Abstract
Background
To define the factors associated with increased risk of isolated locoregional failure that may justify postmastectomy radiotherapy in patients with T1/2 breast cancer and 1–3 positive lymph nodes.
Methods
Between 1990 and 2002, 248 patients who had pT1–2 breast cancer and 1–3 positive lymph nodes were treated with mastectomy without radiotherapy (age 32–84, median 54).
Results
Median follow-up time was 82 months (range 2–189 months). For all patients, the 8-year isolated locoregional failure-free rate was 95 %. In univariate analysis, hormone receptor status and administration of hormone therapy were statistically significant factors, and vascular invasion was the borderline significant factor for isolated locoregional failure-free rates (P = 0.0377, 0.0181, and 0.0555, respectively). The 8-year isolated locoregional failure-free rates were 98 % for patients with positive hormone receptor status and 90 % for patients with negative hormone receptor status, 97 % for patients who received hormone therapy and 89 % for patients who did not receive hormone therapy, 92 % for patients with vascular invasion and 97 % for patients without vascular invasion. In multivariate analysis for hormone receptor status and vascular invasion, the former was statistically significant (P = 0.0491) and the latter was borderline significant (P = 0.0664). When patients had both negative hormone receptor and positive vascular invasion status, the 8-year isolated locoregional failure-free rates decreased to 83 %.
Conclusions
With regard to patients who had pT1/2 breast cancer and 1–3 positive lymph nodes, isolated locoregional failure was not common in general; however, patients who had both negative hormone receptor status and vascular invasion were comparatively high-risk patients for isolated locoregional failure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Postmastectomy radiation therapy (PMRT) improves locoregional control and disease-free and overall survival rates in patients with high-risk breast cancer [1–4]. In meta-analysis of PMRT randomization trials, not only a reduction of locoregional failure but also survival benefit was observed in the group that received PMRT [5, 6].
American Society of Clinical Oncology (ASCO) guidelines recommend PMRT only for patients with four or more positive axillary lymph nodes, patients who have T3 tumors with positive axillary nodes, and patients with operable stage III tumors [7]. According to the ASCO guidelines, there is insufficient evidence to make recommendations or suggestions for the routine use of PMRT in patients with T1/2 breast cancer and 1–3 positive lymph nodes. On the other hand, according to National Comprehensive Cancer Network (NCCN) guidelines, PMRT should be considered also for patients with T1/2 breast cancer and 1–3 positive lymph nodes [8]. On the basis of previous reports with large sample size, the 10-year locoregional failure rates of patients who had T1/2 breast cancer and 1–3 positive lymph nodes and received mastectomy without PMRT were 4.3–12.7 % [9, 10]. The St. Gallen consensus conference recommended PMRT for women with a 20 % or greater risk of locoregional failure [11]. On the basis of this consensus, this percentage may be too small to justify the routine use of PMRT. In addition, a study based on the Surveillance, Epidemiology, and End Results program demonstrated that PMRT reduced mortality only when the number of positive lymph nodes was seven or more with regard to patients with T1/2 breast cancer [12]. However, some authors reported that there were subgroups with comparatively high risk of locoregional failure after mastectomy without PMRT even in patients who had pT1–2 breast cancer and 1–3 positive lymph nodes [9, 10, 13–15]. In addition, PMRT seemed to be useful for the reduction of locoregional failure in these high-risk subgroups [13, 14].
Identification of the factors associated with increased risk of isolated locoregional failure that may justify PMRT (around 20 % or greater risk of locoregional failure) in patients with T1/2 breast cancer and 1–3 positive lymph nodes is useful to establish the indication of PMRT. Because of the lack of Japanese data concerning this issue, we examined the incidence of isolated locoregional failure and assessed risk factors for isolated locoregional failure with regard to Japanese women who had pT1/2 breast cancer and 1–3 positive lymph nodes and received mastectomy without PMRT.
Materials and methods
Between 1990 and 2002, 1271 female patients with untreated unilateral stage 0–III breast cancer were treated with mastectomy in the National Hospital Organization Shikoku Cancer Center. Before 2003, adjuvant therapy for high-risk patients was mainly chemotherapy with or without hormone therapy. PMRT was rarely performed even for the patients with four or more positive lymph nodes and/or with a primary tumor of pT3–4 in our institution before 2003. Only 15 of 1271 (1.2 %) patients received PMRT. Fifteen patients who received PMRT and 80 patients whose sufficient information of histopathologic feature or clinical course was not available (60 patients and 20 patients, respectively) were excluded. Among the 1176 evaluable patients, 248 patients had pT1/2 breast cancer and 1–3 positive lymph nodes (age 32–84, median 54) and were reviewed in this study (Table 1). The number of removed lymph node ranged from 6 to 37 (median 17). Except for three patients (1 %), the number of removed lymph nodes was seven or more.
In the evaluation of hormone receptor, the titers of hormone receptor including estrogen receptor (ER) and progesterone receptor (PgR) were analyzed by enzyme immunoassay methods. When titers of ER and PgR were less than 5 fmol/mg protein, the tumor was determined hormone receptor negative. Patients with negative hormone receptor status often received hormone therapy in the 1990s in our institution. Among 72 patients with negative hormone receptor status, 36 patients received hormone therapy.
Evaluated factors were pT (pT1 vs. pT2), age (≤40 vs. >40 years), lymphatic invasion (positive vs. negative), vascular invasion (positive vs. negative), nuclear grade (G3 vs. G1–2/unknown), hormone receptor status (negative for both ER and PgR vs. positive for ER and/or PgR, unknown hormone receptor status), percentage of positive lymph nodes (positive lymph nodes/dissected lymph nodes <10 vs. ≥10 %), administration of adjuvant chemotherapy (yes vs. no/unknown), and administration of adjuvant hormone therapy (yes vs. no/unknown). Concerning the percentage of positive lymph nodes, 25 % was used as the cutoff point between the higher-rate group and the lower-rate group in the previous representative reports [9, 15]; however, the number of patients whose percentage of positive lymph nodes was 25 % or higher was only five (2 %) in our series. This was due to the comparatively large number of removed lymph nodes (median 17) in our institution. Because the median percentage of positive lymph nodes was 8 %, we used 10 % as the cutoff point in our present study. Because HER2 overexpression was not examined routinely in the 1990s in our institution, the factor of HER2 overexpression could not be assessed in this study.
Statistical analysis
Isolated locoregional failure was defined as initial failure in the chest wall and/or regional lymph nodes. When distant metastases were pointed out within 3 months after locoregional failure as the initial failure, the failure pattern was classified as distant failure. Isolated locoregional failure-free rates, distant failure-free rates, and failure-free survival rates were calculated by the Kaplan–Meier method. Univariate analysis was performed by log-rank test, and multivariate analysis was performed by Cox’s proportional hazard model.
Results
Follow-up time ranged from 2 to 189 months (median 82 months). The percentage of positive lymph nodes (the ratio of the number of positive lymph nodes to the number of dissected lymph nodes) ranged from 3 to 33 % (median 8 %).
During the follow-up time, isolated locoregional failure as the initial failure occurred in 10 patients, and distant failure (with or without locoregional failure) as the initial failure occurred in 25 patients.
For all 248 patients, the 8-year isolated locoregional failure-free rate was 95 % (Fig. 1). The 8-year isolated locoregional failure-free rates and results of univariate analysis are shown in Table 2. In univariate analysis, hormone receptor status (P = 0.0377) and administration of hormone therapy (P = 0.0181) were statistically significant factors for isolated locoregional failure-free rates. Vascular invasion status was a borderline significant factor for isolated locoregional failure-free rates (P = 0.0555). The 8-year isolated locoregional failure-free rates were 98 % for patients with positive/unknown hormone receptor status and 90 % for patients with negative hormone receptor status (Fig. 2), and 97 % for patients who received hormone therapy and 89 % for patients who did not receive hormone therapy, and 92 % for patients with vascular invasion and 97 % for patients without vascular invasion.
Among statistically significant or borderline significant factors on univariate analysis, hormone receptor status and administration of hormone therapy overlapped largely. Therefore, hormone receptor status and vascular invasion status were assessed in multivariate analysis. In multivariate analysis, hormone receptor status was the only statistically significant independent factor for isolated locoregional failure (P = 0.0491). Vascular invasion was a borderline significant factor (P = 0.0664) (Table 3).
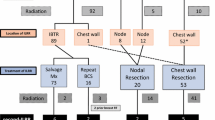
In our patients, the combination of these risk factors on multivariate analysis increased the risk of isolated locoregional failure. The 8-year isolated locoregional failure-free rate decreased to 83 % for patients with both negative hormone receptor and positive vascular invasion status (n = 22), whereas it was 97 % for other patients (n = 226) (Fig. 3).
With regard to distant failure-free rates, pT was the only statistically significant factor on univariate analysis (pT1, 97 % at 8 years; pT2, 84 % at 8 years; P = 0.0066). Vascular invasion status (negative, 91 % at 8 years; positive, 84 % at 8 years; P = 0.0613) and administration of hormone therapy (yes, 95 % at 8 years; no, 87 % at 8 years; P = 0.0560) were borderline significant factors on univariate analysis. The 8-year distant failure-free rates did not differ among factors: age (P = 0.5076), lymphatic invasion status (P = 0.0914), nuclear grade (P = 0.8897), hormone receptor status (P = 0.9222), percentage of positive lymph nodes (P = 0.6420), and chemotherapy (P = 0.2428). The 8-year distant failure-free rates were 90, 88, and 87 % for patients with one, two, and three positive lymph nodes (P = 0.5512).
Among the statistically significant factor and borderline significant factors on univariate analysis, pT was the only statistically significant independent factor for distant failure-free rates on multivariate analysis (pT, P = 0.0252; hormone therapy, P = 0.1066; vascular invasion, P = 0.3834). Although the combination of negative hormone receptor and positive vascular invasion status decreased the isolated locoregional failure-free rates, it did not increase the distant failure significantly. The 8-year distant failure-free rates were 89 % for patients with both negative hormone receptor and positive vascular invasion status (n = 22), and 89 % for other patients (n = 226) (P = 0.8716).
Discussion
Indication of PMRT for patients who had pT1/2 breast cancer and 1–3 positive lymph nodes is still controversial issue. As previously reported, the incidence of locoregional failure after mastectomy without PMRT was comparatively low in these patients. In recent reports from the M. D. Anderson Cancer Center, the 10-year locoregional failure rate of patients who had T1/2 breast cancer and 1–3 positive lymph nodes was 4.3 % [10]. In our series, the 8-year isolated locoregional failure rate for all 248 patients was 5 %. On the basis of these results, the routine use of PMRT for all patients who had T1/2 breast cancer and 1–3 positive lymph nodes could not be justified because of the low incidence of locoregional failure. However, on the basis of recent studies, moderately high-risk subgroups for locoregional failure seemed to exist in patients who had T1/2 breast cancer and 1–3 positive lymph nodes. With regard to patients who had T1/2 breast cancer and 1–3 positive lymph nodes, various clinical and pathologic risk factors for locoregional failure (negative hormone receptor status [9, 13, 14], younger age (<40 or <45 years) [9, 10], ratio of positive lymph nodes to dissected lymph nodes >25 % [9, 15], existence of lymphovascular invasion [14], pT2 [13], two or three positive lymph nodes (compared to one positive node) [13], and a medial tumor location [9]) were reported.
On the basis of our results, negative hormone receptor status (negative for both ER and PgR) and no hormone therapy were statistically significant risk factors for isolated locoregional failure on univariate analysis. Negative hormone receptor status was also identified as a risk factor for locoregional failure in some previous reports [9, 13, 14]. On the basis of these reports and our results, hormone receptor-associated factors seemed to be the important risk factors for locoregional failure in patients with pT1/2 breast cancer and 1–3 positive lymph nodes. However, the incidence of isolated locoregional failure of patients with negative hormone receptor status and patients who did not receive hormone therapy was not high enough to justify the routine use of PMRT in these patients. In our series, 8-year isolated locoregional failure rates were 10 % for patients with negative hormone receptor status and 11 % for patients who did not receive hormone therapy. Although hormone receptor-associated factors were significant for isolated locoregional failure in patients with T1/2 breast cancer and 1–3 positive lymph nodes, it seemed that the hormone receptor-associated factor alone did not cause frequent isolated locoregional failure in patients with T1/2 breast cancer and 1–3 positive lymph nodes (approximately 10 % at 8 years). However, when negative hormone receptor status was combined with positive vascular invasion status, the 8-year isolated locoregional failure rate increased to 17 %. Among patients who had T1/2 breast cancer and 1–3 positive lymph nodes, patients who had both negative hormone receptor and positive vascular invasion status seemed to be potential candidates for PMRT.
Although it has been reported that younger age, high percentage of positive lymph nodes, and a larger primary tumor were associated with locoregional failure, these factors were not statistically significant risk factors in our series. In addition, the incidence of isolated locoregional failure in patients with previously reported risk factors was low in our patients. The 8-year isolated locoregional failure-free rate of patients with pT2 breast cancer, patients with three positive lymph nodes, patients 40 years or younger, patients with grade 3 tumors, and patients with a higher percentage of positive lymph nodes (≥10 %) were 94, 95, 100, 95, and 94 %, respectively. Because of the small number of patients studied, further studies are necessary to determine the comparatively high-risk subgroup in patients with pT1/2 breast cancer and 1–3 positive lymph nodes.
Among the assessed factors in our present study, only pT2 (vs. pT1) was a risk factor for distant failure. Other assessed factors including administration of hormone therapy and vascular invasion status were not statistically significant independent factors for the distant failure-free rates. In addition, the combination of negative hormone receptor and positive vascular invasion status did not increase the distant failure significantly in patients who had pT1/2 breast cancer and 1–3 positive lymph nodes. It seemed that the high-risk subgroup for isolated locoregional failure differed from the high-risk subgroup for distant failure in patients with pT1/2 breast cancer and 1–3 positive lymph nodes. A high incidence of distant failure may deteriorate the benefit from reduction of locoregional failure. Previous reports indicated that survival benefit of PMRT is not necessarily large in patients with high risk of both locoregional failure and distant failure [16, 17]. Therefore, prevention of locoregional failure may be beneficial especially for the patients who had both negative hormone receptor and positive vascular invasion status.
At present, indication of PMRT is mainly determined by the number of positive lymph nodes and the T factor. As a result, patients who had pT1/2 breast cancer and 1–3 positive lymph nodes often do not receive PMRT. However, isolated locoregional failure might not be negligible when patients have both factors of negative hormone receptor and positive vascular invasion status. Further studies are needed to assess the usefulness of PMRT in patients who have pT1/2 breast cancer with 1–3 positive lymph nodes and a combination of negative hormone receptor and positive vascular invasion status.
Unfortunately, we could not assess the overall survival in patients with pT1/2 breast cancer and 1–3 positive lymph nodes. The lack of analysis of overall survival was one of the limitations of our present study. Further studies are necessary to determine the survival benefit of PMRT in the subgroup with a combination of negative hormone receptor and positive vascular invasion status in patients who had pT1/2 breast cancer and 1–3 positive lymph nodes.
In conclusion, the routine use of PMRT in patients who had pT1/2 breast cancer and 1–3 positive lymph nodes could not be justified because of the comparatively low incidence of isolated locoregional failure. However, the incidence of isolated locoregional failure was comparatively high in patients with a combination of negative hormone receptor and positive vascular invasion status.
References
Overgaard M, Hansen PS, Overgaard J, Rose C, Andersson M, Bach F, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med. 1997;337:949–55.
Overgaard M, Jensen MB, Overgaard J, Hansen PS, Rose C, Andersson M, et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen. Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet. 1999;353:1641–8.
Ragaz J, Olivotto IA, Spinelli JJ, Phillips N, Jackson SM, Wilson KS, et al. Locoregional radiation therapy in patients with high-risk breast cancer receiving adjuvant chemotherapy: 20-year results of the British Columbia randomized trial. J Natl Cancer Inst. 2005;97:116–26.
Whelan TJ, Julian J, Wright J, Jadad AR, Levine ML. Does locoregional radiation therapy improve survival in breast cancer? A meta-analysis. J Clin Oncol. 2000;18:1220–9.
Overgaard M. Overview of randomized trials in high risk breast cancer patients treated with adjuvant systemic therapy with or without postmastectomy irradiation. Semin Radiat Oncol. 1999;9:292–9.
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomized trials. Lancet. 2005;366:2087–106.
Recht A, Edge SB, Solin LJ, Robinson DS, Estabrook A, Fine RE, et al. Postmastectomy radiotherapy: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2001;19:1539–69.
NCCN Clinical Practice Guidelines in Oncology. Version 1.2012. Breast Cancer. http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed 25 March 2012.
Truong PT, Olivotto IA, Kader HA, Panades M, Speers CH, Berthelet E, et al. Selecting breast cancer patients with T1–T2 tumors and one to three positive axillary nodes at high postmastectomy locoregional recurrence risk for adjuvant radiotherapy. Int J Radiat Oncol Biol Phys. 2005;61:1337–47.
Sharma R, Bedrosian I, Lucci A, Hwang RF, Rourke LL, Qiao W, et al. Present-day locoregional control in patients with t1 or t2 breast cancer with 0 and 1 to 3 positive lymph nodes after mastectomy without radiotherapy. Ann Surg Oncol. 2010;17:2899–908.
Goldhirsch A, Glick JH, Gelber RD, Senn HJ. Meeting highlights: international consensus panel on the treatment of primary breast cancer. J Natl Cancer Inst. 1998;90:1601–8.
Smith BD, Smith GL, Haffty BG. Postmastectomy radiation and mortality in women with T1–2 node-positive breast cancer. J Clin Oncol. 2005;23:1409–19.
Wu SG, He ZY, Li FY, Wang JJ, Guo J, Lin Q, et al. The clinical value of adjuvant radiotherapy in patients with early stage breast cancer with 1 to 3 positive lymph nodes after mastectomy. Chin J Cancer. 2010;29:668–76.
Yang PS, Chen CM, Liu MC, Jian JM, Horng CF, Liu MJ, et al. Radiotherapy can decrease locoregional recurrence and increase survival in mastectomy patients with T1 to T2 breast cancer and one to three positive nodes with negative estrogen receptor and positive lymphovascular invasion status. Int J Radiat Oncol Biol Phys. 2010;77:516–22.
Truong PT, Berthelet E, Lee J, Kader HA, Olivotto IA. The prognostic significance of the percentage of positive/dissected axillary lymph nodes in breast cancer recurrence and survival in patients with one to three positive axillary lymph nodes. Cancer. 2005;103:2006–14.
Overgaard M, Nielsen HM, Overgaad J. Is the benefit of postmastectomy irradiation limited to patients with four or more positive nodes, as recommended in international consensus reports? A subgroup analysis of the DBCG 82 b&c randomized trials. Radioter Oncol. 2007;82:247–53.
Kyndi M, Overgaard M, Nielsen HM, Sørensen FB, Knudsen H, Overgaard J. High local recurrence risk is not associated with large survival reduction after postmastectomy radiotherapy in high-risk breast cancer: a subgroup analysis of DBCG 82 b&c. Radioter Oncol. 2009;90:74–9.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Hamamoto, Y., Ohsumi, S., Aogi, K. et al. Are there high-risk subgroups for isolated locoregional failure in patients who had T1/2 breast cancer with one to three positive lymph nodes and received mastectomy without radiotherapy?. Breast Cancer 21, 177–182 (2014). https://doi.org/10.1007/s12282-012-0369-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-012-0369-7