Abstract
Background
OncotypeDX® (ODX) is a well-validated assay for breast cancer treatment planning. We explored whether the conventional pathological factors could pick up high risk patients without the help of the ODX.
Methods
The ODX was performed on 139 hormone receptor-positive invasive breast cancers in a single Japanese institution. The recurrence risk was compared between the ODX and the St. Gallen Consensuses. The correlations were analyzed between the Recurrence Score (RS) measured by ODX and the pathological factors. In addition, we performed a follow-up survey and examined the association of the RS with the confirmed recurrence or death.
Results
The ODX classified 68 (49 %) as low RS, 52 (37 %) as intermediate RS, and 19 (14 %) as high RS cases. Correlations were noted between RS and progesterone receptor (PR) (r = −0.53), Ki-67 (r = 0.42), and nuclear grade (NG) (r = 0.41). None had a high RS with PR(3+) or NG1. Only one high RS patient had a Ki-67 (<20 %). The combinations of high RS with PR(0)/Ki-67 (≥20 %) and PR(1+)/Ki-67 (≥20 %) were 70 and 58 %, respectively. The combinations with high RS and PR(0)/NG3, PR(0)/NG2, and PR(1+)/NG3 were 83, 75, and 75 %, respectively. The median follow-up was 39.1 months (range 24.0–67.8). There were one low RS (1 %), four intermediate RS (8 %), and three high RS patients (16 %) who developed local or distant recurrence.
Conclusion
Hormone receptor-positive invasive breast cancers are stratified with the combinations of PR/Ki-67 or PR/NG. Some of the high recurrence risk cases might be identified without the ODX.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hormone receptor status is one of several clinicopathological tumor characteristics used for treatment planning and for assessing prognosis of early breast cancer. Hormone receptor-positive breast cancers generally do not benefit from chemotherapy. Only 15 % of patients with hormone receptor positive early breast cancers treated with tamoxifen alone recur over a 10-year period. Therefore, an estimated 85 % of these patients would be overtreated if adjuvant cytotoxic chemotherapy were universally administered [1]. The utilization of molecular genomic profiling has increased in recent years. Perou et al. [2] suggested that each breast cancer subtype might reflect intrinsic molecular differences in mammary epithelial biology. Sørlie et al. [3] suggested that the luminal epithelial estrogen receptor-positive group could be classified into at least two subgroups as defined by both hormone receptor and HER2 expression into luminal subtype A and luminal subtype B. Luminal A breast cancers have a low risk of relapse and luminal B breast cancers show a worse prognosis [4]. In addition, the clinical and pathologic response to chemotherapy is higher in the luminal B subtype than in the luminal A subtype [5]. For these reasons the distinction between luminal type breast cancers is of great clinical interest for treatment planning.
The OncotypeDX® (ODX) is a clinically validated, 21-gene assay that predicts both the likelihood of distant recurrence and the magnitude of adjuvant chemotherapy benefit for patients with hormone receptor-positive breast cancer [1, 6]. The St. Gallen Expert Consensus, the National Comprehensive Cancer Network, and the American Society of Clinical Oncology guidelines have all described the application of both pathological markers and genomic profiling for breast cancer management [7–9].
In this study, we compared the results of the ODX with those of the St. Gallen Conferences. We also investigated the relationship between the Recurrence Score (RS) measured by the ODX and commonly used pathological factors to assess whether high recurrence risk cases could be identified without the ODX. In addition we performed a follow-up survey in this cohort.
Patients and methods
From October 2007 to October 2010, the ODX assay was performed on 139 hormone receptor-positive invasive breast cancer patients in our institution. To confirm the prognostic value of the ODX results, the risk categories were compared with the well-known St. Gallen 2007, 2009, and 2011 Consensuses [7, 10, 11]. In the St. Gallen 2007 Consensus, the use of nuclear grade was allowed [10]. The pathological evaluation with nuclear grading has clinically been widespread in Japanese institutions and mentioned in “General Rules for Clinical and Pathological Recording of Breast Cancer”. We used the practical nuclear grading for the St. Gallen 2009 instead of histological grading. Second, the correlations between the RS and the conventional pathological factors were analyzed. The pathological factors consisted of tumor size (T), lymph node metastasis (N), nuclear grade (NG), lymphatic and vascular invasion (LI, VI), estrogen receptor (ER), progesterone receptor (PR), HER2, and Ki-67. The expression of ER and PR was measured with the Allred score. In brief, an Allred score 0 or 2 equated to 0, score 3 or 4 to 1+, score 5 or 6 to 2+, and score 7 or 8 to 3+. HER2 expression was evaluated by the HercepTest (Dako, Glostrup, Denmark). Ki-67 was identified with the MIB-1 antibody (Dako, Glostrup, Denmark) and was automatically scored with an Ariol-SL50 instrument (Applied Imaging) at Genetic Laboratory Co., Hokkaido, Japan. In brief, Ariol-SL50 was set up to remove stromal cells, inflammation cells by the nuclear shape and size. The intraductal lesion was excluded from the counting area. Ki-67 was calculated as the ratio of Ki-67-positive cancer cells to total cancer cells. The measurement counted more than 1,000 cancer cells/spot and was performed at 5 hot spots. The Ki-67 labeling index was calculated by the average of 5 hot spots. In addition we used the same tissue sections to examine Ki-67 and ODX. The cutoff value of Ki-67 was 14 % according to the St. Gallen 2011 Conference [11]. However, the Ki-67 staining and counting methods are different in each institution. A Ki-67 cutoff value of 20 % was the most approved of the St. Gallen 2013 expert panels for defining luminal B subtype [12]. In this study we adopted the practical and simple cutoff value of 20 %. The pathological diagnosis was performed under the supervision of one experienced pathologist (K.S.).
The patient characteristics are summarized in Table 1. The ages ranged from 25 to 73 years with a mean of 50 years. The numbers of premenopausal and postmenopausal patients were 82 (59 %) and 57 (41 %), respectively. Mastectomy specimens were available for 134 patients (96 %), and core biopsy samples were used for the others. Eighty patients (58 %) had tumors less than 2 cm in diameter. Eighty-three patients (60 %) had negative axillary nodes, 12 patients (9 %) had isolated tumor cells (ITC), five patients (4 %) had micrometastasis (pN1mi), and 34 patients (24 %) were pN1. Five patients (4 %) had more than four positive nodes. Sixty patients (43 %) were NG1, 44 patients (32 %) were NG2, and 35 patients (25 %) were NG3. Seventy-three patients were LI0 (53 %), and 122 patients were VI0 (88 %). In terms of the biological markers, 120 (86 %) women were ER(3+) and 79 (57 %) were PR(3+). Only one patient (1 %) had HER2 overexpression. Fifty-one patients (37 %) had low Ki-67 expression (<20 %) and 88 patients (63 %) had high Ki-67 expression (≥20 %).
In 68 low RS cases, 67 patients were treated with adjuvant hormonal therapy alone and one patient received no treatment. In 52 intermediate RS cases, 15 patients were treated with adjuvant chemotherapy followed by hormonal therapy and the others received hormonal therapy alone. In 19 high RS cases, all patients were treated with adjuvant chemotherapy followed by hormonal therapy.
Spearman rank correlation coefficients were calculated. When the r was >0.4 or <−0.4 for two factors, they were considered correlated. Kaplan–Meier analysis was used to calculate and visually display disease free survival curves; a log-rank test was used to compare curves. These analyses were performed with StatView for Windows version 5 and IBM SPSS Statistics version 20.
Results
RS and the St. Gallen Conferences
The ODX assay revealed 68 (49 %) low RS cases, 52 (37 %) intermediate RS cases, and 19 (14 %) high RS cases. The comparison between the St. Gallen 2007 and RS is described in Fig. 1a. Nearly all of the cases in the intermediate risk group were incorrectly classified under the St. Gallen criteria. The comparison between the St. Gallen 2009 and RS is shown in Fig. 1b. We used the practical nuclear grading for the evaluation of tumor proliferation, but histological grading was only mentioned in the St. Gallen 2009 Consensus. HT denotes a relative indication for hormonal therapy alone. CT denotes a relative indication for chemotherapy. The high RS cases were appropriately classified into the HT + CT group; however, some low RS cases were also included in this group. The comparison between the St. Gallen 2011 and RS is shown in Fig. 1c. Most of the high RS cases were included in the luminal B group; however, some low RS cases were also classified into the luminal B group.
a Comparison between St. Gallen 2007 and RS. Nearly all of the cases in the intermediate risk group were incorrectly classified under the St. Gallen criteria. b Comparison between St. Gallen 2009 and RS. We used the practical nuclear grading for the evaluation of tumor proliferation, but histological grading was only mentioned in the St. Gallen 2009 Consensus. HT denotes a relative indication for hormonal therapy alone. CT denotes a relative indication for chemotherapy. The high RS cases were appropriately classified into the HT + CT group. However, some low RS cases were also included in this group. c Comparison between St. Gallen 2011 and RS. Most of the high RS cases were included in the luminal B group. However, some low RS cases were also classified into the luminal B group
RS and pathological factors
PR and RS were negatively correlated (r = −0.53). No high RS had PR(3+) (Fig. 2a). Correlations between RS and Ki-67 or NG were also identified (r = 0.42 and 0.41, respectively) (Fig. 2b, c). There was no high RS patient with NG1. There was only one high RS patient (2 %) with Ki-67 (<20 %). On the other hand, the correlations with T, N, LI, VI, ER, and HER2 were weaker (r ranging from −0.33 to 0.22) (Table 2).
a Correlation between PR and RS was negatively correlated with RS (r = −0.53). No patients had high RS with PR(3+). b A correlation between Ki-67 and RS was observed (r = 0.42). Only one patient (2 %) had high RS with Ki-67 (<20 %). c Correlation between NG and RS was observed (r = 0.41). There was no high risk patient in the NG1
PR and Ki-67 in the high RS cases
The rate of high RS with PR(0) and Ki-67 (≥20 %) was 70 %, that of PR(1+) and Ki-67 (≥20 %) was 58 %, and that of PR(2+) and Ki-67 (≥20 %) was 21 % (Fig. 3a).
a PR and Ki-67 in the high RS cases. The rate of high RS with PR(0) and Ki-67 (≥20 %) was 70 %, that of PR(1+) and Ki-67 (≥20 %) was 58 %, and that of PR(2+) and Ki-67 (≥20 %) was 21 %. b PR and NG in the high RS cases. Among the high RS cases, the rate of PR(0) and NG3 was 83 %, that of PR(0) and NG2 was 75 %, and that of PR(1+) and NG3 was 75 %. There was no high RS patient with PR(3+) or NG1
PR and NG in the high RS cases
Among the high RS cases, the rate of PR(0) and NG3 was 83 %, that of PR(0) and NG2 was 75 %, and that of PR(1+) and NG3 was 75 %. There was no high RS patient with PR(3+) or NG1 (Fig. 3b).
Disease free survival
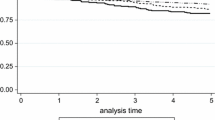
The median follow-up for all patients after the operation was 39.1 months (range 24.0–67.8). Kaplan–Meier curves for disease free survival of the St. Gallen Consensuses are shown in Fig. 4. There was no recurrence case in the low risk group of the St. Gallen 2007, the HT group of the St. Gallen 2009 and the luminal A group of the St. Gallen 2011 (a log-rank test was not available). There were one low RS (1 %, RS = 17), four intermediate RS (8 %, RS = 28, 25, 24, and 19), and three high RS patients (16 %, RS = 48, 46, and 33) who developed local or distant recurrence (Fig. 4d). Of these cases only one high RS patient (RS = 46) was dead 9 months following surgery as a result of multiple bone and lung metastases.
Discussion
Cheang et al. [13] reported that breast cancers could, in clinical practice, be classified into subtypes based on the immunohistochemical (IHC) evaluation of ER, PR, HER2, and Ki-67. They also defined the cutoff value of the Ki-67 labeling index at 13.25 % to classify the luminal type breast cancers. Cuzick [14] also reported that these four IHC biomarkers (IHC4 score) provide prognostic information, which could be considered at least equivalent to the RS. By using these IHC biomarkers many physicians risk stratify each patient and plan treatment on the basis of risk and biomarkers.
Molecular genomic profiling is integral to the postoperative treatment planning of breast cancer patients. Eighty-four percent of the Expert Panel of the 12th St. Gallen Consensus Meeting approved the ODX to predict the effectiveness of adjuvant chemotherapy in hormone receptor-positive disease. In this study, we compared the risk classification of the St. Gallen Conferences with the ODX and found that the St. Gallen Consensuses were of limited usefulness for the risk classification of the luminal B subgroup because the treatment strategies were not suitable for all patients. The St. Gallen Consensuses require further refinement in order to prevent over- and undertreatment of luminal B breast cancer patients.
Most molecular profiling assays are costly and generally not covered by medical insurance, which poses a barrier to universal adoption. Klein et al. [15] reported that the use of pathology-generated equations could be used to estimate the RS for breast cancer patients. Ingoldsby et al. [16] also suggested that the combinations of traditional pathological parameters and biomarkers corresponding to 10 genes (ER, PR, Ki-67, HER2, BCL2, CD68, aurora A kinase, surviving, cyclin B1, and BAG1) could be used as an alternative to the RT-PCR assay to reduce the number of patients that need further analysis by the ODX. In light of their studies we assessed whether commonly used pathological factors could substitute for the RS. Our results indicated that the RS was moderately correlated with PR, Ki-67, and NG. Cancello et al. [17] reported that the ER(+)/PR(−)/HER2(−) subgroup was associated with a reduced breast cancer-related survival and overall survival when compared with the ER(+)/PR(+)/HER2(−) subgroup. They concluded that the loss of PR identified luminal B breast cancer subgroups at higher risk of relapse and death, both with HER2-positive and HER2-negative disease. Kurebayashi et al. [18] indicated that hormonal therapy alone could not prevent distant metastasis with PR-negative breast cancers and/or with cancers showing marked lymphovascular invasion or high Ki-67 labeling index in a Japanese multi-institute cohort study. The significance of IHC assessment of PR was also emphasized in the St. Gallen International Breast Cancer Conference 2013 [12]. Prat et al. [19] reported that the new proposed IHC-based definition of luminal A tumors was hormone receptor-positive/HER2-negative/Ki-67 less than 14 % and PR more than 20 %. With respect to hormonal therapy, the ER(+)/PR(+) subgroup shows a better response to selective ER modulator therapy than ER(+)/PR(−) cancers. PR is a marker of a functional ER, and the expression of PR approximates ER activity. In addition, it has been suggested that the absence of PR may reflect hyperactive cross talk between ER and growth factor signaling pathways [20]. These observations increase the value of PR in the risk stratification of hormone receptor-positive breast cancer.
Both NG and Ki-67 are proliferation factors. Cancer cells express Ki-67 during the G1, S, G2, and M phases, but not during the resting phase G0. In particular, the expression level is low in the G1 and S phases and peaks in mitosis [21]. Nuclear grade is defined as the sum of both nuclear atypia and mitotic count. For these reasons, NG correlates with Ki-67 expression. Ki-67 is widely used to risk stratify breast cancer [13]; however, our data failed to show a perfect correlation between Ki-67 and the RS, suggesting that Ki-67 itself is insufficient for risk stratification. We combined PR and Ki-67 or PR and NG and found that this combination of factors resulted in comparable risk stratification as obtained with ODX. In contrast, N, T, LI, and VI, which are also highly prognostic clinical factors in early breast cancers, did not correlate with the RS in this study. With regard to lymph node metastasis, it was reported that the routine use of IHC to look for low volume metastasis was not indicated, because the presence of micrometastasis did not change management [11]. We also consider that lymph node metastasis is not so fatal to hormone receptor-positive breast cancers because lymph node status was poorly relative to RS (r = −0.193). Although the node-positive postmenopausal patients are eligible for the ODX, the node-positive premenopausal patients are not; however, 20 node-positive premenopausal cases (24 %) are actually included in this study. We should ascertain the eligibility of the ODX for the premenopausal node-positive breast cancer patients in the RxPONDER trial (SWOG S1007), which is an ongoing clinical trial designed to address this question.
This study was limited in terms of generalizability by the selection of patients from a single institution. All cases, however, were reviewed and analyzed by a single pathologist, which resulted in consistent scoring.
We propose that the combinations of PR/NG or PR/Ki-67 be used to select patients for further risk stratification via ODX.
Conclusions
Hormone receptor-positive invasive breast cancers are stratified with the combinations of PR/Ki-67 or PR/NG. Some of the high recurrence risk cases might be identified without the ODX.
References
Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–26.
Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–52.
Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–74.
Parker JS, Mullins M, Cheang MC, Leung S, Voduc D, Vickery T, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27:1160–7.
Carey LA, Dees EC, Sawyer L, Gatti L, Moore DT, Collichio F, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13:2329–34.
Paik S, Tang G, Shak S, Kim C, Baker J, Kim W, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24:3726–34.
Goldhirsch A, Ingle JN, Gelber RD, Coates AS, Thürlimann B, Senn HJ, et al. Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer. Ann Oncol. 2009;20:1319–29.
NCCN. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology™. Breast Cancer. 2011; NCCN® Practice Guidelines in Oncology. Version 2.
Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25:5287–312.
Goldhirsch A, Wood WC, Gelber RD, Coates AS, Thürlimann B, Senn HJ. Progress and promise: highlights of the international expert consensus on the primary therapy of early breast cancer 2007. Ann Oncol. 2007;18:1133–44.
Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn HJ, et al. Strategies for subtypes-dealing with the diversity of breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736–47.
Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013;24:2206–23.
Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009;101:736–50.
Cuzick J, Dowsett M, Pineda S, Wale C, Salter J, Quinn E, et al. Prognostic value of a combined estrogen receptor, progesterone receptor, Ki-67, and human epidermal growth factor receptor 2 immunohistochemical score and comparison with the Genomic Health Recurrence Score in early breast cancer. J Clin Oncol. 2011;29:4273–8.
Klein ME, Dabbs DJ, Shuai Y, Brufsky AM, Jankowitz R, Puhalla SL, et al. Prediction of the Oncotype DX Recurrence Score: use of pathology-generated equations derived by linear regression analysis. Mod Pathol. 2012;26:658–64.
Ingoldsby H, Webber M, Wall D, Scarrott C, Newell J, Callagy G. Prediction of Oncotype DX and TAILORx risk categories using histopathological and immunohistochemical markers by classification and regression tree (CART) analysis. Breast. 2013;22:879–86.
Cancello G, Maisonneuve P, Rotmensz N, Viale G, Mastropasqua MG, Pruneri G, et al. Progesterone receptor loss identifies luminal B breast cancer subgroups at higher risk of relapse. Ann Oncol. 2013;24:661–8.
Kurebayashi J, Kanomata N, Shimo T, Yamashita T, Aogi K, Nishimura R. Marked lymphovascular invasion, progesterone receptor negativity, and high Ki67 labeling index predict poor outcome in breast cancer patients treated with endocrine therapy alone. Breast Cancer. 2012. doi:10.1007/s12282-012-0380-z.
Prat A, Cheang MC, Martin M, Parker JS, Carrasco E, Caballero R. Prognostic significance of progesterone receptor-positive tumor cells within immunohistochemically defined luminal A breast cancer. J Clin Oncol. 2013;31:203–9.
Cui X, Schiff R, Arpino G, Osborne CK, Lee AV. Biology of progesterone receptor loss in breast cancer and its implications for endocrine therapy. J Clin Oncol. 2005;23:7721–35.
Yerushalmi R, Woods R, Ravdin PM, Hayes MM, Gelmon KA. Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol. 2010;11:174–83.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Onoda, T., Yamauchi, H., Yagata, H. et al. The value of progesterone receptor expression in predicting the Recurrence Score for hormone-receptor positive invasive breast cancer patients. Breast Cancer 22, 406–412 (2015). https://doi.org/10.1007/s12282-013-0495-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-013-0495-x