Abstract
Purpose
In order to evaluate the diagnostic and therapeutic potential of mammary ductoscopy and watchful follow-up for treating bloody nipple discharge, we investigated the incidence of cancer evolving from the location related to the affected duct and the disappearance of nipple discharge.
Patients and methods
Between April 1998 and March 2008, we assessed 709 lesions among 624 patients without a diagnosis of malignancy at the time of 6 months after mammary ductoscopy. The median follow-up time was 5.5 years. We reviewed the subjects’ charts retrospectively and investigated the dates on which discharge-related cancer was diagnosed and the disappearance of discharge was noted after the initial examination with mammary ductoscopy.
Results
The incidence of cancer evolving from the location related to the pathological duct was 11 % (78/709). Nipple discharge disappeared in 480 (85.1 %) of the 564 followed up lesions, with the exception of 78 breast cancers and 67 resected benign lesions. The rate of disappearance for nipple discharge in the cases of intraductal papilloma at the first examination was 82.5 %. In cases in which no obvious lesions were observed on mammary ductoscopy, there was a 90 % probability that the nipple discharge would disappear, and the rate of evolving breast cancer in the cases of atypical papillary lesions at the first examination was significantly higher than that observed in the cases of intraductal papilloma, at 50 and 8.9 %, respectively.
Conclusions
Information revealed by mammary ductoscopy is useful for differentiating patients who should be subjected to intensive examinations and those who should expect disappearance of their discharge. Mammary ductoscopy and watchful follow-up can substitute microdochectomy in patients with bloody nipple discharge.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Approximately 5 % of patients report nipple discharge as their chief complaint during their first visit to a breast clinic. Discharge can be an important clue for the diagnosis of early-stage breast cancer, along with microcalcification on a mammogram, small tumors on ultrasonography and nipple erosion. Bloody nipple discharge can be an important clue for the diagnosis of early-stage breast cancer [1]. Nipple touch smear cytology is sometimes conducted for screening; however, the sensitivity of smear cytology is very low. Ductal lavage and nipple aspiration of breast fluid have been reported to be useful for detecting breast cancer, in place of touch smear cytology, although special instruments are required for these procedures [2, 3]. In addition, positive cytology does not indicate the location of suspicious lesions. Unless associated findings are also detected on a mammogram or ultrasonography, galactography is needed to detect intraductal lesions. Microdochectomy was used to diagnose nipple discharge. However, since 1989, mammary ductoscopy has been applied in Japan [4, 5]. Mammary ductoscopy is valuable for diagnosis because it allows for the visualization of intraductal lesions and approaches the lesion directly without surgery.
Because the gold standard for analyzing nipple discharge is a pathological examination, surgical resection is usually performed immediately after mammary ductoscopy in both Europe and the United States [6–12]. In contrast, in Japan, the modality was developed as a tool to evaluate nipple discharge, instead of microdochectomy, and physicians often consider various diagnostic modalities together. The cost of mammary ductoscopy in Japan has been covered by medical care insurance since approximately 1995; therefore, it is considered a routine examination for assessing nipple discharge in the outpatient setting.
At our institution, in order to determine whether follow-up is justified in individual cases, we confirm that the smear or duct wash cytology is benign, even if an intraductal breast biopsy sample [5] is diagnosed as a benign papillary lesion. Moreover, we search for lesions in the peripheral region of the affected duct on breast imaging and confirm the diagnosis of benign lesions using an image-guided needle biopsy, if necessary [13]. Many patients who suffer from nipple discharge and are diagnosed to have no malignancy are followed up every 6 months if they wish to avoid surgical resection. Follow-up allows clinicians to evaluate not only the diagnostic potential of endoscopic classification, but also the natural history of nipple discharge [14, 15]. Therefore, we investigated the disappearance of nipple discharge and cancer evolving from the location related to the pathological duct.
Patients and methods
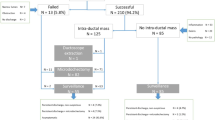
Between April 1998 and March 2008, 1,262 ducts exhibiting clinically bloody discharge (examined if necessary using an occult blood test) were examined using mammary ductoscopy, a total of 1,643 times among 1,139 patients at the Cancer Institute Hospital, Tokyo, Japan. All patients were also examined with mammography and ultrasonography. Prior to performing mammary ductoscopy, touch smear cytology of the affected nipple was conducted. In cases in which the lesion was detected on mammography or ultrasonography, an image-guided needle biopsy or fine-needle aspiration cytology specimen was obtained. The indication for mammary ductoscopy was the presence of abundant, continuous or bloody discharge. Because the finding of duct cell-rich smear cytology or dilated ducts on ultrasonography with or without intraductal echogenic spots suggests the existence of intraductal lesions, we also performed mammary ductoscopy. A total of 218 breast cancers in 216 patients were diagnosed prior to mammary ductoscopy. In these cases, mammary ductoscopy was performed in order to evaluate the degree of cancer spread toward the nipple and determine the indications for preserving the nipple areolar complex during partial mastectomy. Excluding the 171 breast cancers diagnosed within 6 months, 49 benign lesions resected within 6 months and 115 lesions among 104 patients followed up for less than 6 months, we enrolled 709 lesions in 624 patients followed up for more than 6 months without a diagnosis of malignancy at the time of 6 months after mammary ductoscopy. All enrolled patients were diagnosed with benign lesions at the initial examination, as we followed benign cases for 6 months as a rule. They were followed until May 2014, and the median follow-up time was 5.5 years. Most of the patients followed up for less than 6 months received no additional visits because they lived too far from our hospital and were consulted for mammary ductoscopy.
Mammary ductoscopic images were obtained using an Optiscope III fiberscope (Clinical Supply Co., Gifu, Japan; 10,000 pixels, 0.8 mm in diameter of its tip) or MS series fiberscope (FiberTech Co., Tokyo, Japan; 10,000 pixels, 0.8 mm in diameter of its tip) and recorded on digital videotapes or digital video discs. Informed consent was obtained from each patient. The procedure used for mammary ductoscopy was as follows: first, a bougie (Bowman lacrimal dilator) was inserted into the affected duct, and the duct was dilated with progressively larger dilators. An 18-Ga Surflo intravenous catheter (Terumo Co., Tokyo, Japan) was then inserted and left in the duct. In order to ensure good endoscopic resolution and establish analgesia, the inside of the duct was washed with a lidocaine solution containing 1 % epinephrine. The liquid inside the duct was then collected via aspiration for a cytological evaluation (duct wash cytology). Next, the fiberscope was inserted through a hemostasis valve (Y8199, SI-BEN, Clinical Supply Co., Gifu, Japan) into the catheter, and the whole duct system was visualized by inflating the duct with air.
If a polypoid lesion was confirmed inside the duct, an intraductal breast biopsy [5] was performed via aspiration of the lesion using an 18-Ga Surflo intravenous catheter or needle designed for an intraductal biopsy (18-Ga AKO needle or 18-Ga JN needle, Hakko Shoji Co., Japan). The aspirated tissue sample was treated with 10 % formalin within the catheter and collected as a tissue fragment. The tissue samples were then fixed and embedded in paraffin, after which they were sectioned and stained with hematoxylin and eosin (HE) in a routine manner.
The endoscopic findings consisted of elevated (polypoid) type lesions and/or superficial type lesions. The endoscopic classification of intraductal lesions was divided into five categories (Fig. 1). The polypoid type represented the localized expansive growth lesions that were often spherical, pedunculated or hemispherical. If the polypoid lesion was solitary in the duct, the case was classified as being of the polypoid-solitary type. In contrast, the multiple lesions type was composed of multiple elevated lesions and/or superficial lesions, while the superficial type presented as superficial spreading lesions, such as those involving continuous luminal irregularity, redness and/or an erosive surface, without any obvious polypoid type lesions. The previously reported “combined type” [16] was included in the multiple lesions type in this study due to the similarities between the types. In each case, we allocated one endoscopic classification to one affected duct lobular unit. When the examination was performed successfully and no obvious lesions were detected inside the entire duct, the case was classified as “no lesion.” Meanwhile, when proximal stenosis of the main duct resulted in the inability to examine the duct thoroughly or when the observed lesions could not be classified into one of the above four types, the case was designated as “unclassified.”
With respect to the intraductal breast biopsy, smear or duct wash cytology, we searched for other lesions in the peripheral region of the affected duct again using breast imaging techniques, including magnetic resonance imaging (MRI) and ultrasonography, and confirmed the diagnosis of a benign lesion using an image-guided needle biopsy, if necessary. If we deemed follow-up to be appropriate for the patient, and she wished to avoid surgical resection, she was followed at 6-month intervals. All patients were evaluated, and findings of malignancy and/or symptoms of nipple discharge were recorded in the patient’s medical chart at every visit. If the patient exhibited no bloody discharge, and no smear cytology was performed, we determined that the nipple discharge had disappeared and defined the day of the visit as the date of confirmation of the disappearance of the discharge.
Furthermore, we defined cancers evolving from locations identical to or neighboring the affected duct as discharge-related cancers on the assumption that the breast could be divided into nine locations, as follows: upper, upper-outer, outer, lower-outer, lower, lower-inner, inner, upper-inner and central (Fig. 2). The diagnosis of breast cancer was made depending on the histological diagnosis or fine-needle aspiration cytology of the discharge-related lesions when the patient was treated at another hospital. We reviewed the charts retrospectively and investigated the date on which the discharge-related cancer was diagnosed and the date on which disappearance of discharge was noted after the initial examination with mammary ductoscopy. The Chi square test was used for the statistical analysis. A p value of <0.05 was considered to be statistically significant.
Results
Characteristics of the cases (Table 1)
The patient’s age at the initial mammary ductoscopy examination ranged from 16 to 79 years, with a median age of 47 years. The case distribution of the initial endoscopic classification with the number of lesions classified as polypoid-solitary, multiple lesions, superficial, no lesion or unclassified was 319 (45.0 %), 63 (8.9 %), 19 (2.7 %), 235 (33.1 %) and 73 (10.3 %), respectively. In approximately 30 % of patients with nipple discharge, the examination was performed successfully, although there were no obvious lesions inside the entire duct or dilated ductal lumen.
Two hundred and sixty-eight lesions (37.7 %) were diagnosed histologically based on intraductal breast biopsies performed at the first examination. The remaining 441 lesions were not diagnosed as breast cancer using fine-needle aspiration cytology, duct wash cytology and so on. Among the 268 cases diagnosed histologically, there was a total of eight atypical papillary lesions, 257 cases of intraductal papilloma and three benign papillary lesions.
Breast cancers evolving from the location related to the pathological duct
Table 2 shows the relationship between the clinical/pathological factors and the outcomes, while Table 3 shows the incidence of evolving cancer and the probability of the discharge disappearing. The proportion of cases of breast cancer diagnosed after 6 months, resected benign lesions, discharge that continued and discharge that disappeared was 11.0, 9.4, 11.8 and 67.7 %, respectively.
During the follow-up period, 78 breast cancers were diagnosed more than 6 months after the initial mammary ductoscopy examination (Fig. 3). Sixty-three (80.8 %) of these cases were detected chiefly with ultrasonography of the peripheral region of the affected duct. Although nipple discharge had disappeared at the time of diagnosis in 29 cases, 42 patients (53.8 %) were re-examined with mammary ductoscopy, 34 of whom displayed abnormal findings. Seven intraductal breast biopsies revealed breast cancer. The median time between mammary ductoscopy and the diagnosis of breast cancer was 2.2 years in the 78 cases. A total of 59.0 % (46/78) of these breast cancer cases were diagnosed within 3 years after the initial mammary ductoscopy examination. However, four breast cancer cases were diagnosed later than 10 years after initial examination.
The rate of cancer evolving from the location related to the pathological duct was 11.0 % (78/709). The 78 breast cancers diagnosed after 6 months consisted of 32 cases of ductal carcinoma in situ (DCIS), 43 invasive breast cancers and three unspecified cases treated at other hospitals. Thirteen breast cancers exhibited lymph node metastasis.
The rate of cancer evolving from the location related to the pathological duct in the polypoid-solitary, multiple lesions, superficial, no lesion and unclassified types was 8.5, 19.0, 31.6, 7.2 and 21.9 %, respectively. The rate of evolving breast cancer among the cases of atypical papillary lesions at the first examination was higher than that observed among the cases of intraductal papilloma, at 50 % (4/8) and 8.9 % (23/257), respectively (p = 0.0046).
Disappearance of nipple discharge in the follow-up cases
Sixty-seven lesions were resected and proven to be benign during the follow-up period. Among these 67 lesions, the case distribution of the type of surgery with the number of cases of excisional biopsies (tumorectomy), microdochectomy, partial mastectomy and mastectomy due to breast cancer evolving from another location was 37 (55.2 %), seven (10.4 %), four (6 %) and 19 (28.6 %), respectively. There was a total of 42 cases of intraductal or intracystic papilloma, one cyst and 24 benign lesions.
Nipple discharge disappeared in 480 (85.1 %) of the 564 followed up lesions, with the exception of 78 breast cancers and 67 resected benign lesions. The median time between mammary ductoscopy and the disappearance of nipple discharge was 1.9 years among the 480 cases with disappearance of discharge. The relationship between the cumulative incidence of disappearance of discharge and the interval after the first examination is displayed in Fig. 4. Approximately 90 % of the cases experienced disappearance of nipple discharge up to 5 years after the first examination, although the rate of disappearance within the first 2 years was approximately 50 %.
The probability of discharge disappearing in the polypoid-solitary, multiple lesions, superficial, no lesion and unclassified types was 84.8, 78.3, 77.8, 87.9 and 83.0 %, respectively. In cases in which no obvious lesions were observed on mammary ductoscopy, there was a 90 % probability that the nipple discharge would disappear. Meanwhile, the rate of disappearance of nipple discharge in the cases of intraductal papilloma at the first examination was 82.5 % (170/206).
Discussion
Nipple discharge that is bilateral and nonspontaneous and that emanates from multiple ducts after breast manipulation or stimulation is classified as benign (physiologic). However, nipple discharge that is unilateral, from a single duct, spontaneous and persistent is classified as “pathologic” [1]. The presence of clinically bloody discharge (including occult blood test positive) is considered to be a “pathologic” condition and it may be an important clue for the diagnosis of early-stage breast cancer. The issue of follow-up for bloody nipple discharge and the treatment potential of intraductal breast biopsies in cases of intraductal papilloma were recently reported [14, 15, 17, 18]. In the present study, we evaluated both the safety of follow-up without surgery and the therapeutic potential of intraductal breast biopsies. We restricted the enrollment to only follow-up cases without a diagnosis at the time of 6 months after mammary ductoscopy. During the study period, the incidence of cancer evolving from the location related to the pathological duct was 11.0 %. In contrast, the rate of evolving cancer after central duct resection for nipple discharge has been reported to be 5.3 % [19]. In this period, the rates of a history of breast cancer, contralateral breast cancer and ipsilateral breast cancer without relation to nipple discharge were 5.8, 5.1 and 3 %, respectively. However, we considered the incidence of evolving cancer to be slightly high within an acceptable range, as patients with proliferative intraductal lesions have a risk of breast cancer [20]. It would be extreme to conclude that all patients with bloody nipple discharge should undergo microdochectomy, as such patients have an approximate 10 % risk of breast cancer. It is therefore important to keep in mind the fact that cancers can evolve from the location related to the affected duct more than 10 years after onset. Because the number of evaluated cases was limited during the routine pathological investigation at that time, positive findings for ER and PgR were noted in 94.0 and 89.6 % of the 67 evaluated cases, respectively, and there were no cases with an HER2-positive status among the 40 evaluated cases. Only one case out of the 30 evaluated cases was classified as nuclear grade 3, and belonged to the superficial type. However, there was no significant correlation between the endoscopic classification and expression of biomarkers. The cancers evolving from the location related to the affected duct during the follow-up period tended to be non-aggressive, and slow growing in nature.
Mammary ductoscopy is valuable for diagnosis because it allows for the visualization of intraductal lesions, while intraductal biopsies make it possible to diagnose the lesion histologically without surgery. If a case is classified as belonging to the polypoid-solitary type and diagnosed as intraductal papilloma based on an intraductal biopsy, the nipple discharge will likely disappear within a few years and surgical resection need not be performed. We believe that follow-up is acceptable for the management of nipple discharge in these cases because single papillomas are less likely to progress to breast cancer than multiple papillomas [21, 22]. In contrast, lesions classified as multiple type or superficial type or atypical papillary lesions diagnosed using intraductal breast biopsies must be followed up carefully due to the association with malignancy. Although it has yet to be proven why nipple discharge disappears in intraductal papilloma cases, this phenomenon may be due to the nature of intraductal papillomas rather than the therapeutic potential of intraductal breast biopsies. We previously reported that papillomas frequently consist of various colored parts and contain areas of papillary, papillotubular or degenerated cells histologically [23]. If the components of papillomas change gradually from exhibiting papillary to degenerated characteristics, then the amount of discharge from the papilloma is likely to decrease, eventually resulting in the disappearance of the nipple discharge.
The endoscopic findings consist of elevated (polypoid) type lesions and/or superficial type lesions. Regarding elevated lesions, the number of lesions is important for differentiating whether the lesions are malignant or benign [13, 16]. We attempted to confirm whether a given lesion was a floating piece or an originating polypoid lesion using a Surflo catheter similar to a stick. We also attempted to confirm the presence of other lesions behind the occlusive polypoid lesion. With regard to superficial lesions, such lesions detected focally under the orifice of the duct can be considered artefacts arising from bougie operation. Meanwhile, focal superficial lesions in peripheral regions apart from the orifice are designated as the unclassified type rather than the superficial type. Only when a superficial spreading lesion is observed to have widely spread throughout the peripheral region, do we classify it as belonging to the superficial type.
Although superficial lesions accompanied by continuous luminal irregularity, redness and/or an erosive surface are easy to recognize, it can be difficult to detect such lesions in cases in which the vessels are hidden beneath the luminal surface. The visibility of vessels is often superior in cases involving a larger duct lumen that can be observed as a normal lumen with a shiny surface. The invisibility of vessels is difficult to detect, although this finding is consistent with low-papillary intraductal carcinoma, which includes cells with a high degree of cytological atypism [24]. Fortunately, superficial type cases tend to have positive findings on duct wash cytology.
In this study, the proportion of cases of breast cancer diagnosed after 6 months, resected benign lesions, discharge that continued and discharge that disappeared was 11.0, 9.4, 11.8 and 67.7 %, respectively. In addition, the proportions of the five types of endoscopic classification were compared, and the multiple lesions type and superficial type were found to be more likely to be diagnosed as breast cancer, while the no lesion and polypoid-solitary types were more likely to be associated with the disappearance of nipple discharge. Endoscopic classification is therefore useful for differentiating patients who should be subjected to intensive examinations and those who should expect disappearance of their discharge. This modality is also therefore to provide guidance for the management of patients with nipple discharge. In one study, because the gold standard for evaluating nipple discharge is a pathological examination, surgical resection was carried out immediately after mammary ductoscopy in order to assess the usefulness of endoscopic classification in Canada, although the authors failed to show the significance of endoscopic classification [25]. In that study, if the cases had been restricted to only resected cases, the polypoid-solitary type would have exhibited a higher frequency of malignancy, and the usefulness of differentiation would have diminished.
The number of diagnostic resections will likely decrease in the future due to the use of image-guided needle biopsies. The exception will be lesions such as intracystic papillomas located in the peripheral region, which must be removed surgically because they cannot be reached with a mammary ductoscope. Peripherally located intraductal lesions that cause bloody nipple discharge generally cannot be detected using mammary ductoscopy, although other modalities are useful in such cases, including ultrasonography, MRI and so on. Although mammary ductoscopy has a weak point in that it cannot reach peripheral lesions and could not detect lesions in 33.1 % of the cases, the number of diagnostic resections will decrease due to the use of mammary ductoscopy and watchful follow-up by repeatedly searching for other lesions in the peripheral region of the affected duct.
Missing clues for diagnosis can result in cancer progression. In fact, the proportion of invasive cancers diagnosed after 6 months from the initial mammary ductoscopy examination in the present study was slightly higher than that of breast cancers diagnosed within 6 months in this period (43/75, 57.3 % vs 71/154, 46.1 %). Therefore, caution must be exercised to ensure that lesions in the peripheral region of the affected duct are not missed. Furthermore, the majority of breast cancers diagnosed after 6 months in the current study were detected chiefly with ultrasonography. When nipple discharge continues, repeat examinations with mammary ductoscopy are also effective for providing a diagnosis. MRI is also effective in such cases due to its high sensitivity for detecting lesions [26]. Breast cancers presenting with nipple discharge are usually thought to be latent in the breast and in an initial stage of the disease. After spreading throughout the ducts, they form areas of palpable induration and ultimately large tumors. Although only three cases of breast cancer in the present study were diagnosed using intraductal biopsy only, with no findings on MMG, ultrasonography or MRI, between April 2005 and March 2008, all cases were DCIS. During the same period, 159 breast cancers presenting with nipple discharge were detected on ultrasonography or MRI. Among these lesions, the proportion of DCIS in patients with and without palpable induration or tumors was 45.5 and 35.4 %, respectively. Therefore, it is necessary to keep in mind that breast cancer presenting with nipple discharge should be diagnosed at an early stage before it forms a palpable tumor.
We conduct an intraductal biopsy to make the final histological diagnosis instead of surgical resection, unless the lesion is suspected to be malignant on another modality or based on the cytological examination. Intraductal biopsies were performed 897 times among 1,643 examinations in this study period, and the number of sampling procedures in which there was no material, insufficient material and sufficient material for diagnosis was 106, 116 and 675, respectively. Consequently, the probability of obtaining a histological diagnosis via intraductal biopsy was 75.3 % for the intended lesions. With respect to diagnosis via intraductal biopsy, the number of cancers, suspected malignancies, papillomas, atypical lesions and absence of malignant tissue was 79, 19, 477, 52 and 52, respectively, and benign intraductal papilloma was diagnosed in more than half of the 897 affected ducts with intraductal lesions. It is worth noting that most benign lesions are proven to be non-malignant without surgery, sparing patients the need for such procedures. However, the size of the intraductal sample is very small compared to that obtained with a core needle biopsy. Because the samples are picked up by suction, they cannot be identified with respect to their original location within the affected duct-lobular unit. Although the target lesion can be precisely identified using imaging during an image-guided needle biopsy and resected on demand, the diagnosis obtained with an intraductal biopsy reflects only the area of the discharge-related lesion, and a clinical diagnosis should be made after confirming that no other lesions are present in the peripheral region of the affected duct. In fact, 23 of the 257 initial intraductal biopsy samples that exhibited intraductal papilloma in this series were finally diagnosed as breast cancer, while four of the eight intraductal biopsy samples that suggested atypical papillary lesions were finally diagnosed as cancer.
In conclusion, mammary ductoscopy is valuable for diagnosis by allowing for the visualization of intraductal lesions, and intraductal biopsies make it possible to diagnose the lesion histologically without surgery in the outpatient setting. The information revealed on mammary ductoscopy is therefore useful for differentiating patients who should be subjected to intensive examinations and those who should expect disappearance of their discharge and is thought to help guide the management of nipple discharge. In cases involving a classification of no lesion or the polypoid-solitary type, it is likely that the nipple discharge will disappear within a few years, and surgical resection need not be performed. Hence, the number of diagnostic resections will likely decrease in the future with the advent of newer technology. At present, mammary ductoscopy and watchful follow-up can be substituted for microdochectomy in patients with bloody nipple discharge.
References
Cabioglu N, Hunt KK, Singletary SE, Stephens TW, Marcy S, Ross MI, et al. Surgical decision making and factors determining a diagnosis of breast carcinoma in women presenting with nipple discharge. J Am Coll Surg. 2003;196(3):354–64.
Dooley WC, Ljung B-M, Veronesi U, Cazzaniga M, Elledge RM, O’Shaughnessy JA, et al. Ductal lavage for detection of cellular atypia in women at high risk for breast cancer. J Natl Cancer Inst. 2001;93(21):1624–32.
Wrensch MR, Petrakis NL, King EB, Miike R, Mason L, Chew KL, et al. Breast cancer incidence in women with abnormal cytology in nipple aspirates of breast fluid. Am J Epidemiol. 1992;135(2):130–41.
Teboul M. A new concept inbreast investigation; echo-histological acino-ductal analytic echography. Biomed Pharmacother. 1988;42:289–96.
Makita M, Sakamoto G, Akiyama F, Namba K, Sugano H, Kasumi F, et al. Duct endoscopy and endoscopic biopsy in the evaluation of nipple discharge. Breast Cancer Res Treat. 1991;18:179–88.
Tang SS, Twelves DJ, Isacke CM, Gui GP. Mammary ductoscopy in the current management of breast disease. Surg Endosc. 2011;25(6):1712–22.
Hunerbein M, Schwarz LE, Schneider U, Schlag PM. Evaluation of pathologic nipple discharge with ductoscopy. J Am Coll Surg. 2003;197:697–8.
Shen RW, Wu J, Lu JS, Han QX, Shen ZZ, Nguyen M, et al. Fiberoptic ductoscopy for patients with nipple discharge. Cancer. 2000;89(7):1512–9.
Dominguez-Cunchillos F, Armendariz P, Perez-Cabanas I, Artieda C, Oteiza F, Sanz MA. Endoscopic technique for the localization of intraduct papillomas. Brit J Surg. 1999;86:1470–1.
Pereira B, Mokbel K. Mammary ductoscopy: past, present, and future. Int J Clin Oncol. 2005;10:112–6.
Al Sarakbi W, Salhab M, Mokbel K. Does mammary ductoscopy have a role in clinical practice? Int Semin Surg Oncol. 2006;3:16.
Dooley WC. Routine operative breast endoscopy during lumpedomy. Ann Surg Oncol. 2003;10:38–42.
Makita M, Namba K, Aoyama E, Mizutani M, Murata H, Yamao R, et al. Endoscopic diagnosis of intraductal lesions in patients with nipple discharge. Jpn J Breast Cancer. 1996;11(1):134–41 (in Japanese with English summary).
Dillon MF, Mohd Nazri SR, Nasir S, Mc Dermott EW, Evoy D, Crotty TB, et al. The role of major duct excision and microdochectomy in the detection of breast carcinoma. BMC Cancer. 2006;6:164. doi:10.1186/1471-2407-6-164.
Kamali S, Bender O, Kamali GH, Aydin MT. Karatepe, and Yuney E:Diagnostic and therapeutic value of ductoscopy in nipple discharge and intraductal proliferations compared with standard methods. Breast Cancer. 2014;21(2):154–61.
Makita M, Akiyama F, Gomi N, Ikenaga M, Yoshimoto M, Kasumi F, et al. Endoscopic classification of intraductal lesions and histological diagnosis. Breast Cancer. 2002;9(3):220–5.
Ashfaq A, Senior D, Pockaj BA, Wasif N, Pizzitola VJ, Giurescu ME, et al. Validation study of a modern treatment algorithm for nipple discharge. Am J Surg. 2014. doi:10.1016/j.amjsurg.2013.12.035.
Matsunaga T, Kawakami Y, Namba K, et al. Intraductal biopsy for diagnosis and treatment of intraductal lesions of the breast. Cancer. 2004;101(10):2164–9.
Nelson RS, Hoehn JL. Twenty-year outcome following central duct resection for bloody nipple discharge. Ann Surg. 2006;243(4):522–4.
Dupont WD, Page DL. Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med. 1985;312(3):146–51.
Haagensen CD (1986) Solitary intraductal papilloma, multiple intraductal papilloma. Disease of the breast, 3rd edn. Philadelphia: W.B. Saunders Co.; 1986. p. 136–191.
Ali-Fehmi R, Carolin K, Wallis T, Visscher DW. Clinicopathologic analysis of breast lesions associated with multiple papillomas. Hum Pathol. 2003;34:234–9.
Makita M, Akiyama F, Gomi N, Iwase T, Kasumi F, Sakamoto G. Endoscopic and histological findings of intraductal lesions presenting with nipple discharge. Breast J. 2006;12(s2):s210–7.
Makita M, Akiyama F, Kimura K, et al. Mammary ductoscopic diagnosis of intraductal spread of breast cancer. Jpn J Breast Cancer. 2001;16(3):274–8 (in Japanese with English summary).
Simpson JS, Connolly EM, Leong WL, Escallon J, McCready D, Reedijk M, et al. Mammary ductoscopy in the evaluation and treatment of pathologic nipple discharge: a Canadian experience. Can J Surg. 2009;52(6):E245–8.
Ballesio L, Maggi C, Savelli S, Angeletti M, De Felice C, Meggiorini ML, et al. Role of breast magnetic resonance imaging (MRI) in patients with unilateral nipple discharge: preliminary study. Radiol Med. 2008;113(2):249–64.
Acknowledgments
This study was supported by a Grant from Foundation for Promotion of Cancer Research.
Conflict of interest
No authors have any conflict of interest to declare in association with this study.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Makita, M., Akiyama, F., Gomi, N. et al. Mammary ductoscopy and watchful follow-up substitute microdochectomy in patients with bloody nipple discharge. Breast Cancer 23, 242–251 (2016). https://doi.org/10.1007/s12282-014-0561-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-014-0561-z