Abstract
Background
Trastuzumab following anthracycline causes cardiotoxicity in up to 28% of patients. Although the cardiotoxicity is often irreversible once cardiac dysfunction is detected, the early predictor has not been established yet.
Methods
We prospectively observed breast cancer patients treated with anthracycline or trastuzumab at Tonan Hospital. All patients underwent echocardiography and blood sampling at baseline, and every three months during chemotherapy. Cardiotoxicity was defined as a decline in left ventricular ejection fraction >10% points.
Results
Of 40 patients, 34 patients (85%) were treated with anthracycline (epirubicin), 18 (45%) with trastuzumab, and 12 (30%) with both agents. Cardiotoxicity was observed in four patients (10%), who were all treated with both agents. The absolute levels of high-sensitive troponin T (hs-TnT) were increased in all four patients with cardiotoxicity, and all the highest points were observed before or at the time of detection of cardiotoxicity. The highest level of hs-TnT was not significantly different in patients with and without cardiotoxicity. “Hs-TnT increment from baseline to the highest value” and “hs-TnT integration value above baseline” were significantly greater in patients with cardiotoxicity (0.039 vs. 0.007 ng/mL, P = 0.046, 0.113 vs. 0.022 ng months/mL, P = 0.013, respectively). The integration value had 100% sensitivity and specificity with a cutoff level at 0.070 ng months/mL.
Conclusions
Hs-TnT assay may be able to predict anthracycline- and trastuzumab-induced cardiotoxicity in breast cancer patients, and the hs-TnT increment or hs-TnT integration value above baseline was more reliable than the absolute value.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chemotherapy-induced cardiotoxicity (CTIC) is one of the most feared adverse events. CTIC has become a major issue for long-term cancer survivors including breast cancer patients [1, 2]. Modern comprehensive management of breast cancer including surgery, chemotherapy with molecular targeted agents, and radiotherapy has resulted in prolonged survival especially in patients receiving adjuvant treatment [3]. However, the gain in life expectancy might be countered by increased mortality due to cardiac failure. Anthracycline and trastuzumab are widely used, and are the most frequent causes of chemotherapy-induced cardiomyopathy as cardiotoxicity, in breast cancer therapy for both early and advanced diseases [3, 4]. The incidence of CTIC from anthracycline sequentially with trastuzumab has been reported to be up to 28%, varying according to the definition and an observation period in different studies [4,5,6,7]. Anthracycline immediately before trastuzumab considerably increases the cardiotoxicity. At the point when cardiac systolic dysfunction is detected with echocardiography, CTIC from the two agents is often already irreversible [4, 8]. Trastuzumab-induced cardiotoxicity is not dose dependent, different from anthracycline-induced one [4]. Early detection of myocardial damage is then crucial since it may improve cardiac prognosis [9].
Early predictors of CTIC have not been established yet. Cardiac troponin T and I are biomarkers of myocardial damage. Over the last few years, a new generation of troponin assays has become available, which is referred to as high-sensitive assays, able to detect very low concentrations of troponin [10]. Several reports showed that high-sensitive troponin I (hs-TnI) could predict trastuzumab-induced cardiomyopathy [11, 12]. However, accurate biomarkers predicting CTIC remain to be established. Conventional troponin T assays could not predict CTIC [13], and the role of high-sensitive troponin T (hs-TnT) in CTIC has not been investigated in a large number of patients.
High-sensitive troponin assays have recently been established as the best biomarkers for the early identification of microdamage of cardiomyocytes due to acute myocardial infarction [14]. Furthermore, hs-TnT has been reported to be able to predict cardiac prognosis of patients with cardiac failure or hypertension; slight increase of troponin T was the negative predictive factor [15,16,17,18,19]. This slight increase can be detected only with high-sensitive assays. In this study, we prospectively investigated whether hs-TnT assay can detect subclinical myocardial damage from chemotherapy including anthracycline or trastuzumab, and how accurately the assay can predict CTIC. We created some indices based on the data to improve the diagnostic accuracy.
Patients and methods
Study population
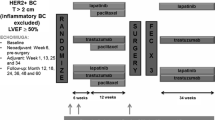
We enrolled consecutive patients with histologically or cytologically confirmed breast cancer undergoing chemotherapy including anthracycline or trastuzumab at Tonan Hospital, Sapporo, Japan, between April and December 2014. Patients were required to have acceptable bone marrow, hepatic, and renal functions for chemotherapy and adequate Eastern Cooperative Oncology Group performance status of 0–2. Exclusion criteria included symptomatic cardiac failure. Trastuzumab was administrated triweekly with a loading dose of 8 mg/kg followed by a maintenance dose of 6 mg/kg.
Study protocol
We prospectively observed troponin T levels and cardiac functions. All patients underwent chest radiography and electrocardiogram at baseline to screen signs of cardiac failure, cardiomyopathy, conduction disturbances, and QT prolongation. All patients further underwent blood sampling and transthoracic echocardiography at baseline, every three months during chemotherapy, and every six months during the first year after the end of trastuzumab administration. In case of cardiotoxicity, chemotherapy was discontinued and renin–angiotensin system inhibitor or ß-blocker was administrated as cardioprotective therapy. Echocardiography and blood sampling were performed monthly during the first three months after cardiotoxicity. In case of recovery, it was at our discretion whether to resume chemotherapy or not. The institutional review board of Tonan Hospital approved the protocol, and all participating patients provided written informed consent.
Definition of cardiotoxicity
The endpoint was cardiotoxicity, which was defined as a decline in LVEF >10% points from baseline [20], symptomatic cardiac failure, acute coronary syndrome, or life-threatening arrhythmias. LVEF was determined from two-dimensional echocardiograms according to the established criteria, the modified Simpson method [21]. Parasternal and apical views were obtained with a standard echocardiograph (Aplio 500, Toshiba Medical Systems Co., Ltd., Otawara, Japan).
Measurement and evaluation of biomarker
Plasma troponin T concentration was determined by electro-chemiluminescence immunoassay analyzer (EcLusys high-sensitivity Troponin T, Roche Diagnostics, Co., Ltd., Tokyo, Japan). The troponin T level was assessed as three indices: the highest level, hs-TnT increment from baseline to the highest value, and hs-TnT integration value above baseline. Figure 1 shows the last index we created, hs-TnT integration value of the increment from baseline, defined as an area under the troponin T level and over the baseline level during chemotherapy until the detection of cardiotoxicity. Brain natriuretic peptide (BNP) levels in plasma were measured with a chemiluminescent immunoassay (BNP-JP Architect, Abbott Japan Co., Ltd., Tokyo, Japan).
Definition of integration value of high-sensitive cardiac troponin above baseline. This index is shown as a gray area over the baseline level during chemotherapy. We calculated the area of triangles or trapezoids with the troponin values and the measurement months above baseline, and added the values. For example, this integration value above baseline is calculated as follows: (y 3 − y0) × x 3′/2 + (y 3 − y 0) + (y 4 − y 0) × x 4/2. x 3′ = x 3 × (y 3 − y 0)/(y 3 − y 2)
Statistical analysis
This study was conducted as a preliminary assessment of the diagnostic accuracy of hs-TnT. Continuous variables were expressed as mean ± standard deviation (SD), mean ± standard error (SE), or median with interquartile range (IR), as appropriate. Welch’s t test was used to compare differences of normally distributed continuous variables in mean values. Fisher’s exact was used to compare differences of proportions between independent groups. All statistical tests were two sided, and P value <0.05 was considered statically significant. All analyses were performed using SPSS Statistics 22 (IBM Japan, Ltd., Tokyo, Japan).
Results
Patient characteristics
A total of 43 patients were enrolled, of which three patients could not be followed up. Table 1 summarizes baseline clinical characteristics available for analysis in patients with and without cardiotoxicity. All 40 analyzed patients were Japanese women with good performance status. Thirty-four patients (85%) received anthracycline, 18 (45%) received trastuzumab, and 12 (30%) received both agents. Epirubicin was used in all patients receiving anthracycline, practically all patients received it as FEC 100 regimen: 5-fluorouracil 500 mg/m2, epirubicin 100 mg/m2, and cyclophosphamide 500 mg/m2. Cumulative doses of epirubicin were 300–400 mg/m2 in most patients (91%) receiving epirubicin. Patients receiving both anthracycline and trastuzumab were all treated with epirubicin for 3 months followed by trastuzumab for 12 months as a perioperative adjuvant treatment. Taxane was used in 36 patients, of which 32 (89%) received preceding anthracycline. Four patients (10%) had distant metastases. LVEF and BNP level were 70 ± 3.5% and 19 ± 11.4, respectively (mean ± SD). Practically no patients had chronic heart disease, and risk factors of cardiac diseases were observed in 21 patients (53%). Cardioprotective drugs were used in five patients (13%), who were not administrated ß-blocker. No patients had a previous history of chest radiation therapy. Treatment and follow-up time was 9 (IR, 3–15) months in the whole population. Patients with cardiotoxicity had significantly the longer time, 17 (IR, 3–15) months, than without, 6 (IR, 3–12) months. Significantly more patients received trastuzumab and that with epirubicin in patients with cardiotoxicity than without. The other characteristics were similar between patients with and without cardiotoxicity (Table 1).
Cardiotoxicity and BNP level
Cardiotoxicity was observed in four patients (10%), who all were treated with both anthracycline and trastuzumab as a perioperative adjuvant treatment. All cardiotoxicity was an asymptomatic decline in LVEF during trastuzumab monotherapy following anthracycline-containing regimen. Lowest LVEF during chemotherapy in patients with cardiotoxicity was 55 ± 7.3% (mean ± SD). All patients completely recovered after 1–3 months of trastuzumab discontinuation, and could restart and complete trastuzumab therapy after the recovery without any adverse events and hs-TnT elevation.
Baseline BNP levels were not significantly different in patients with and without cardiotoxicity (Table 1). The maximum levels during chemotherapy were also not significantly different in patients with and without cardiotoxicity (20 ± 6.0 vs. 18 ± 2.2 pg/mL; mean ± SE; P = 0.70).
Hs-TnT level
Baseline hs-TnT levels were not significantly different in patients with and without cardiotoxicity (Table 1). Figure 2 shows timeline of hs-TnT levels in patients with and without cardiotoxicity. Hs-TnT levels were increased in all patients with cardiotoxicity (Fig. 2a). All the highest points were observed before or at the time of detection of cardiotoxicity. In contrast, the troponin T level was stable in patients without cardiotoxicity (Fig. 2b, ESM1). Elevated hs-TnT level in all four patients with cardiotoxicity returned to their baseline level within their LVEF recovery time. Figure 3 shows differences with and without cardiotoxicity in two indices, highest level of hs-TnT during chemotherapy and the hs-TnT increment. The highest level was not significantly higher in patients with cardiotoxicity than without (0.044 ± 0.0109 vs. 0.013 ± 0.0014 ng/mL; mean ± SE; P = 0.067). The hs-TnT increment was significantly greater in patients with cardiotoxicity (0.039 ± 0.0096 vs. 0.007 ± 0.0013 ng/mL; mean ± SE; P = 0.046). Figure 4 shows the difference of hs-TnT integration value above baseline, which was also significantly greater in patients with cardiotoxicity (0.113 ± 0.0178 vs. 0.022 ± 0.0038 ng months/mL; mean ± SE). Furthermore, hs-TnT integration value above baseline had not only the lowest P value, 0.013, but also 100% sensitivity and specificity with cutoff level at 0.070 ng months/mL (Fig. 4).
Timeline of high-sensitives cardiac troponin T level in patients with and without cardiotoxicity. Line graph with cardiotoxicity (a) ends at the time of detection of cardiotoxicity (arrow). Each value without cardiotoxicity (b) is expressed as mean ± standard deviation. Case 4 reached only 0.014 ng/mL as the highest level and 0.012 ng/mL as the increment; however, the elevation continued for 12 months until the detection of cardiotoxicity
High-sensitive cardiac troponin T level in patients with cardiotoxicity. Highest level of high-sensitive cardiac troponin T (left) does not show significant difference with and without cardiotoxicity. The increment from baseline to the top value (right) shows the significant difference. Each value is expressed as mean ± standard error
Increased integration value of high-sensitive cardiac troponin T above baseline in two types of graph. This index is defined in Fig. 1. The integration value shows significant difference with and without cardiotoxicity, having the lowest P value of three troponin T indices. Each value in the left bar graph is expressed as mean ± standard error. Right scatter graph shows 100% sensitivity and specificity with a cutoff level. Each case with cardiotoxicity corresponds to that in Fig. 2a
Discussion
Absolute highest level of hs-TnT
In this study, we made two important clinical observations. First, hs-TnT assay may be able to predict CTIC from anthracycline and trastuzumab in breast cancer patients. Cardiac troponin is an early organ-specific biomarker of myocardial damage. In several cardiac biomarkers, only high-sensitive troponin assays are shown to detect not only immediately after the onset of acute myocardial infarction, but also poor cardiac prognosis of patients with cardiac failure or hypertension [14,15,16,17,18,19]. In regard to CTIC, several studies showed hs-TnI levels elevated after trastuzumab following anthracycline administration [11, 12, 23]. Conventional troponin T assays could not predict CTIC [13]. However, whether “hs-TnT” assay can predict CTIC due to anthracycline and trastuzumab is not investigated in a large number of patients. It is well established that anthracycline causes myocardial necrosis, from the cumulative dose versus cardiac failure relationship [4], from troponin I elevation after anthracycline administration, predicting a decline in LVEF [22]. One preliminary study, whose number of candidates was 19, has recently suggested that hs-TnT could predict CTIC due to administration of anthracycline and trastuzumab [24]. These findings are compatible with our results. Our results have demonstrated hs-TnT could detect preclinical myocardial damage due to anthracycline and trastuzumab. High-sensitive assays are essential to evaluate the levels specific to individual patients. High-sensitive assays including the one we used (EcLusys high-sensitivity Troponin T, Roche Diagnostics) have smaller coefficient of variation than the conventional ones; the coefficient of the range including 99th percentile is under 10% [10]. The detection limit of conventional troponin T assays is around 0.010 ng/mL, which is not sensitive to detect the slight increase from baseline. Only few healthy people over the detection limit can be conventionally detected at baseline. In one of the four patients with cardiotoxicity in this study, Case 4 (Fig. 2a), hs-TnT increased to the level of only 0.014 ng/mL. The precisely detected absolute, highest levels of hs-TnT would reflect the risk of CTIC, as with hs-TnI [11, 12, 22]. This slight increase can be detected only with high-sensitive assays. Indeed, highest level of hs-TnT was significantly higher in patients with than without cardiotoxicity in this study.
Hs-TnT increment from baseline to the highest value and hs-TnT integration value above baseline
The second clinical observation is that the hs-TnT increment and hs-TnT integration value above baseline were more reliable than the absolute value. Baseline levels of hs-TnT have great interindividual variability, affected not only by cardiac failure, but also by hypertension, cardiac hypertrophy, and renal dysfunction [17,18,19, 25]. Lower limit of hs-TnT assay is very low, 0.003 ng/mL, that this assay can assess the baseline level of each patient with sufficient precision. The absolute highest level of hs-TnT highly depends on the baseline level, and just the absolute level does not directly reflect myocardial damage due to chemotherapy. We then considered “hs-TnT increment from baseline to the highest value” to be more suitable to evaluate the elevation and the myocardial damage. This index seems unaffected by the baseline level, and thus we believe it to directly reflect CTIC. Indeed, the hs-TnT increment in this study was also significantly greater in patients with cardiotoxicity than without; patients without cardiotoxicity recorded hardly any increase in their levels of hs-TnT. Furthermore, cardiac troponin is a deviation enzyme, and it is better for the evaluation to test at multiple time points rather than at only one. We adopted “integration value” as an evaluation method. Integration value is well established to clinically measure the size of myocardial infarction, using creatine phosphokinase activity [26]. On the other hand, we had already pointed out the problem of great interindividual variability of baseline levels of hs-TnT, and to solve it we then created another new index, “hs-TnT integration value above baseline”. This index was also significantly higher in patients with cardiotoxicity than without; 100% sensitivity and specificity with cutoff level at 0.070 ng months/mL. That is, all patients developed cardiotoxicity over 0.070 ng months/mL in hs-TnT integration value above baseline, and never developed under the value. This method of evaluating hs-TnT levels or such a precise predictive biomarker of CTIC had never been published before. These fine results cannot be accomplished without a precise measurement of baseline levels of hs-TnT, and initial analysis of the baseline value has to be conducted. A slight change of the baseline level has a substantial influence on the hs-TnT integration value above baseline. Patients without cardiac failure have very low troponin levels and, therefore, the levels cannot be detected without high-sensitive assays [17,18,19].
High-sensitive cardiac troponin assay as biomarker of CTIC
High-sensitive cardiac troponin assays have two characteristics that can help define a more aggressive strategy for prevention of CTIC. First, myocardial damage can be evaluated moment to moment with high-sensitive cardiac troponin assays. This test can be easily performed with routine blood sampling. Pathophysiology of CTIC can be interpreted with cardiac troponin. For example, hs-TnT elevation was observed exclusively in patients with trastuzumab following anthracycline despite normal LVEF or an asymptomatic decline in this study, suggesting subclinical myocardial damage due to anthracycline that would have otherwise remained unrecognized. BNP could not predict CTIC in several studies including ours [27, 28]. The elevated high-sensitive cardiac troponin levels in other studies were gradually normalized even if trastuzumab was not discontinued [12, 24]. This is one of the reasons why trastuzumab in combination with anthracycline is considered to promote or elicit anthracycline-induced cardiomyopathy. The second characteristic of high-sensitive cardiac troponin is that it may also be a reasonable approach to developing a surrogate marker of CTIC. The approach is expected to be cost-effective and sophisticated enough to help prevent CTIC. Highly specialized and expensive imaging that requires highly specialized expertise such as three-dimensional echocardiography, cardiac strain imaging, cardiovascular magnetic resonance, and radionuclide angiography, is not a generally applicable strategy in routine medical care. High-sensitive cardiac troponin may allow us to indicate those at high risk of CTIC and to specifically tailor the therapy based on the value. Early detected anthracycline-induced cardiomyopathy appears to be reversible [9]. Cardioprotective therapy of carvedilol and enalapril has recently shown to be beneficial in patients with a hematological malignancy at very high risk of CTIC [29]. This is why early detection of CTIC is becoming more important. Based on the cutoff level given from our results of hs-TnT integration value above baseline, 0.070 ng month/mL, examples of patients with very high risk of CTIC from anthracycline and trastuzumab are as follows: highest level of hs-TnT ≥0.040 ng/mL, hs-TnT elevation increment from baseline ≥0.030 ng/mL × 2 months, 0.025 ng/mL × 3 months, or 0.015 ng/mL × 4 months. Expert consensus of American Society of Echocardiography and the European Association of Cardiovascular imaging recommends measurement of cardiac troponin at baseline and during chemotherapy [30]. When abnormal, a consultation with cardiologist is also recommended. Oncologists should consider discontinuing trastuzumab when cardiac troponin is significantly or continuously elevated, and collaborating with cardiologists during treatment. Early suspension of trastuzumab may facilitate patient’s ability to resume and complete trastuzumab therapy after recovery from subclinical myocardial damage; all four patients with cardiotoxicity in this study could do so successfully.
Study limitation
This study has two limitations. First, the definition of CTIC is different from a recently stated consensus [30]. The consensus is a decline in LVEF >10% points, “and to a value <53% (normal reference value for two-dimensional echocardiography)”. It is a natural consequence of the rather fragmented landscape of CTIC research that the reported frequency of CTIC varies with the definition: a number of different definitions of CTIC have been used historically [31]. Despite the widespread use of the current consensus, the clinical importance is unknown. In this study, we adopted the most sensitive criteria of CTIC, as in BCIRG-006 study regarding trastuzumab [20]. The criteria are a decline in LVEF >10% points including asymptomatic decline to a value ≥53%. Of the 12 patients using both anthracycline and trastuzumab in this study, 4 as many as 33% developed cardiotoxicity, despite the almost no concomitant risk factors: high baseline LVEF, practically no cardiovascular diseases, and no chest radiation therapy. All the cardiotoxicity was an asymptomatic decline in LVEF to a value >50%, which is not known whether to affect the cardiac prognosis of breast cancer patients [31]. Epidemiological evidence suggests that, even without an overt decline in LVEF, anthracycline carries a substantial long-term risk of cardiac failure, especially for older patients [32]. Moreover, the previously described consensus states that even an asymptomatic decline in LVEF <10% points includes subclinical left ventricular dysfunction [30].
Second limitation of this study is that hs-TnT was measured only every 3 months. Of the four patients with cardiotoxicity, two patients (50%) had hs-TnT elevation at the time of detection of cardiotoxicity (Fig. 2). In theory, cardiac troponin elevation precedes declines in LVEF. More frequent measurement is required to predict and prevent CTIC, to validate this study, and to determine the threshold for abnormality. Measuring monthly or at the beginning of each treatment cycle is more reasonable.
Conclusion
Hs-TnT assay may be able to predict CTIC from anthracycline and trastuzumab in breast cancer patients, and hs-TnT increment from baseline to the highest value and hs-TnT integration value above baseline were more reliable than the absolute value. If periodic high-sensitive cardiac troponin measurement is included in daily medical practice during chemotherapy at high risk of cardiomyopathy, it may help to develop a more aggressive strategy for prevention of CTIC. However, independent validation is necessary before hs-TnT application in clinical practice. We suggest that future studies include the concept of increment from baseline or the integration value to assess myocardial damage of CTIC from high-sensitive cardiac troponin assays.
References
Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, Childhood Cancer Survivor Study, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–82.
Hooning MJ, Botma A, Aleman BM, Baaijens MH, Bartelink H, Klijn JG, et al. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J Natl Cancer Inst. 2007;99:365–75.
Chen MH, Colan SD, Diller L. Cardiovascular disease: cause of morbidity and mortality in adult survivors of childhood cancers. Circ Res. 2011;108:619–28.
Bovelli D, Plataniotis G, Roila F, ESMO Guidelines Working Group. Cardiotoxicity of chemotherapeutic agents and radiotherapy-related heart disease: ESMO Clinical Practice Guidelines. Ann Oncol. 2010;21:v277–82.
Seidman A, Hudis C, Pierri MK, Shak S, Paton V, Ashby M, et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. 2002;20:1215–21.
Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92.
Guarneri V, Lenihan DJ, Valero V, Durand JB, Broglio K, Hess KR, et al. Long-term cardiac tolerability of trastuzumab in metastatic breast cancer: the M.D. Anderson Cancer Center experience. J Clin Oncol. 2006;24:4107–15.
Telli ML, Hunt SA, Carlson RW, Guardino AE. Trastuzumab-related cardiotoxicity: calling into question the concept of reversibility. J Clin Oncol. 2007;25:3525–33.
Cardinale D, Colombo A, Lamantia G, Colombo N, Civelli M, De Giacomi G, et al. Anthracycline-induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol. 2010;55:213–20.
Apple FS. A new season for cardiac troponin assays: it’s time to keep a scorecard. Clin Chem. 2009;55:1303–6.
Ky B, Putt M, Sawaya H, French B, Januzzi JL Jr, Sebag IA, et al. Early increases in multiple biomarkers predict subsequent cardiotoxicity in patients with breast cancer treated with doxorubicin, taxanes, and trastuzumab. J Am Coll Cardiol. 2014;63:809–16.
Sawaya H, Sebag IA, Plana JC, Januzzi JL, Ky B, Tan TC, et al. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circ Cardiovasc Imaging. 2012;5:596–603.
Fallah-Rad N, Walker JR, Wassef A, Lytwyn M, Bohonis S, Fang T, et al. The utility of cardiac biomarkers, tissue velocity and strain imaging, and cardiac magnetic resonance imaging in predicting early left ventricular dysfunction in patients with human epidermal growth factor receptor II-positive breast cancer treated with adjuvant trastuzumab therapy. J Am Coll Cardiol. 2011;57:2263–70.
Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, et al. Management of acute coronary syndromes in patients presenting without persistent ST-segment Elevation of the European Society of Cardiology. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:267–315.
Latini R, Masson S, Anand IS, Missov E, Carlson M, Vago T, et al. Val-HeFT investigators. prognostic value of very low plasma concentrations of troponin T in patients with stable chronic heart failure. Circulation. 2007;116:1242–9.
Peacock WF 4th, De Marco T, Fonarow GC, Diercks D, Wynne J, Apple FS, et al. ADHERE Investigators. Cardiac troponin and outcome in acute heart failure. N Engl J Med. 2008;358:2117–26.
Saunders JT, Nambi V, de Lemos JA, Chambless LE, Virani SS, Boerwinkle E, et al. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study. Circulation. 2011;123:1367–76.
deFilippi CR, de Lemos JA, Christenson RH, Gottdiener JS, Kop WJ, Zhan M, et al. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA. 2010;304:2494–502.
Otsuka T, Kawada T, Ibuki C, Seino Y. Association between high-sensitivity cardiac troponin T levels and the predicted cardiovascular risk in middle-aged men without overt cardiovascular disease. Am Heart J. 2010;159:972–8.
Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, et al. Breast Cancer International Research Group. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–83.
Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Chamber Quantification Writing Group; American Society of Echocardiography’s Guidelines and Standards Committee; European Association of Echocardiography, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63.
Cardinale D, Sandri MT, Martinoni A, Borghini E, Civelli M, Lamantia G, et al. Myocardial injury revealed by plasma troponin I in breast cancer treated with high-dose chemotherapy. Ann Oncol. 2002;13:710–5.
Mokuyasu S, Suzuki Y, Kawahara E, Seto T, Tokuda Y. High-sensitivity cardiac troponin I detection for 2 types of drug-induced cardiotoxicity in patients with breast cancer. Breast Cancer. 2015;22:563–9.
Katsurada K, Ichida M, Sakuragi M, Takehara M, Hozumi Y, Kario K. High-sensitivity troponin T as a marker to predict cardiotoxicity in breast cancer patients with adjuvant trastuzumab therapy. Springerplus. 2014;3:620.
Tsutamoto T, Kawahara C, Yamaji M. Relationship between renal function and serum cardiac troponin T in patients with chronic heart failure. Eur J Heart Fail. 2009;11:653–8.
Norris RM, Whitlock RM, Barratt-Boyes C, Small CW. Clinical measurement of myocardial infarct size. Modification of a method for the estimation of total creatine phosphokinase release after myocardial infarction. Circulation. 1975;51:614–20.
Meinardi MT, van Veldhuisen DJ, Gietema JA, Dolsma WV, Boomsma F, van den Berg MP, et al. Prospective evaluation of early cardiac damage induced by epirubicin-containing adjuvant chemotherapy and locoregional radiotherapy in breast cancer patients. J Clin Oncol. 2001;19:2746–53.
Daugaard G, Lassen U, Bie P, Pedersen EB, Jensen KT, Abildgaard U, et al. Natriuretic peptides in the monitoring of anthracycline induced reduction in left ventricular ejection fraction. Eur J Heart Fail. 2005;7:87–93.
Bosch X, Rovira M, Sitges M, Domènech A, Ortiz-Pérez JT, de Caralt TM, et al. Enalapril and carvedilol for preventing chemotherapy-induced left ventricular systolic dysfunction in patients with malignant hemopathies: the OVERCOME trial (preventiOn of left Ventricular dysfunction with Enalapril and caRvedilol in patients submitted to intensive ChemOtherapy for the treatment of Malignant hEmopathies). J Am Coll Cardiol 2013;61:2355–62.
Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2014;15:1063–93.
Khouri MG, Douglas PS, Mackey JR, Martin M, Scott JM, Scherrer-Crosbie M, et al. Cancer therapy-induced cardiac toxicity in early breast cancer: addressing the unresolved issues. Circulation. 2012;126:2749–63.
Doyle JJ, Neugut AI, Jacobson JS, Grann VR, Hershman DL. Chemotherapy and cardiotoxicity in older breast cancer patients: a population-based study. J Clin Oncol. 2005;23:8597–605.
Acknowledgements
The authors would like to thank the patients and ultrasonographers who participated in this study. We also thank Dr. Hiroyuki Shikishima for patient recruitment and follow-up, and Mr. David Hochman for reviewing the language of our article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no competing interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
12282_2017_778_MOESM1_ESM.tif
Timeline of high-sensitives cardiac troponin T level of each patient without cardiotoxicity (n =36). Median treatment and follow up time is 6 months (interquartile range, 3-12). Troponin level after three months from chemotherapy initiation is 0.013 ±0.0092 ng/mL (mean ±standard deviation), while integration value of the troponin level above baseline is <0.070 ng months/mL in every patient without cardiotoxicity (TIFF 1052 kb)
About this article
Cite this article
Kitayama, H., Kondo, T., Sugiyama, J. et al. High-sensitive troponin T assay can predict anthracycline- and trastuzumab-induced cardiotoxicity in breast cancer patients. Breast Cancer 24, 774–782 (2017). https://doi.org/10.1007/s12282-017-0778-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-017-0778-8