Abstract
Background
Bone-modifying agents are effective for treatment of breast cancer patients with bone metastases. Since their action is mediated through suppression of the osteoclast function, their efficacy can be determined by monitoring bone turnover markers. However, the clinical significance of these markers is yet to be compared.
Methods
For this study, 52 breast cancer patients with bone metastases treated with zoledronic acid (n = 36) or denosumab (n = 22) were enrolled (6 patients were treated sequentially with both agents). Serum tartrate-resistant acid phosphatase-5b (TRACP-5b), pyridinoline cross-linked carboxyterminal telopeptide of type I collagen (1CTP), N-terminal cross-linking telopeptides of type I collagen (NTX) and bone-specific alkaline phosphatase (BAP) were measured at pretreatment and 1, 3 and 6 months after treatment.
Results
Serum TRACP-5b (p < 0.0001), NTX (p = 0.0007) and BAP (p = 0.0032) decreased significantly after treatment. The baseline median value of TRACP-5b (457.5 mU/dL, range 173–1630 mU/dL) decreased to 137 mU/dL (91–795 mU/dL) 1 month after treatment. Reduction in serum NTX and BAP was greatest after 3 and 6 months, respectively. TRACP-5b, NTX and BAP were above normal levels at baseline in 62.5, 25 and 35.3 % of patients, respectively, and nearly 80 % of these patients attained normal levels during the treatment.
Conclusions
Although bone-modifying agents reduced the baseline levels of TRACP-5b, NTX and BAP significantly, the reduction patterns differed. TRACP-5b appears to affect levels most quickly and sensitively, possibly due to its direct link to the number and activity of osteoclasts. These findings suggest that the efficacy of TRACP-5b is clinically significant when considering which bone-modifying agents to use for breast cancer patients with bone metastases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bone metastases are most common in patients with primary metastatic or recurrent breast cancers and usually cause disability and morbidity accompanied by pain and neurological disorders [1–3]. In clinical settings, the disability is assessed in terms of skeletal-related events (SREs), which include pathogenic fractures, spinal cord compression, the need for palliative radiation therapy for bone, and hypercalcemia of malignancy [4]. Bone-modifying agents, including bisphosphonates and denosumab, have therefore been developed for the treatment of bone metastases and their efficacy for these SREs has been established. The key effects on SREs induced by bone-modifying agents are identified by determining the degree of suppression of osteoclast function.
According to a 2012 Cochrane systematic review, bisphosphonates including zoledronic acid have been used most frequently for breast cancer patients with bone metastases [5]. It was reported in this review that bisphosphonates significantly reduced the incidence and rate of SREs [risk ratio (RR) 0.85; 95 % confidence interval (CI) 0.77–0.94; p = 0.001] as compared to the control. Although this reduction is likely to be observed irrespective of the agent, RR of zoledronic acid was as small as 0.59. In addition, denosumab, a fully human antibody targeting the receptor activator of the nuclear factor-kappa B (RANK)-ligand, which disrupts activation of osteoclasts, has also been developed for the treatment of bone metastases [6]. In a randomized double-blind study involving 2046 breast cancer patients with bone metastases, time to first and subsequent SREs was significantly better for the denosumab than for the zoledronic acid group [hazard ratio (HR) 0.82; 95 % CI 0.71–0.95; p = 0.01] [7].
Although the effects of bone-modifying agents are evident, biomarkers for monitoring of treatment efficacy of these agents for bone metastases are yet to be established. Since breast cancer metastasis in the bone promotes turnover of bone remodeling, thus constituting a vicious cycle [8], changes in the biomarkers are well known to be linked with existing bone metastases [9, 10]. It has also been reported that bone turnover marker levels, including those of deoxypyridinoline (DPD), N-terminal cross-linking telopeptides of type I collagen (NTX), pyridinoline cross-linked carboxyterminal telopeptide of type I collagen (1CTP) and bone-specific alkaline phosphatase (BAP), correlate with the number of skeletal segments with metastatic disease [11]. Since these findings suggest that a correlation exists between the level of a bone turnover marker and extent of disease in the bone, bone turnover markers seem to have a significant prognostic value. In support of this notion, Ali et al. reported that elevated serum NTX levels (>26 nM BCE) were related to a shorter time to progression (TTP) [odds ratio (OR) 1.76; p = 0.0008] and overall survival (OS) (OR 1.71; p = 0.003) of breast cancer patients with bone-only or bone plus soft tissue metastasis [12]. Similarly, the prognostic values of NTX as well as BAP levels at baseline were demonstrated for solid tumors including breast cancer [13–16]. Interestingly, a normalized NTX group after a 3-month treatment with zoledronic acid for bone metastases of breast cancers had significantly lower risk for a first SRE, a first fracture or surgery to bone or death as compared to a group of persistently elevated urinary NTX [4]. Consistent with this report, normalization of urinary NTX during treatment with zoledronic acid or pamidronate for 3 months of 578 breast cancers was found to correlate with improved OS [17]. These results indicate the significance of NTX levels for breast cancer patients with bone metastases, not only at baseline, but also of the changes in these levels during treatment with bone-modifying agents. They also strongly suggest that monitoring bone turnover markers is clinically useful, not only for predicting prognosis but also for determining treatment efficacy.
Tartrate-resistant acid phosphatase-5b (TRACP-5b) has recently become clinically available as a new marker of bone resorption [18, 19]. In contrast to other markers which indirectly reflect bone turnover status, serum TRACP-5b, a proteolytically cleaved subunit derived from osteoclasts, has been established as a direct marker showing osteoclast number and activity. This marker is thus expected to be more sensitive and specific than other markers for breast cancer patients with bone metastases [20, 21].
However, the issue as to which bone turnover marker is clinically most useful to indicate the frequency of abnormal elevation in relation to time course as well as rate of normalization during the treatment with bone-modifying agents remains unresolved. Since a novel assay of TRACP-5b was established and has recently become clinically available [22], we have investigated bone resorption markers including 1CTP, NTX and TRACP-5b as well as a bone forming marker BAP, by focusing on the time course during the treatment with bone-modifying agents, to compare the significance of these markers as indicators of treatment efficacy.
Patients and methods
Eligible patients and treatments
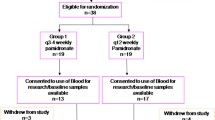
For this retrospective study, 52 breast cancer patients with bone metastases from Hyogo College of Medicine were recruited, comprising patients with primary metastatic cancers (n = 17) and recurrences (n = 35), who were treated with bone-modifying agents between July 2008 and November 2014. Zoledronic acid and denosumab were administered as first bone-modifying agents to 36 and 16 patients, respectively. For six patients, denosumab was used as the second bone-modifying agent after zoledronic acid, and data for zoledronic acid and denosumab treatment outcomes were calculated separately. Zoledronic acid (4 mg intravenously) and denosumab (120 mg subcutaneously) were administered to patients every 3–4 weeks and every 4 weeks, respectively. These patients were 32–84 years old (median age 61) and male breast cancer patients were excluded. All bone metastases were diagnosed using whole-body bone scintigraphy or 2-[(18)F]-fluoro-2-deoxy-d-glucose positron emission tomography (FDG-PET) followed by confirmation with magnetic resonance imaging. Chemotherapy (n = 22), endocrine therapy (n = 32), everolimus plus exemestane (n = 2) or trastuzumab (n = 1) were also administered in combination with bone-modifying agents as clinically required. One patient received only bone-modifying agent without other treatment for breast cancer. Zoledronic acid was changed to denosumab for six patients due to SRE.
Blood tests for the tumor markers carcinoembryonic antigen (CEA) and carbohydrate antigen 15-3 (CA15-3), physical examinations and examinations of clinical symptoms such as pain, neurological abnormality and disorder of movement were performed at 1- to 3-month intervals, when the patients visited our hospital. Serum levels of bone turnover markers including 1CTP, NTX, BAP and TRACP-5b were determined at baseline, and at 1, 3 and 6 months after treatment with bone-modifying agents. 1CTP, NTX and BAP were examined between July 2008 and March 2014, while TRACP-5b was measured between September 2013 and November 2014. This study was approved by the Ethics Committee of Hyogo College of Medicine (No. 1969).
Assay of bone turnover markers
Serum 1CTP, NTX, BAP and TRACP-5b were measured at SRL Inc. (Tokyo, Japan). For the 1CTP assay, the two-antibody radioimmunoassay method using a kit provided by Orion Diagnostica Oy (Espoo, Finland) was employed and radioactivity was determined with a γ-counter (Wallac1460SRL; Wallac Co., Ltd., Turku, Finland) [23]. Serum NTX levels were measured with the enzyme-linked immunosorbent assay method using a kit provided by Alere Medical Co., Ltd. (Tokyo, Japan) and an automated EIA system (EMS-01; Nippon Advanced Technology Co., Ltd., Ibaraki, Japan) [24]. Serum BAP was measured with a chemiluminescence enzyme immunoassay method using the Beckman Access Ostase assay kit with a UniCel DxI 800 system (Beckman Coulter K.K., Tokyo, Japan) [25, 26]. The serum level of TRACP-5b was determined with an enzyme immunoassay using the Osteolinks TRAP-5b kit provided by Nittobo Medical Co., Ltd. (Fukushima, Japan) following a previously described method [22].
The normal ranges of serum 1CTP, NTX and TRACP-5b were defined as 0–4.5 ng/mL, 7.5–16.5 nmol BCE/L and 120–420 mU/dL, respectively. The normal range of BAP was set at 2.9–14.5 μg/L for premenopausal and at 3.8–22.6 μg/L for postmenopausal women. Blood samples were obtained at baseline (before treatment with bone-modifying agents), and subsequently 1, 3 and 6 months after treatment.
Statistical analysis
Changes in bone turnover markers during the treatment course were statistically analyzed with Friedman’s test. Differences were considered statistically significant if p < 0.05, and the JMP Pro 10 software (SAS Institute Inc., Cary, NC, USA) was used for all statistical analyses.
Results
Patients’ characteristics and bone turnover marker levels according to clinical features
In this study, we collected data from 58 cases treated with zoledronic acid or denosumab in 52 breast cancer patients with bone metastases. The clinical characteristics of these 52 patients and baseline levels of bone turnover markers in each clinical feature are shown in Table 1. A total of 17 primary advanced and 35 recurrent breast cancers were included. Half of these patients had bone-only metastasis and 14 and 38 patients had single and multiple bone metastases, respectively. Out of 52 breast cancers, 43 (82.7 %) were estrogen receptor-positive and 42 out of 51 breast cancers (84.0 %) were human epidermal growth factor receptor 2-negative. We did not calculate significant differences in bone turnover markers in the different subsets because the number of these patients was not sufficient for analysis.
Normalization of bone turnover markers during treatment with bone-modifying agents
Although 15 out of 24 (62.5 %) cases showed elevated levels of TRACP-5b at baseline, only two (8.3 %) patients had consistently elevated TRACP-5b 1 month after treatment with bone-modifying agents (Fig. 1). This suppression of TRACP-5b continued for 6 months. Similarly, ratios for patients with elevated NTX (25.0 %) and BAP (35.3 %) at baseline maximally decreased to 3.8 % after 3 months and 7.1 % after 6 months, respectively. On the other hand, elevation of 1CTP levels at 58.8 % at baseline did not change during 6 months of treatment with bone-modifying agents.
Frequencies of elevation of bone turnover markers above the upper limit of normal range at baseline in patients after 1 month (1 M), 3 months (3 M) and 6 months (6 M) of treatment with bone-modifying agents. a TRACP-5b (tartrate-resistant acid phosphatase-5b), b 1CTP (pyridinoline cross-linked carboxyterminal telopeptide of type I collagen), c NTX (N-terminal cross-linking telopeptides of type I collagen), d BAP (bone-specific alkaline phosphatase)
Changes in bone turnover markers during treatment with bone-modifying agents
Next, we analyzed changes in bone turnover markers for each time course with the results shown in Fig. 2. TRACP-5b at baseline (median 457.5 mU/dL, range 173–1630 mU/dL) had decreased significantly (p < 0.0001) by 1 month after treatment (137 mU/dL, 91–795 mU/dL) and remained at low levels during the treatment course (Fig. 2a). Similarly, a significant reduction was obtained for both NTX (p = 0.0007; Fig. 2c) and BAP (p = 0.0032; Fig. 2d). The baseline levels of NTX (16.45 nmol BCE/L, 6.1–52.2 nmol BCE/L) showed the greatest reduction 3 months after treatment (9.55 nmol BCE/L, 6.4–56.0 nmol BCE/L) and BAP levels of baseline (14.15 μg/L, 6.4–81.3 μg/L) decreased to a maximum of 8.3 μg/L (4.7–43.5 μg/L) after 6 months. On the other hand, no significant decrease in 1CTP levels was detected during the experimental course (p = 0.83).
Changes in bone turnover markers at baseline and 1 month (1 M), 3 months (3 M) and 6 months (6 M) after treatment with bone-modifying agents. a TRACP-5b (tartrate-resistant acid phosphatase-5b), b 1CTP (pyridinoline cross-linked carboxyterminal telopeptide of type I collagen), c NTX (N-terminal cross-linking telopeptides of type I collagen), d BAP (bone-specific alkaline phosphatase). Boxes show median and quartile range
To assess the effect of systemic therapies combined with bone-modifying agents, changes in bone turnover markers were divided into those for the chemotherapy and endocrine therapy groups as shown in Fig. 3. A significant reduction in TRACP-5b was observed in the endocrine group (p < 0.0001, Fig. 3a). A similar reduction pattern was also observed in the chemotherapy group, but we did not perform statistical analysis due to the small number of patients (n = 8). Although only NTX showed a significant and discernible decrease in the chemotherapy group (p = 0.012; Fig. 3c) and BAP in the endocrine therapy group (p = 0.0097; Fig. 3d), the number of these groups was small.
Changes in bone turnover markers at baseline and 1 month (1 M), 3 months (3 M) and 6 months (6 M) after treatment with bone-modifying agents of two patient groups: chemotherapy (CT) and endocrine therapy (ET). a TRACP-5b (tartrate-resistant acid phosphatase-5b), b 1CTP (pyridinoline cross-linked carboxyterminal telopeptide of type I collagen), c NTX (N-terminal cross-linking telopeptides of type I collagen), d BAP (bone-specific alkaline phosphatase). Boxes shows median and quartile range
Serum levels of bone turnover markers at each time point are shown separately in Table 2 after zoledronic acid and denosumab treatment. Although only five cases could be investigated and statistical analysis was infeasible, TRACP-5b had decreased 1 month after treatment with zoledronic acid, which was similar to the reduction for those treated with denosumab. As for 1CTP, NTX and BAP, the number of patients treated with denosumab was too small for further analysis of the relevant data.
Discussion
In this study, we showed that serum levels of TRACP-5b, NTX and BAP, but not of 1CTP of patients with bone metastases, were significantly reduced during 6 months of treatment with bone-modifying agents. The levels of TRACP-5b were found to be elevated in a greater proportion of patients (62.5 %) than were NTX (25 %) and BAP (35.3 %). Interestingly, a reduction in TRACP-5b occurred within 1 month after the start of treatment and remained at reduced levels for 6 months. On the other hand, maximum reduction was not attained until after 3 months for NTX and 6 months for BAP. It has been reported that urinary NTX at baseline exceeded the normal range in 51.1–59.7 % of breast cancer patients with bone metastases [4, 16, 17]. In addition, according to studies assessing serum NTX, abnormal NTX at baseline for patients with bone metastases was found in 24 % [12] and 50 % [15]. According to the findings of our study, elevation of levels above the upper normal range was observed most frequently for TRACP-5b than for other markers. Wada et al. reported that the diagnostic values of TRACP-5b in breast cancer patients with bone metastases were 91.0 % for specificity, 65.7 % for sensitivity and 83.2 % for accuracy [27]. In addition, Chao et al. established by analyzing receiver operator characteristic (ROC) curves that 4.026 units/L of TRACP-5b for identification of patients with extensive bone metastases yielded a specificity of 98 % and a sensitivity of 93 % [28]. These data appear to suggest that the diagnostic value of TRACP-5b for breast cancer patients with bone metastases is superior to that of other bone turnover markers.
The prognostic significance of NTX at baseline as well as interval changes after treatment has been the subjects of reports on breast cancer patients with bone metastases treated with bone-modifying agents [4, 12–17]. The OS of breast cancer patients with bone metastases was found to be significantly poorer for patients with higher baseline TRACP-5b and when a cutoff value was used to delineate the highest tertile (HR 3.524; p < 0.0001), but OS was significantly better for those patients who showed a reduction in serum TRACP-5b after treatments (p = 0.0015) [29]. Thus, both NTX and TRACP-5b seem to be clinically valuable as prognostic factors for breast cancer patients with bone metastases. However, since no comparative study of the two markers has been reported, it has remained unknown which marker is superior for predicting patients’ prognosis.
According to Chung et al.’s report, responders showed a significant decrease in TRACP-5b after treatment (p < 0.0001) [30], which was more prominent in TRACP-5b than in NTX. In addition, changes in TRACP-5b levels after treatment were marginally significantly associated with SRE (HR 0.40; 95 % CI 0.14–1.10; p = 0.076) [31]. It is conceivable that the treatment combines a systemic therapy, such as chemotherapy or endocrine therapy, with a bone-modifying agent. Since treatment efficacy was achieved by such a combination of agents, we could not evaluate the effect of a single bone-modifying agent by means of bone turnover markers accurately. The association between a decrease of TRCP-5b and clinical response induced by systemic treatment including chemotherapy or endocrine therapy is also needed to be investigated in future studies.
Of the 15 patients with elevated TRACP-5b at baseline, 13 (86.7 %) showed normal levels after only 1 month of treatment. On the other hand, maximum normalization of elevated NTX and BAP at baseline was achieved 3 months (85.7 %) and 6 months (75 %), respectively, after treatment with bone-modifying agents. Thus, the rates of normalization for these bone turnover markers were similar, but the pattern of reduction varied, and TRACP-5b appears to reflect the efficacy of bone-modifying agents most quickly and sensitively. According to two reports, 76 and 81 %, respectively, of patients with elevated urinary NTX at baseline normalized after 3 months of treatment with zoledronic acid [4, 17]. Consistent with this finding, in another study the frequency of 50 % of elevated serum NTX at baseline was reduced to 32 % after 3 months of treatment using low or conventional dosage of zoledronic acid [15]. These data show that normalization of elevated NTX at baseline was consistently achieved after 3 months of treatment with bisphosphonate. Barnadas et al., who evaluated the time course of various bone turnover markers, demonstrated that the maximum reduction in NTX was obtained after 9 months [16] and, consistent with our finding, the mean BAP level after 6 months of treatment with zoledronic acid (18.4 μg/L) was lower than that after 3 months (24.8 μg/L).
Although they are not conclusive, these data indicate that reductions in NTX and BAP levels can be obtained after 3 months of treatment with bone-modifying agents. It is well established that bone turnover markers increase in patients with osteoporosis and reduction of these markers is obtained by treatment with bone-modifying agents [32]. According to previous studies, TRACP-5b has been decreased by 1-month treatment with denosumab in patients with osteoporosis [33, 34]. Similar to these reports, we demonstrate that the reduction in TRACP-5b occurred very early, that is, within 1 month of treatment for patients with bone metastases. Since TRACP-5b is a specific marker of osteoclasts, its serum levels are known to reflect the number of active systemic osteoclasts. This direct link to the activity of osteoclasts accounts for the earlier response seen in TRACP-5b as compared to other bone turnover markers, which indirectly initiates the process of bone resorption or bone formation. One limitation of this study is that we assessed the effects of bone-modifying agents only in terms of reduction in bone turnover markers. Although the relationship between reductions in marker levels and SRE needs to be investigated, this was not feasible in the present study due to the small number of patients. This issue should thus be investigated in a future study with a larger number of patients. The other limitation is that assessment of the response by patients in term of changes in levels of TRACP-5b and other markers was not the same and most of these patients were treated with either denosumab or zoledronic acid, respectively. Although the basis of these assays is therefore different, we believe the differences in the time course for these markers after treatment could be determined accurately, irrespective of bone-modifying agents. However, this point also needs to be further analyzed.
In conclusion, we showed that baseline values of bone turnover markers TRACP-5b, NTX and BAP decreased significantly after treatment with bone-modifying agents combined with chemotherapy or endocrine therapy, but that the time course of reduction for these three markers varied. TRACP-5b appears to reflect the efficacy of bone-modifying agents most quickly and sensitively, possibly due to its direct link to the number and activity of osteoclasts. These findings provide evidence of the usefulness of TRACP-5b when the efficacy of various bone-modifying agents is being considered in clinical practice.
References
Manders K, van de Poll-Franse LV, Creemers GJ, Vreugdenhil G, van der Sangen MJ, Nieuwenhuijzen GA, et al. Clinical management of women with metastatic breast cancer: a descriptive study according to age group. BMC Cancer. 2006;6:179.
Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108.
Coleman RE. Adjuvant bisphosphonates in breast cancer: are we witnessing the emergence of a new therapeutic strategy? Eur J Cancer. 2009;45(11):1909–15.
Lipton A, Cook RJ, Major P, Smith MR, Coleman RE. Zoledronic acid and survival in breast cancer patients with bone metastases and elevated markers of osteoclast activity. Oncologist. 2007;12(9):1035–43.
Wong MH, Stockler MR, Pavlakis N. Bisphosphonates and other bone agents for breast cancer. Cochrane Database Syst Rev. 2012;2:CD003474. doi:10.1002/14651858.CD003474.pub3.
Bekker PJ, Holloway DL, Rasmussen AS, Murphy R, Martin SW, Leese PT, et al. A single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal women. 2004. J Bone Miner Res. 2005;20(12):2275–82.
Stopeck AT, Lipton A, Body JJ, Steger GG, Tonkin K, de Boer RH, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol. 2010;28(35):5132–9.
Mundy GR. Mechanisms of bone metastasis. Cancer. 1997;80(8 suppl):1546–56.
Massidda B, Ionta MT, Foddi MR, Mascia L, Bruder F, Aloi MB, et al. Usefulness of pyridinium crosslinks and CA 15-3 as markers in metastatic bone breast carcinoma. Anticancer Res. 1996;16(4B):2221–3.
Tähtelä R, Thölix E. Serum concentrations of type I collagen carboxyterminal telopeptide (ICTP) and type I procollagen carboxy-and aminoterminal propeptides (PICP, PINP) as markers of metastaticbone disease in breast cancer. Anticancer Res. 1996;16(4B):2289–93.
Demers LM, Costa L, Lipton A. Biochemical markers and skeletal metastases. Cancer. 2000;88(12 Suppl):2919–26.
Ali SM, Demers LM, Leitzel K, Harvey HA, Clemens D, Mallinak N, et al. Baseline serum NTx levels are prognostic in metastatic breast cancer patients with bone-only metastasis. Ann Oncol. 2004;15(3):455–9.
Brown JE, Cook RJ, Major P, Lipton A, Saad F, Smith M, et al. Bone turnover markers as predictors of skeletal complications in prostate cancer, lung cancer, and other solid tumors. J Natl Cancer Inst. 2005;97(1):59–69.
Coleman RE, Major P, Lipton A, Brown JE, Lee KA, Smith M, et al. Predictive value of bone resorption and formation markers in cancer patients with bonemetastases receiving the bisphosphonate zoledronic acid. J Clin Oncol. 2005;23(22):4925–35.
Zhao X, Xu X, Zhang Q, Jia Z, Sun S, Zhang J, et al. Prognostic and predictive value of clinical and biochemical factors in breast cancer patients with bone metastases receiving “metronomic” zoledronic acid. BMC Cancer. 2011;22(11):403. doi:10.1186/1471-2407-11-403.
Barnadas A, Manso L, de la Piedra C, Meseguer C, Crespo C, Gómez P, et al. Bone turnover markers as predictive indicators of outcome in patients with breast cancer and bone metastases treated with bisphosphonates: results from a 2-year multicentre observational study (ZOMAR study). Bone. 2014;68:32–40.
Lipton A, Cook R, Saad F, Major P, Garnero P, Terpos E, et al. Normalization of bone markers is associated with improved survival in patients with bone metastases from solid tumors and elevated bone resorption receiving zoledronic acid. Cancer. 2008;113(1):193–201.
Halleen JM, Alatalo SL, Suominen H, Cheng S, Janckila AJ, Väänänen HK. Tartrate-resistant acid phosphatase 5b: a novel serum marker of bone resorption. J Bone Miner Res. 2000;15(7):1337–45.
Janckila AJ, Takahashi K, Sun SZ, Yam LT. Tartrate-resistant acid phosphatase isoform 5b as serum marker for osteoclastic activity. Clin Chem. 2001;47(1):74–80.
Chao TY, Ho CL, Lee SH, Chen MM, Janckila A, Yam LT. Tartrate-resistant acid phosphatase 5b as a serum marker of bone metastasis in breast cancer patients. J Biomed Sci. 2004;11(4):511–6.
Halleen JM, Alatalo SL, Janckila AJ, Woitge HW, Seibel MJ, Väänänen HK. Serum tartrate-resistant acid phosphatase 5b is a specific and sensitive marker of bone resorption. Clin Chem. 2001;47(3):597–600.
Ohashi T, Igarashi Y, Mochizuki Y, Miura T, Inaba N, Katayama K, et al. Development of a novel fragments absorbed immunocapture enzyme assay system for tartrate-resistant acid phosphatase 5b. Clin Chim Acta. 2007;376(1–2):205–12.
Kiyohara T, Kuroe K, Chichibu K, Fukunaga H. Radioimmunoassay of 1CTP (cross-linked carboxyterminal telopeptide of type I collagen) in serum. (Japansese) Horumon to Rinshou. 1994;42:1189–93.
Woitge HW, Pecherstorfer M, Li Y, Keck AV, Horn E, Ziegler R, et al. Novel serum markers of bone resorption: clinical assessment and comparison with established urinary indices. J Bone Miner Res. 1999;14(5):792–801.
Overgaard K, Alexandersen P, Riis BJ, Christiansen C. Evaluation of a new commercial IRMA for bone-specific alkaline phosphatase during treatment with hormone replacement therapy and calcitonin. Clin Chem. 1996;42(6 Pt 1):973–4.
Kress BC, Mizrahi IA, Armour KW, Marcus R, Emkey RD, Santora AC 2nd. Use of bone alkaline phosphatase to monitor alendronate therapy in individual postmenopausal osteoporotic women. Clin Chem. 1999;45(7):1009–17.
Wada N, Ishii S, Ikeda T, Enomoto K, Kitajima M. Serum tartrate resistant acid phosphatase as a potential marker of bone metastasis from breast cancer. Anticancer Res. 1999;19(5C):4515–21.
Chao TY, Yu JC, Ku CH, Chen MM, Lee SH, Janckila AJ, et al. Tartrate-resistant acid phosphatase 5b is a useful serum marker for extensive bone metastasis in breast cancer patients. Clin Cancer Res. 2005;11(2 Pt 1):544–50.
Wu YY, Janckila AJ, Ku CH, Yu CP, Yu JC, Lee SH, et al. Serum tartrate-resistant acid phosphatase 5b activity as a prognostic marker of survival in breast cancer with bone metastasis. BMC Cancer. 2010;10:158. doi:10.1186/1471-2407-10-158.
Chung YC, Ku CH, Chao TY, Yu JC, Chen MM, Lee SH. Tartrate-resistant acid phosphatase 5b activity is a useful bone marker for monitoring bone metastases in breast cancer patients after treatment. Cancer Epidemiol Biomark Prev. 2006;15(3):424–8.
Mountzios G, Terpos E, Syrigos K, Papadimitriou C, Papadopoulos G, Bamias A, et al. Markers of bone remodeling and skeletal morbidity in patients with solid tumors metastatic to the skeleton receiving the biphosphonate zoledronic acid. Transl Res. 2010;155(5):247–55.
Bandeira F, Costa AG, Soares Filho MA, Pimentel L, Lima L, Bilezikian JP. Bone markers and osteoporosis therapy. Arq Bras Endocrinol Metabol. 2014;58(5):504–13.
Eastell R, Christiansen C, Grauer A, Kutilek S, Libanati C, McClung MR, et al. Effects of denosumab on bone turnover markers in postmenopausal osteoporosis. J Bone Miner Res. 2011;26(3):530–7.
Inage K, Orita S, Yamauchi K, Sakuma Y, Kubota G, Oikawa Y, et al. The time course changes in bone metabolic markers after administering the anti-receptor activator of nuclear factor-kappa B ligand antibody and drug compliance among patients with osteoporosis. Asian Spine J. 2015;9(3):338–43.
Acknowledgments
This study was supported by a Grant from the Hyogo College of Medicine.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
YM. received honoraria and research funds from AstraZeneca K.K., Novartis Pharma K.K., Daiichi Sankyo Co., Ltd. and Nittobo Medical Co., Ltd. The other authors declare that they have no conflict of interest.
About this article
Cite this article
Nishimukai, A., Higuchi, T., Ozawa, H. et al. Different patterns of change in bone turnover markers during treatment with bone-modifying agents for breast cancer patients with bone metastases. Breast Cancer 24, 245–253 (2017). https://doi.org/10.1007/s12282-016-0695-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-016-0695-2