Abstract
MicroRNAs (miRs) are short and highly conserved non-coding RNAs molecules consisting of 18–25 nucleotides that regulate gene expression at post-transcriptional level by direct binding to complementary binding sites within the 3′untranslated region (3′UTR) of target mRNAs. New evidences have demonstrated that miRNAs play an important role in diverse physiological processes, including regulating cell growth, apoptosis, metastasis, drug resistance, and invasion. In chromosomes 11 and 22 of the miR-130 family, paralogous miRNA sequences, miR-130a and miR-130b are situated, respectively. MiR-130a has participated in different pathogenesis, including hepatocellular carcinoma, cervical cancer, ovarian cancer, glioblastoma, prostate carcinoma, leukemia, etc. Most important of all, more and more evidences indicate that miR-130a is associated with drug resistance and acts as an intermediate in PI3 K/Akt/PTEN/mTOR, Wnt/β-catenin and NF-kB/PTEN drug resistance signaling pathways. Drug resistance has emerged as a major obstacle to successful treatment of cancer nowadays and in this review, we will reveal the function of miR-130a in cancer, especially in drug resistance. Therefore, it will provide a new therapeutic target for the treatment of cancer, especially in chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Based on the latest world cancer statistics, global cancer burden ascends to 14.1 million new cases and 8.2 million people cancer deaths occurred in 2012 [1]. However, growing evidences have confirmed that miRNAs serve as oncogenes or tumor suppressor genes, and also serve an important role in carcinogenesis and tumor progression [2, 3]. MiRNAs are a class of short, highly conserved non-coding RNAs that regulate gene expression by inhibiting translation or inducing mRNA degradation at the post-transcriptional level [4,5,6]. MiRNAs participate in diverse biological processes by directly binding to the 3′untranslational region (3′-UTR) of target mRNAs, including growth, metabolic processes and tumorigenesis and also in drug resistance [7]. MiR-130a is located on chromosome 11 and its mature sequence is distributed in the nucleotide at position 11 [8]. It has been reported that the expression of miR-130a is aberrant in several types of cancer, overexpressed in adult T cell leukemia (ATL) [9], basal cell carcinoma [10], gastric cancer [11], non-small cell lung cancer [12], osteosarcoma [13], and esophageal cancer [14] tissue, but downexpressed in bladder [15], ovarian cancer [16], hepatocellular carcinoma (HCC) cells [17], breast cancer[18], cervical cancer [19], glioblastoma [20], prostate carcinoma [21], and chronic lymphocytic leukemia [22]. In different types of cancer, miR-130a has different roles as oncogenes or tumor suppressor gene to mediate diverse biological processes by regulating various canonical pathways or target genes (Table 1). The data also imply various roles of miR-130a in tumorigenesis. Furthermore, chemotherapy is still an important treatment method for cancer, but the intrinsic or acquired drug resistance, especially multidrug resistance (MDR) is considered to be the major reason for chemotherapy failure; similarly, several studies have found that miR-130a expression is also abnormal in drug-resistant cancer cells. MiR-130a is overexpressed in Adriamycin-resistant breast cancer [23], platinum-resistant ovarian cancer [24], Gefitinib-resistant non-small cell lung cancer [25], R-CHOP-resistant B-cell lymphoma patients [26], but downexpressed in gemcitabine- or cisplatin-resistant hepatoma cells [27, 28], and paclitaxel-resistant prostate cancer [29]. In addition, new evidences indicate that miR-130a acts as an intermediate in PI3 K/Akt/PTEN/mTOR, Wnt/β-catenin, NF-κB/TNF-α, and NF-kB/PTEN drug-resistant signaling pathways. These results suggest miR-130a might modulate the resistance of cancer cells to chemotherapeutic agents. Therefore, this review will focus on the role of miR-130a in the development of cancer, especially in drug resistance, and on the role to be a potential therapeutic target for cancer in future.
MiR-130a in cell proliferation and apoptosis
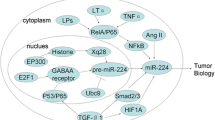
Increasing reports have confirmed that the miR-130a can regulate cancer cells proliferation and apoptosis. Rab5a is a member of the Rab subfamily of small GTPases, and its association with diverse cellular functions, including growth, differentiation, gene expression, and signal transduction has been confirmed [30]. Moreover, overexpression of miR-130a inhibits breast cancer cell proliferation and apoptosis by targeting Rab5a [18]. The Runt family of transcription factors includes RUNX1, RUNX2, and RUNX3. Runt family plays an important role in both the natural biological processes and carcinogenesis [31]. RUNX3 is located on human chromosome 1p36 and acts as a tumor suppressor in lung cancer, bladder cancer, and gastric cancer [11]. MiR-130a is deemed to be a potential oncomiR candidate in gastric cancer cells, and increases cell proliferation and inhibits cell apoptosis by targeting RUNX3 [11]. The mechanism may be because of the upregulation of RUNX3 target genes, Bim and p21, which increases cell proliferation. Some studies have also reported that miR-130a promotes cancer cell growth by targeting NF-kB/PTEN/AKT pathway. NF-κB constitutes five subunits: RelA, RelB, c-Rel, p50, and p52; nuclear factor-κB (NF-κB) is a nuclear transcription factor that regulates a series of gene expression, associated with inflammation, tissue damage and repair, cell differentiation, apoptosis and tumor growth [32,33,34,35,36]. NF-kB may upregulate miR-130a expression, which inhibits the expression of PTEN, and then initiates protein kinase B (AKT) pathway activation, consistent with promoting cell growth in cervical cancer [37]. In addition, TNF-α is one form of TNF [38], and it acts as a proinflammatory cytokine. Some studies showed TNF-α could promote cell growth in low concentration [39]. Growing evidences have also confirmed that TNF-α can activate NF-κB activity that upregulates miR-130a expression, and then miR-130a targets and oppositely inhibits TNF-α expression, the negative feedback regulation of NF-κB/miR-130a/TNF-α/NF-κB may provide insight into the carcinogenesis of cervical cancer [36] (Fig. 1).
MiR-130a in invasion and metastasis
Migration and invasion are important processes for cells, and miR-130a has been confirmed to regulate both metastatic and invasive activities in cancer cells. Rab5a has been confirmed to act as an oncogene to participate in cellular functions [18]. Moreover, Rab5a, a direct target gene of miR-130a, plays an accelerating role in the invasion and migration. Some studies showed TNF-α could enhance breast cancer cells invasion and metastasis by depending on matrix metalloproteases [40]. MMPs are family members of extracellular proteinases that regulate cellular biological processes, including cell proliferation, invasion, and migration [41, 42]; miR-130a inhibits cancer cell proliferation and migration by inhibiting TNF-α expression. Smad4, one of the direct targets of miR-130a, plays a critical role in the TGF-β–signaling pathway [43]; moreover, downexpression of Smad4 decreases E-cadherin expression, but increases Vimentin and MMP2 expression. In addition, downexpression of Smad4 reverses EMT to MET in HCC and GR cells [27]. The epithelial-to-mesenchymal transition (EMT) could impair cancer cells’ epithelial features transiently, including the loss of apico-basal polarity, disintegrating tight and adherent junctions, and obtaining mesenchymal traits, inducing higher invasion and metastasis ability [44]. Consequently, we can conclude that miR-130a inhibits Smad4 expression, and then further regulates E-cadherin, Vimentin and MMP2 expression and reverses EMT to MET, and ultimately inhibits cell migration and invasion. However, in gastric cancer and osteosarcoma, miR-130a promotes cell migration and invasion by targeting RUNX3 and PTEN, respectively. Previous findings have shown that a loss or substantial decline in RUNX3 expression may be associated with the occurrence of gastric cancer. Furthermore, PTEN acts as tumor suppressor [45], which regulates the invasive and metastatic ability in osteosarcoma cells. Overexpression of PTEN reverses the promoting effects of miR-130a overexpression on regulating the EMT process and metastatic behavior [46, 47]. Therefore, miR-130a may act as an oncogene in gastric cancer and osteosarcoma to promote cell migration and invasion (Fig. 2).
miR-130a regulates tumor cells invasion and metastasis. MiR-130a inhibits cancer cell proliferation and migration by inhibiting TNF-α, Smad4, and RAB5A, Furthermore, miR-30a reverses EMT to MET to control cell invasion and metastasis. However, miR-130a may act as an oncogene in gastric cancer and osteosarcoma to promote cell migration and invasion by targeting RUNX3 and PTEN
MiR-130a regulates drug susceptibility through Wnt signaling
Cisplatin is a common chemotherapeutic drug used for hepatocellular carcinoma. However, the resistance of hepatocellular carcinoma cells (HCC) to cisplatin frequently leads to the subsequent recurrence and metastasis of cancer. It has been reported that miR-130a regulates cisplatin susceptibility through Wnt signaling in cisplatin-treated HCC cell. Wnt signaling pathway is frequently dysregulated in various tumor types and plays important roles in tumor development and progression [48]. According to the ligand, Wnt signaling is divided into two arms: canonical and noncanonical. Canonical Wnt signaling pathway (Wnt/β-catenin pathway) is initially performed by the bipartite transcription factor complex β-catenin/TCF (T cell factor) or LEF1 (lymphoid enhancer factor) to activate or inhibit the target genes [49, 50]. However, the noncanonical arm is performed by activating the molecules of downstream, such as PRKC and Rho [51]; both of them are all well-known molecules involved in breast cancer progression [52]. Based on research results, it is found that the expression of miR-130a is significantly increased in HCC patients treated with cisplatin-based chemotherapy. Similarly, miR-130a expression is also upregulated in cisplatin-resistant Huh7 cells compared to Huh7 cells themselves; whereas knockdown of miR-130a significantly increases cisplatin-induced inhibition of cell proliferation [28]. Therefore, overexpression of miR-130a increases cisplatin resistance in Huh7 cell. Furthermore, β-catenin expression is higher in cisplatin-resistant Huh7 cells than Huh7 cells, and little nuclear accumulation of β-catenin is found in Huh7 cells; however, nuclear β-catenin is very obvious in cisplatin-resistant Huh7 cells. As we all known Axin2 is a standard marker to assess β-catenin activation, and Axin2 mRNA levels are significantly higher in cisplatin-resistant Huh7 cells than Huh7 cells. Besides, luciferase activity assay also shows that RUNX3 is the target of miR-130a. Subsequently it is found that RUNX3 overexpression inhibits miR-130a upregulation-induced cell proliferation, and inhibition of Wnt signaling also increases the chemosensitivity of hepatocellular carcinoma cells to cisplatin [28]. Consequently, we can conclude that miR-130a increases drug resistance by regulating RUNX3 and Wnt signaling in cisplatin-treated HCC cell (Fig. 3).
miR-130a regulates drug susceptibility through Wnt signaling, PTEN signaling and other relevant target genes. The expression of miR-130a is significantly increased in HCC patients treated with cisplatin-based chemotherapy, which increases cisplatin resistance in Huh7 cell. RUNX3 overexpression inhibits miR-130a upregulation-induced cell proliferation, and inhibition of Wnt signaling also increases the chemosensitivity of hepatocellular carcinoma cells to cisplatin. PTEN participates in multiple cellular functions by regulating the activity of the PI3 K/AKT/mTOR signaling pathway. Overexpression of miR-130a may promote the proliferation of epithelial platinum-resistant ovarian cancer by inhibiting PTEN expression, which activates PI3 K/AKT signal pathway that increases BCL-2 expression to decrease tumor cell apoptosis. Furthermore, miR-130a overexpression also increases the expression of MDR1 and P-gp in platinum-resistant ovarian cancer cell, which are correlated with drug resistance of tumor cells. Furthermore, miR-130a also regulates drug susceptibility by inhibiting other relevant target genes. Met protein is the receptor tyrosine kinase for hepatocyte growth factors, and downexpression of Met could rescue the functions of miR-130a to induce cell apoptosis and inverse gefitinib resistance by inhibiting Akt and ERKs signaling. Furthermore, miR-130a could modulate the miR-221 and miR-222 expression levels by AP1 to increase cell apoptosis. In addition, miR-130a could inverse drug resistance by inhibiting XIAP and regulating caspase-8
MiR-130a regulates drug susceptibility through PTEN signaling
The PTEN gene, located on chromosome 10q23.3, is composed of 9 exons plus a 5-Kb variable exon [53], and it is a lipid phosphatase that deletes phosphate groups from pivotal intracellular phosphoinositide signaling molecules [54]. In addition, PTEN counteracts the activation of PI3-kinase by de-phosphorylating the phosphatidylinositol (3,4,5)-trisphosphates (PIP3) [54]. Similarly, the PIP3 phospholipid restricts certain kinases in the cell membrane, including AKT and PDK1 [55, 56]. AKT is activated in the membrane, through the phosphorylation of serine residue 473 by the rapamycin-insensitive complex 2 (mTORC2) [57, 58]. PTEN participates in multiple cellular functions such as cell proliferation, differentiation, survival, metastasis, and invasion by regulating the activity of the PI3 K signaling pathway [59]. Furthermore, the loss of PTEN function in tumor cells will result in increasing cellular proliferation. It is found that the expression of miR-130a and BCL-2 are significantly higher in epithelial ovarian cancer patients treated with compared with platinum-sensitive; but lower expression of PTEN in platinum-resistant than patients treated with platinum-sensitive, which leads to increasing cells proliferation. PTEN is a direct target of miR-130a [60, 61] and BCL-2 is the anti-apoptotic protein of the PI3 K/AKT signaling pathway downstream. Overexpression of miR-130a may regulate the proliferation of epithelial platinum-resistant ovarian cancer by inhibiting PTEN expression, which activates PI3 K/AKT signal pathway that increases BCL-2 expression to decrease tumor cell apoptosis. Furthermore, compared with A2780 s cell, the expression of MDR1 and P-gp is significantly higher in platinum-resistant ovarian cancer cell (A2780/DDP), concomitant with lower expression of PTEN [62]. The overexpression of MDR1/P-glycoprotein (P-gp) has been discovered and served as a molecular mechanism of drug resistance [63, 64]. The ABC (ATP-binding cassette) is drug transporter, consisting of P-glycoprotein, MRP1, and BCRP, which is correlated with resistance of tumor cells to anticancer drugs [63,64,65]. P-gp is a 170-kDa transmembrane glycoprotein encoded by MDR1 gene on human chromosome 7p21 [60]. Consequently, miR-130a may be associated with drug resistance and play the role in drug resistance pathways of PI3 K/Akt/PTEN and ABC superfamily drug transporters in ovarian cancer cells (Fig. 3).
MiR-130a regulates drug susceptibility by inhibiting other relevant target genes
In addition, the target genes of miR-130a, X-linked inhibitor of apoptosis (XIAP) and Met are all involved in drug resistance. XIAP is one of the most potent inhibitors of caspases and apoptosis [66]. Furthermore, XIAP acts as an important regulator in cisplatin-induced apoptosis in ovarian cancer cells, downexpression of XIAP sensitizes cells to cisplatin to increase cell apoptosis [16, 67]. Met protein is the receptor tyrosine kinase for hepatocyte growth factors, and it has been reported that Met expression and phosphorylation are connected with resistance to EGFR-TKI therapies in NSCLC patients [68, 69]. Overexpression of miR-130a increases cell apoptosis and inhibits cell proliferation after treatment with gefitinib in gefitinib-resistant NSCLC cell lines. Furthermore, downexpression of Met could rescue the functions of miR-130a to induce cell apoptosis and inverse gefitinib resistance [25]. In addition, Met receptor is able to directly activate Akt and ERKs signaling [70]. Indeed, the activation of MET could enhance miR-221 and miR-222 expression through JNK and AP1, and overexpression of miR-221 and miR-222 could decrease cell apoptosis [71]. However, overexpression of miR-130a not only decreases Akt and ERK1/2 phosphorylation levels in A549 lung cancer cells, but also modulates the miR-221 and miR-222 expression levels by AP1, by acting on MET expression. Furthermore, downexpression of miR-221 and miR-222 could sensitize NSCLC to TRAIL-inducing apoptosis [72]. Consequently, miR-130a could target Met and induce TNF-related apoptosis-inducing ligand (TRAIL) sensitivity in NSCLC by down-regulating miR-221 and miR-222 expression. Besides, miR-130a is down-regulated in paclitaxel-resistant cells, and miR-130a overexpression could increase the sensitivity to paclitaxel and inhibit cell growth by activating apoptotic signaling through activation of caspase-8 in paclitaxel-resistant cell line [29] (Fig. 3).
Conclusion
In this review, it is learned that miR-130a dysregulation is a general feature in many cancers. However, miR-130 plays different roles in cancer depending on the tumor type. In most cancers, miR-130 may act as tumor suppressor genes to regulate tumor cells’ various biological processes, including inhibiting cell proliferation, migration and invasion, and inducing cell apoptosis. However, in gastric cancer and osteosarcoma, miR-130a may act as an oncogene to promote cell migration and invasion, which may be connected with the genes of RUNX3 and PTEN. Most important of all, miR-130 is involved in various canonical pathways or target genes to enhance the sensitivity of drug-resistant cells, and then promotes cell apoptosis combined with anticancer drug. Therefore, it is advised that miR-130a could be regarded as a new therapeutic target in the treatment of cancer.
References
Torre Lindsey A, Bray Freddie, Siegel Rebecca L, et al. Global cancer statistics. CA Cancer J Clin. 2015;65:87–108.
Xiong J, Wei B, Ye Q, Liu W. MiR-30a-5p/UBE3C axis regulates breast cancer cell proliferation and migration. Biochem Biophys Res Commun. 2016;pii:S0006–291X(16)30381–3. doi:10.1016/j.bbrc.2016.03.069.
Wang D, Qiu C, Zhang H, Wang J, Cui Q, Yin Y. Human microRNA oncogenes and tumor suppressors show significantly different biological patterns: from functions to targets. PLoS One. 2010;5:e13067.
Giannakakis A, Coukos G, Hatzigeorgiou A, Sandaltzopoulos R, Zhang L. miRNA genetic alterations in human cancers. Expert Opin Biol Ther. 2007;7:1375–86.
Zhang HD, Jiang LH, Sun DW, Tang JH. MiR-139-5p: promising biomarker for cancer. Tumour Biol. 2015;36:1355–65.
Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–33.
Chen X, Lu P, Wang DD, Yang SJ, Wu Y, Shen HY. The role of miRNAs in drug resistance and prognosis of breast cancer formalin-fixed paraffin-embedded tissues. Gene. 2016;595:221–6.
Suresh S, McCallum L, Lu W, Lazar N, Perbal B, Irvine AE. MicroRNAs 130a/b are regulated by BCR-ABL and downregulate expression of CCN3 in CML. J Cell Commun Signal. 2011;5:183–91.
Ishihara K, Sasaki D, Tsuruda K, Inokuchi N, Nagai K, Hasegawa H, et al. Impact of miR-155 and miR-126 as novel biomarkers on the assessment of disease progression and prognosis in adult T-cell leukemia. Cancer Epidemiol. 2012;36:560–5.
Sand M, Skrygan M, Sand D, Georgas D, Hahn SA, Gambichler T, et al. Expression of microRNAs in basal cell carcinoma. Br J Dermatol. 2012;167:847–55.
Jiang H, Yu WW, Wang LL, Peng Y. MiR-130a acts as a potential diagnostic biomarker and promotes gastric cancer migration, invasion and proliferation by targeting RUNX3. Oncol Rep. 2015;34:1153–61.
Wang XC, Tian LL, Wu HL, Jiang XY, Du LQ, Zhang H, et al. Expression of miRNA-130a in nonsmall cell lung cancer. Am J Med Sci. 2010;340:385–8.
Chen J, Yan D, Wu W, et al. MicroRNA-130a promotes the metastasis and epithelial-mesenchymal transition of osteosarcoma by targeting PTEN. Oncol Rep. 2016;35:3285–92.
Liu SG, Qin XG, Zhao BS, Zhu J, Ye W, Shu Q. Differential expression of miRNAs in esophageal cancer tissue. Oncol Lett. 2013;5:1639–42.
Ratert N, Meyer HA, Jung M. miRNA profiling identifies candidate mirnas for bladder cancer diagnosis and clinical outcome. J Mol Diagn. 2013;15:695–705.
Zhang X, Huang L, Zhao Y, Tan W. Downregulation of miR-130a contributes to cisplatin resistance in ovarian cancer cells by targeting X-linked inhibitor of apoptosis (XIAP) directly. Acta Biochim Biophys Sin (Shanghai). 2013;45:995–1001.
Li B, Huang P, Qiu J, Liao Y, Hong J, Yuan Y. MicroRNA-130a is down-regulated in hepatocellular carcinoma and associates with poor prognosis. Med Oncol. 2014;31:230.
Pan Y, Wang R, Zhang F, Chen Y, Lv Q, Long G, et al. MicroRNA-130a inhibits cell proliferation, invasion and migration in human breast cancer by targeting the RAB5A. Int J Clin Exp Pathol. 2015;8:384–93.
He L, Wang HY, Zhang L, Huang L, Li JD, Xiong Y, et al. Prognostic significance of low DICER expression regulated by miR-130a in cervical cancer. Cell Death Dis. 2014;5:e1205.
Qiu S, Lin S, Hu D, Feng Y, Tan Y, Peng Y. Interactions of miR-323/miR-326/miR-329 and miR-130a/miR-155/miR-210 as prognostic indicators for clinical outcome of glioblastoma patients. J Transl Med. 2013;11:10.
Boll K, Reiche K, Kasack K, Mörbt N, Kretzschmar AK, Tomm JM, et al. MiR-130a, miR-203 and miR-205 jointly repress key oncogenic pathways and are downregulated in prostate carcinoma. Oncogene. 2013;32:277–85.
Kovaleva V, Mora R, Park YJ, Plass C, Chiramel AI, Bartenschlager R, et al. miRNA-130a targets ATG2B and DICER1 to inhibit autophagy and trigger killing of chronic lymphocytic leukemia cells. Cancer Res. 2012;72:1763–72.
Cui XY, Guo YJ, Yao HR. Analysis of microRNA in drug- resistant breast cancer cell line MCF-7/ADR. Nan Fang Yi Ke Da Xue Xue Bao. 2008;28:1813–5.
Chen C, Wang HJ, Yang LY, Jia XB, Xu P, Chen J, et al. Expression of MiR-130a in serum samples of patients with epithelial ovarian cancer and its association with platinum resistance. Sichuan Da Xue Xue Bao Yi Xue Ban. 2016;47:60–3.
Zhou YM, Liu J, Sun W. MiR-130a overcomes gefitinib resistance by targeting met in non-small cell lung cancer cell lines. Asian Pac J Cancer Prev. 2014;15:1391–6.
Yuan WX, Gui YX, Na WN, et al. Circulating microRNA-125b and microRNA-130a expression profiles predict chemoresistance to R-CHOP in diffuse large B-cell lymphoma patients. Oncol Lett. 2016;11:423–32.
Liu Y, Li Y, Wang R, Chao J, Yang X. MiR-130a-3p regulates cell migration and invasion via inhibition of Smad4 in gemcitabine resistant hepatoma cells. J Exp Clin Cancer Res. 2016;35:19.
Xu N, Shen C, Luo Y, Xia L, Xue F, Xia Q, Zhang J. Upregulated miR-130a increases drug resistance by regulating RUNX3 and Wnt signaling in cisplatin-treated HCC cell. Biochem Biophys Res Commun. 2012;425:468–72.
Fujita Y, Kojima T, Kawakami K, Mizutani K, Kato T, Deguchi T, et al. miR-130a activates apoptotic signaling through activation of caspase-8 in taxane-resistant prostate cancer cells. Prostate. 2015;75:1568–78.
Lanzetti L, Palamidessi A, Areces L, Areces L, Scita G, Di Fiore PP. Rab5 is a signalling GTPase involved in actin remodelling by receptor tyrosine kinases. Nature. 2004;429:309–14.
Li QL, Ito K, Sakakura C, Ki Inoue, Chi XZ, Lee KY, et al. Causal relationship between the loss of RUNX3 expression and gastric cancer. Cell. 2002;109:113–24.
Jo MJ, Lee JR, Cho IJ, Kim YW, Kim SC. Roots of Erigeron annuus attenuate acute inflammation as mediated with the inhibition of NF- kappa B-associated nitric oxide and prostaglandin E2 production. Evid Based Complement Alternat Med. 2013;2013:297427.
Park SW, Cho C, Cho BN, Kim Y, Goo TW, Kim YI. 15-deoxy-delta12,14-prostaglandin J 2 down-regulates activin-induced activin receptor, smad, and cytokines expression via suppression of NF- kappa B and MAPK signaling in HepG2 cells. PPAR Res. 2013;2013:751261.
Ling H, Gray CB, Zambon AC, Grimm M, Gu Y, Dalton N, et al. Ca2+/calmodulin-dependent protein kinase II deltamediates myocardial ischemia/reperfusion injury through nuclear factor-kappaB. Circ Res. 2013;112:935–44.
Zhang J, Chen J, Yang J, Xu CW, Pu P, Ding JW, et al. Resveratrol attenuates oxidative stress induced by balloon injury in the rat carotid artery through actions on the ERK1/2 and NF-kappa B pathway. Cell Physiol Biochem. 2013;31:230–41.
Zhang J, Wu H, Li P, Zhao Y, Liu M, Tang H. NF-κB-modulated miR-130a targets TNF-α in cervical cancer cells. J Transl Med. 2014;12:155.
Feng Y, Zhou S, Li G, Hu C, Zou W, Zhang H, et al. Nuclear factor-κB-dependent microRNA-130a upregulation promotes cervical cancer cell growth by targeting phosphatase and tensin homolog. Arch Biochem Biophys. 2016;598:57–65.
Majetschak M, Obertacke U, Schade FU, Bardenheuer M, Voggenreiter G, Bloemeke B, et al. Tumor necrosis factor gene polymorphisms, leukocyte function, and sepsis susceptibility in blunt trauma patients. Clin Diagn Lab Immunol. 2002;9:1205–11.
Balkwill F, Joffroy C. TNF: a tumor-suppressing factor or a tumor promoting factor? Future Oncol. 2010;6:1833–6.
Hagemann T, Robinson SC, Schulz M, Trümper L, Balkwill FR, Binder C. Enhanced invasiveness of breast cancer cell lines upon co-cultivation with macrophages is due to TNF-α dependent up-regulation of matrix metalloproteases. Carcinogenesis. 2004;25:1543–9.
Vu TH, Werb Z. Matrix metalloproteinases: effectors of development and normal physiology. Genes Dev. 2000;14:2123–33.
Zucker S, Vacirca J. Role of matrix metalloproteinases (MMPs) in colorectal cancer. Cancer Metastasis Rev. 2004;23:101–17.
Häger M, Pedersen CC, Larsen MT, Andersen MK, Hother C, Grønbæk K, et al. MicroRNA-130a-mediated down-regulation of Smad4 contributes to reduced sensitivity to TGF-β1 stimulation in granulocytic precursors. Blood. 2011;118:6649–59.
Chang CW, Yu JC, Hsieh YH, Yao CC, Chao JI, Chen PM, et al. MicroRNA-30a increases tight junction protein expression to suppress the epithelial-mesenchymal transition and metastasis by targeting Slug in breast cancer. Oncotarget. 2016;7:16462–78.
Parsons R, Simpson L. PTEN and cancer. Methods Mol Biol. 2003;222:147–66.
Nielsen-Preiss SM, Silva SR, Gillette JM. Role of PTEN and Akt in the regulation of growth and apoptosis in human osteoblastic cells. J Cell Biochem. 2003;90:964–75.
Hu Y, Xu S, Jin W, Yi Q, Wei W. Effect of the PTEN gene on adhesion, invasion and metastasis of osteosarcoma cells. Oncol Rep. 2014;32:1741–7.
Wang Z, Dai X, Chen Y, et al. MiR-30a-5p is induced by Wnt/β-catenin pathway and promotes glioma cell invasion by repressing NCAM. Biochem Biophys Res Commun. 2015;465:374–80.
Wang W, Lin H, Zhou L, Sun C, Zhu Q, Zhao H, et al. MicroRNA-30a-3p inhibits tumor proliferation, invasiveness and metastasis and is downregulated in hepatocellular carcinoma. Eur J Surg Oncol. 2014;40:1586–94.
Li WF, Dai H, Ou Q, Zuo GQ, Liu CA. Overexpression of microRNA-30a-5p inhibits liver cancer cell proliferation and induces apoptosis by targeting MTDH/PTEN/AKT pathway. Tumour Biol. 2016;37:5885–95.
Schlessinger K, Hall A, Tolwinski N. Wnt signaling pathways meet Rho GTPases. Genes. 2009;23:265–77.
Scheel C, Eaton EN, Li SH, Reinhardt F, Kah KJ, Bell G, et al. Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in the breast. Cell. 2011;145:926–40.
Sharrard RM, Maitland NJ. Alternative splicing of the human PTEN/MMAC1/TEP1 gene. Biochim Biophys Acta. 2000;1494:282–5.
Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–8.
Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–27.
Testa JR, Bellacosa A. AKT plays a central role in tumorigenesis. Proc Natl Acad Sci USA. 2001;98:10983–5.
Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, et al. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127:125–37.
Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–68.
Vazquez F, Sellers WR. The PTEN tumor suppressor protein: an antagonist of phosphoinositide 3-kinase signalling. Biochim Biophys Acta. 2000;1470:M21–35.
Yang L, Li N, Wang H, Jia X, Wang X, Luo J. Altered microRNA expression in cisplatin-resistant ovarian cancer cells and upregulation of miR-130a associated with MDR1/P-glycoprotein-mediated drug resistance. Oncol Rep. 2012;28:592–600.
Li NW, Wang HJ, Yang LY, Jia XB, Chen C, Wang X. Regulatory effects and associated mechanisms of miR-130a molecules on cisplatin resistance in ovarian cancer A2780 cell lines. Sichuan Da Xue Xue Bao Yi Xue Ban. 2013;44:865–70.
Li N, Yang L, Wang H, Yi T, Jia X, Chen C, Xu P. MiR-130a and MiR-374a function as novel regulators of cisplatin resistance in human ovarian cancer A2780 cells. PLoS One. 2015;10:e0128886.
Hu M, Liu Y, Deng C, Han R, Jia Y, Liu S, et al. Enhanced invasiveness in multidrug resistant leukemic cells is associated with overexpression of P-glycoprotein and cellular inhibitor of apoptosis protein. Leuk Lymphoma. 2011;52:1302–11.
Gottesman MM, Ling V. The molecular basis of multidrug resistance in cancer: the early years of P-glycoprotein research. FEBS Lett. 2006;580:998–1009.
Li Z, Hu S, Wang J, Cai J, Xiao L, Yu L, et al. MiR-27a modulates MDR1/Pglycoprotein expression by targeting HIPK2 in human ovarian cancer cells. Gynecol Oncol. 2010;119:125–30.
Ma JJ, Chen BL, Xin XY. XIAP gene downregulation by small interfering RNA inhibits proliferation, induces apoptosis, and reverses the cisplatin resistance of ovarian carcinoma. Eur J Obstet Gynecol Reprod Biol. 2009;146:222–6.
Sasaki H, Sheng Y, Kotsuji F, Tsang BK. Down-regulation of X-linked inhibitor of apoptosis protein induces apoptosis in chemoresistant human ovarian cancer cells. Cancer Res. 2000;60:5659–66.
Suda K, Murakami I, Katayama T, Tomizawa K, Osada H, Sekido Y, et al. Reciprocal and complementary role of MET amplification and EGFR T790 M mutation in acquired resistance to kinase inhibitors in lung cancer. Clin Cancer Res. 2010;16:5489–98.
Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–43.
Peruzzi B, Bottaro DP. Targeting the c-Met signaling pathway in cancer. Clin Cancer Res. 2006;12:3657–60.
Garofalo M, Di Leva G, Romano G, Nuovo G, Suh SS, Ngankeu A, et al. miR-221&222 regulate TRAIL resistance and enhance tumorigenicity through PTEN and TIMP3 downregulation. Cancer Cell. 2009;16:498–509.
Acunzo M, Visone R, Romano G, Veronese A, Lovat F, Palmieri D, et al. miR-130a targets MET and induces TRAIL-sensitivity in NSCLC by downregulating miR-221 and 222. Oncogene. 2012;31:634–42.
Chen CX, Hu Y, Li L. NRP1 is targeted by miR-130a and miR-130b, and is associated with multidrug resistance in epithelial ovarian cancer based on integrated gene network analysis. Mol Med Rep. 2016;13:188–96.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
About this article
Cite this article
Zhang, Hd., Jiang, Lh., Sun, Dw. et al. The role of miR-130a in cancer. Breast Cancer 24, 521–527 (2017). https://doi.org/10.1007/s12282-017-0776-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-017-0776-x