obeid LM. Many ceramides. J Biol Chem. 2011;286:27855–62.
Kitatani K, Idkowiak-Baldys J, Hannun YA. The sphingolipid salvage pathway in ceramide metabolism and signaling. Cell Signal. 2008;20:1010–8.
Grassme H, Jekle A, Riehle A, Schwarz H, Berger J, Sandhoff K, et al. CD95 signaling via ceramide-rich membrane rafts. J Biol Chem. 2001;276:20589–96.
Colombini M. Membrane channels formed by ceramide. In: Gulbins E, Petrache I, editors. Sphingolipids: basic science and drug development. Vienna: Springer; 2013. p. 109–26.
Garcia-Gonzalez V, Diaz-Villanueva JF, Galindo-Hernandez O, Martinez-Navarro I, Hurtado-Ureta G, Perez-Arias AA. Ceramide metabolism balance, a multifaceted factor in critical steps of breast cancer development. Int J Mol Sci. 2018;19:2527.
Pewzner-Jung Y, Park H, Laviad EL, Silva LC, Lahiri S, Stiban J, et al. A critical role for ceramide synthase 2 in liver homeostasis: I. alterations in lipid metabolic pathways. J Biol Chem. 2010;285:10902–10.
Mao Z, Sun W, Xu R, Novgorodov S, Szulc ZM, Bielawski J, et al. Alkaline ceramidase 2 (ACER2) and its product dihydrosphingosine mediate the cytotoxicity of N-(4-hydroxyphenyl)retinamide in tumor cells. J Biol Chem. 2010;285:29078–90.
Ledesma MD, Prinetti A, Sonnino S, Schuchman EH. Brain pathology in Niemann Pick disease type A: insights from the acid sphingomyelinase knockout mice. J Neurochem. 2011;116:779–88.
Casasampere M, Ordonez YF, Casas J, Fabrias G. Dihydroceramide desaturase inhibitors induce autophagy via dihydroceramide-dependent and independent mechanisms. Biochim Biophys Acta. 2017;1861:264–75.
Lee AY, Lee JW, Kim JE, Mock HJ, Park S, Kim S, et al. Dihydroceramide is a key metabolite that regulates autophagy and promotes fibrosis in hepatic steatosis model. Biochem Biophys Res Commun. 2017;494:460–9.
Hillig I, Leipelt M, Ott C, Zahringer U, Warnecke D, Heinz E. Formation of glucosylceramide and sterol glucoside by a UDP-glucose-dependent glucosylceramide synthase from cotton expressed in Pichia pastoris. FEBS Lett. 2003;553:365–9.
Liu YY, Patwardhan GA, Xie P, Gu X, Giuliano AE, Cabot MC. Glucosylceramide synthase, a factor in modulating drug resistance, is overexpressed in metastatic breast carcinoma. Int J Oncol. 2011;39:425–31.
Liu YY, Patwardhan GA, Bhinge K, Gupta V, Gu X, Jazwinski SM. Suppression of glucosylceramide synthase restores p53-dependent apoptosis in mutant p53 cancer cells. Cancer Res. 2011;71:2276–85.
Pena LA, Fuks Z, Kolesnick R. Stress-induced apoptosis and the sphingomyelin pathway. Biochem Pharmacol. 1997;53:615–21.
Hertervig E, Nilsson A, Nyberg L, Duan RD. Alkaline sphingomyelinase activity is decreased in human colorectal carcinoma. Cancer. 1997;79:448–53.
Senkal CE, Ponnusamy S, Bielawski J, Hannun YA, Ogretmen B. Antiapoptotic roles of ceramide-synthase-6-generated C16-ceramide via selective regulation of the ATF6/CHOP arm of ER-stress-response pathways. Faseb J. 2010;24:296–308.
Coroneos E, Wang Y, Panuska JR, Templeton DJ, Kester M. Sphingolipid metabolites differentially regulate extracellular signal-regulated kinase and stress-activated protein kinase cascades. Biochem J. 1996;316(Pt 1):13–7.
Hartmann D, Lucks J, Fuchs S, Schiffmann S, Schreiber Y, Ferreiros N, et al. Long chain ceramides and very long chain ceramides have opposite effects on human breast and colon cancer cell growth. Int J Biochem Cell Biol. 2012;44:620–8.
Ruckhaberle E, Holtrich U, Engels K, Hanker L, Gatje R, Metzler D, et al. Acid ceramidase 1 expression correlates with a better prognosis in ER-positive breast cancer. Climacteric. 2009;12:502–13.
Saad AF, Meacham WD, Bai A, Anelli V, Elojeimy S, Mahdy AE, et al. The functional effects of acid ceramidase overexpression in prostate cancer progression and resistance to chemotherapy. Cancer Biol Ther. 2007;6:1455–60.
Takabe K, Paugh SW, Milstien S, Spiegel S. “Inside-out” signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol Rev. 2008;60:181–95.
Tsuchida J, Nagahashi M, Takabe K, Wakai T. Clinical Impact of Sphingosine-1-Phosphate in Breast Cancer. Mediators Inflamm. 2017;2017:2076239.
Nagahashi M, Takabe K, Liu R, Peng K, Wang X, Wang Y, et al. Conjugated bile acid-activated S1P receptor 2 is a key regulator of sphingosine kinase 2 and hepatic gene expression. Hepatology. 2015;61:1216–26.
Young N, Pearl DK, Van Brocklyn JR. Sphingosine-1-phosphate regulates glioblastoma cell invasiveness through the urokinase plasminogen activator system and CCN1/Cyr61. Mol Cancer Res. 2009;7:23–32.
Kawamori T, Osta W, Johnson KR, Pettus BJ, Bielawski J, Tanaka T, et al. Sphingosine kinase 1 is up-regulated in colon carcinogenesis. Faseb J. 2006;20:386–8.
Fyrst H, Saba JD. Sphingosine-1-phosphate lyase in development and disease: sphingolipid metabolism takes flight. Biochim Biophys Acta. 2008;1781:448–58.
Min J, Van Veldhoven PP, Zhang L, Hanigan MH, Alexander H, Alexander S. Sphingosine-1-phosphate lyase regulates sensitivity of human cells to select chemotherapy drugs in a p38-dependent manner. Mol Cancer Res. 2005;3:287–96.
Brizuela L, Ader I, Mazerolles C, Bocquet M, Malavaud B, Cuvillier O. First evidence of sphingosine 1-phosphate lyase protein expression and activity downregulation in human neoplasm: implication for resistance to therapeutics in prostate cancer. Mol Cancer Ther. 2012;11:1841–51.
Wrage M, Ruosaari S, Eijk PP, Kaifi JT, Hollmen J, Yekebas EF, et al. Genomic profiles associated with early micrometastasis in lung cancer: relevance of 4q deletion. Clin Cancer Res. 2009;15:1566–74.
Arana L, Gangoiti P, Ouro A, Trueba M, Gomez-Munoz A. Ceramide and ceramide 1-phosphate in health and disease. Lipids Health Dis. 2010;9:15.
Zeidan YH, Jenkins RW, Hannun YA. Remodeling of cellular cytoskeleton by the acid sphingomyelinase/ceramide pathway. J Cell Biol. 2008;181:335–50.
Yang L, Zheng LY, Tian Y, Zhang ZQ, Dong WL, Wang XF, et al. C6 ceramide dramatically enhances docetaxel-induced growth inhibition and apoptosis in cultured breast cancer cells: a mechanism study. Exp Cell Res. 2015;332:47–59.
Molteni LP, Rampinelli I, Cergnul M, Scaglietti U, Paino AM, Noonan DM, et al. Capecitabine in breast cancer: the issue of cardiotoxicity during fluoropyrimidine treatment. Breast J. 2010;16(Suppl 1):45-8.
Modrak DE, Rodriguez MD, Goldenberg DM, Lew W, Blumenthal RD. Sphingomyelin enhances chemotherapy efficacy and increases apoptosis in human colonic tumor xenografts. Int J Oncol. 2002;20:379–84.
Eichhorst ST, Muerkoster S, Weigand MA, Krammer PH. The chemotherapeutic drug 5-fluorouracil induces apoptosis in mouse thymocytes in vivo via activation of the CD95(APO-1/Fas) system. Cancer Res. 2001;61:243–8.
Ilson DH, Saltz L, Enzinger P, Huang Y, Kornblith A, Gollub M, et al. Phase II trial of weekly irinotecan plus cisplatin in advanced esophageal cancer. J Clin Oncol. 1999;17:3270–5.
Moro K, Nagahashi M, Naito T, Nagai Y, Katada T, Minagawa M, et al. Gastric adenosquamous carcinoma producing granulocyte-colony stimulating factor: a case of a rare malignancy. Surg Case Rep. 2017;3:67.
Denkert C, Liedtke C, Tutt A, von Minckwitz G. Molecular alterations in triple-negative breast cancer-the road to new treatment strategies. Lancet. 2017;389:2430–42.
Noda S, Yoshimura S, Sawada M, Naganawa T, Iwama T, Nakashima S, et al. Role of ceramide during cisplatin-induced apoptosis in C6 glioma cells. J Neurooncol. 2001;52:11–21.
Sassa T, Suto S, Okayasu Y, Kihara A. A shift in sphingolipid composition from C24 to C16 increases susceptibility to apoptosis in HeLa cells. Biochim Biophys Acta. 2012;1821:1031–7.
Lacour S, Hammann A, Grazide S, Lagadic-Gossmann D, Athias A, Sergent O, et al. Cisplatin-induced CD95 redistribution into membrane lipid rafts of HT29 human colon cancer cells. Cancer Res. 2004;64:3593–8.
Gottesman MM. Mechanisms of cancer drug resistance. Annu Rev Med. 2002;53:615–27.
Patel NR, Pattni BS, Abouzeid AH, Torchilin VP. Nanopreparations to overcome multidrug resistance in cancer. Adv Drug Deliv Rev. 2013;65:1748–62.
Chang JE, Cho HJ, Yi E, Kim DD, Jheon S. Hypocrellin B and paclitaxel-encapsulated hyaluronic acid-ceramide nanoparticles for targeted photodynamic therapy in lung cancer. J Photochem Photobiol B. 2016;158:113–21.
Deshpande D, Devalapally H, Amiji M. Enhancement in anti-proliferative effects of paclitaxel in aortic smooth muscle cells upon co-administration with ceramide using biodegradable polymeric nanoparticles. Pharm Res. 2008;25:1936–47.
Daneman R, Prat A. The blood-brain barrier. Cold Spring Harb Perspect Biol. 2015;7:a020412.
Cipriani R, Chara JC, Rodriguez-Antiguedad A, Matute C. FTY720 attenuates excitotoxicity and neuroinflammation. J Neuroinflamm. 2015;12:86.
Mesev EV, Miller DS, Cannon RE. Ceramide 1-Phosphate Increases P-Glycoprotein Transport Activity at the Blood-Brain Barrier via Prostaglandin E2 Signaling. Mol Pharmacol. 2017;91:373–82.
Doan NB, Alhajala H, Al-Gizawiy MM, Mueller WM, Rand SD, Connelly JM, et al. Acid ceramidase and its inhibitors: a de novo drug target and a new class of drugs for killing glioblastoma cancer stem cells with high efficiency. Oncotarget. 2017;8:112662–74.
Che J, Huang Y, Xu C, Zhang P. Increased ceramide production sensitizes breast cancer cell response to chemotherapy. Cancer Chemother Pharmacol. 2017;79:933–41.
Morad SA, Cabot MC. Tamoxifen regulation of sphingolipid metabolism—therapeutic implications. Biochim Biophys Acta. 2015;1851:1134–45.
Morad SA, Ryan TE, Neufer PD, Zeczycki TN, Davis TS, MacDougall MR, et al. Ceramide-tamoxifen regimen targets bioenergetic elements in acute myelogenous leukemia. J Lipid Res. 2016;57:1231–42.
Morad SA, Levin JC, Shanmugavelandy SS, Kester M, Fabrias G, Bedia C, et al. Ceramide—antiestrogen nanoliposomal combinations—novel impact of hormonal therapy in hormone-insensitive breast cancer. Mol Cancer Ther. 2012;11:2352–61.
Winkler F, Kozin SV, Tong RT, Chae SS, Booth MF, Garkavtsev I, et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6:553–63.
Fisher B, Bauer M, Margolese R, Poisson R, Pilch Y, Redmond C, et al. Five-year results of a randomized clinical trial comparing total mastectomy and segmental mastectomy with or without radiation in the treatment of breast cancer. N Engl J Med. 1985;312:665–73.
Linkous AG, Yazlovitskaya EM. Novel radiosensitizing anticancer therapeutics. Anticancer Res. 2012;32:2487–99.
Garcia-Barros M, Paris F, Cordon-Cardo C, Lyden D, Rafii S, Haimovitz-Friedman A, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300:1155–9.
Mesicek J, Lee H, Feldman T, Jiang X, Skobeleva A, Berdyshev EV, et al. Ceramide synthases 2, 5, and 6 confer distinct roles in radiation-induced apoptosis in HeLa cells. Cell Signal. 2010;22:1300–7.
Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA. 2002;99:12293–7.
Parekh VV, Lalani S, Kim S, Halder R, Azuma M, Yagita H, et al. PD-1/PD-L blockade prevents anergy induction and enhances the anti-tumor activities of glycolipid-activated invariant NKT cells. J Immunol. 2009;182:2816–26.
Nagahashi M, Tsuchida J, Moro K, Hasegawa M, Tatsuda K, Woelfel IA, et al. High levels of sphingolipids in human breast cancer. J Surg Res. 2016;204:435–44.
Moro K, Kawaguchi T, Tsuchida J, Gabriel E, Qi Q, Yan L, et al. Ceramide species are elevated in human breast cancer and are associated with less aggressiveness. Oncotarget. 2018;9:19874–90.
Tsuchida J, Nagahashi M, Nakajima M, Moro K, Tatsuda K, Ramanathan R, et al. Breast cancer sphingosine-1-phosphate is associated with phospho-sphingosine kinase 1 and lymphatic metastasis. J Surg Res. 2016;205:85–94.
Jatoi A, Suman VJ, Schaefer P, Block M, Loprinzi C, Roche P, et al. A phase II study of topical ceramides for cutaneous breast cancer. Breast Cancer Res Treat. 2003;80:99–104.
Hon KL, Pong NH, Wang SS, Lee VW, Luk NM, Leung TF. Acceptability and efficacy of an emollient containing ceramide-precursor lipids and moisturizing factors for atopic dermatitis in pediatric patients. Drugs R D. 2013;13:37–42.
Grammatikos G, Schoell N, Ferreiros N, Bon D, Herrmann E, Farnik H, et al. Serum sphingolipidomic analyses reveal an upregulation of C16-ceramide and sphingosine-1-phosphate in hepatocellular carcinoma. Oncotarget. 2016;7:18095–105.
Acknowledgements
This work was supported by the Japan Society for the Promotion of Science (JSPS) Grant-in-Aid for Scientific Research Grant Number 18K19576 for MN, and 16K15610 for TW. MN was supported by the Uehara Memorial Foundation, Takeda Science Foundation, and Tsukada Medical Foundation. KT was supported by NIH/NCI grant R01CA160688 and Susan G. Komen Investigator Initiated Research Grant IIR12222224.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Moro, K., Nagahashi, M., Gabriel, E. et al. Clinical application of ceramide in cancer treatment. Breast Cancer 26, 407–415 (2019). https://doi.org/10.1007/s12282-019-00953-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-019-00953-8