Abstract
Background
Malignant phyllodes tumor of the breast is a rare neoplasm for which clinical findings remain insufficient for determination of optimal management. We examined the clinical behavior of these lesions in an attempt to determine appropriate management. We evaluated long-term outcome and clinical characteristics of malignant phyllodes tumors arising from fibroadenomas of the breast.
Methods
A total of 173 patients were given a diagnosis of phyllodes tumor and underwent surgery at the Cancer Institute Hospital in Japan between January 1980 and December 1999. Of these patients, 39 (22.5%) were given a diagnosis of malignant phyllodes tumor; in three of these cases, detailed medical records were lost. Malignant phyllodes tumors were classified into two groups based on history of malignant transformation. Of the 36 malignant cases, 11 (30.6%) were primary and were given a diagnosis of fibroadenoma, experienced recurrence during the follow-up period, and were diagnosed with malignant phyllodes tumor (cases with a history of fibroadenoma). The other group was defined as cases without history of fibroadenoma and in whom lesions initially occurred as malignant phyllodes tumors. Based on differences between the two groups, overall survival curves were plotted using the Kaplan–Meier method, and statistical comparisons were performed using the log-rank test and Peto and Peto’s test.
Results
The outcome of cases with history of fibroadenoma was significantly better than that of cases without history of fibroadenoma.
Conclusions
Patients with malignant phyllodes tumors but without prior history of malignant transformation who exhibit rapid growth within 6 months require aggressive treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phyllodes tumor of the breast is an uncommon fibroepithelial breast neoplasm that accounts for 0.3–1.0% of cases of female breast carcinoma [1]. On the other hand, fibroadenomas are the most frequent benign tumors of the breast after fibrocystic disease. The histogeneses of fibroadenoma and phyllodes tumor of the breast appear to be closely related. Because of the similarity of the epithelial cells in phyllodes tumors to cells in fibroadenomas, many believe phyllodes tumors to arise from a preexisting fibroadenoma [2, 3]. Whether all phyllodes tumors originate as fibroadenomas or whether they can arise de novo without a preexisting fibroadenoma is a matter of ongoing debate. In a study in 1995, Noguchi et al. [3] reported three cases of fibroadenoma that were diagnosed by excisional biopsy and recurred as benign phyllodes. Clonal analysis showed that all three fibroadenomas were monoclonal in origin. It was speculated that phyllodes tumors begin as fibroadenomas, and that subsequently a single stromal cell undergoes mutation and develops into a phyllodes tumor composed mainly of monoclonal stromal cells but partially of monoclonal epithelial cells. Kuijper et al. [4] studied clonal progression in fibroadenomas and phyllodes tumors and concluded that fibroadenomas can progress in an epithelial direction to carcinoma in situ or in a stromal direction to phyllodes tumors. The incidence of monoclonal fibroadenoma is quite low, and this tumor can subsequently progress to phyllodes tumor. Valdes et al. [5] presented a case of malignant transformation of a fibroadenoma to cystosarcoma phyllodes after 5 years of radiologic stability, and demonstrated that monoclonal fibroadenomas can progress to phyllodes tumors by clonal analysis performed on fine-needle aspiration (FNA) sample. However, performing clonal analysis on all fibroadenomas is time consuming and not cost effective. Malignant phyllodes tumors are very uncommon. Successful management of them will require determination of their clinical characteristics. In this study, we specifically, evaluated the outcomes of and prognostic factors for the malignant transformation from fibroadenomas to malignant phyllodes tumors.
Patients and methods
Patient population
We treated 173 patients with the diagnosis of phyllodes tumors between 1980 and 1999 at the Department of Breast Surgery, Cancer Institute Hospital in Japan and classified the tumors as benign, borderline or malignant using the histological classification (Table 1) proposed by the Japanese Breast Cancer Society, which is similar to that proposed by Pietruszka and Barnes [6]. In total, 39 patients were diagnosed with malignant phyllodes tumors, though three of these patients were excluded because their medical records had been lost. The clinical features of 36 patients with malignant phyllodes tumors were retrospectively reviewed and collated.
Classification of cases
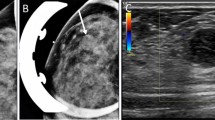
“Malignant transformation” was considered to have occurred when a fibroadenoma became a benign phyllodes tumor, or when a benign phyllodes tumor became a malignant phyllodes tumor. Eleven patients (30.6%) had been diagnosed as having fibroadenoma previously. Ten patients underwent excisional biopsy, exhibited recurrence in the region near the scar, and were diagnosed with malignant phyllodes tumors, while in one patient the tumor was demonstrated histopathologically (Fig. 1). These 11 cases were classified as cases with history of fibroadenoma. Another 25 patients (69.4%) were initially diagnosed with malignant phyllodes tumors. We compared findings for these two groups of patients.
Statistical analysis
For this study, the two groups were compared with respect to age, tumor size, surgical treatment, surgical margins, duration of signs and symptoms, local recurrence, metastases, and survival. Overall survival curves were plotted using the Kaplan–Meier method, and statistical comparisons were performed using the log-rank test and Peto and Peto’s test [7]. Comparisons of clinical background factors were made between those two groups using Welch’s t-test and Fisher’s exact test.
Result
Patient demographics
There were 36 patients diagnosed with malignant phyllodes tumors between the years 1980 and 1999. All were female, with a median age of 43.6 (range 16–88) years. The median size was 91.4 (range 15–320) mm. The median duration of follow-up was 68.5 (range 2–287) months. Of 36 patients, nine (25%) had local recurrence and 14 (39%) had hematogenous metastases.
Outcomes of groups with or without history of fibroadenoma
In the group with history of fibroadenoma, four of 11 (36.4%) patients developed generalized hematogenous metastases. Three of the four died, and the remaining patient underwent three partial resections of lung metastases and remains alive 137 months after mastectomy.
However, in the group without history of fibroadenoma, 13 of 25 (52%) patients died. Ten patients developed generalized hematogenous metastases; nine of these 10 patients died early (at 2–27 months after diagnosis), and only one patient died later, at 134 months after final mastectomy, with pleural effusion and ascites. The other three patients died of other diseases. Moreover, some of the patients without history of fibroadenoma exhibited aggressive tumor growth, and died despite surgical resection. However, these were no early deaths among patients without history of fibroadenoma. The overall 20-year survivals of the two groups are shown in Fig. 2. The outcome of cases with history of fibroadenoma was significantly better than that of cases without history of fibroadenoma (log-rank test p = 0.0551, Peto and Peto’s test [7] p = 0.0409). Multivariate analysis using the Cox model was used for stepwise regression to select the best subset of predictors from candidate covariates, yielding the following statistically significant variables as effective predictors of survival: tumor size, tumor growth rate in duration of symptoms (SIZE/DOS), surgical treatment, and malignancy (Table 2).
Table 3 summarizes tumor-related characteristics according to malignant transformation. The difference between the two groups in DOS was significant (Welch’s t-test p = 0.01437). The cases without history of fibroadenoma had shorter DOS than the cases with history of fibroadenoma. There were no differences in age, tumor size, surgical treatment, surgical margins, local recurrence, metastasis or survival outcome between the two groups.
Discussion
Phyllodes tumor of the breast is a rare fibroepithelial lesion that accounts for less than 1% of all primary breast neoplasms [1]. The majority of phyllodes tumors have been described as benign (35–64%), with the remainder divided between borderline and malignant subtypes. The malignant subtype is found in approximately 25–30% of resected phyllodes tumors [1, 2]. Malignant phyllodes tumor sometimes metastasizes to the lungs. The median rate of metastasis reported after surgery for malignant phyllodes tumors is 25–35% [2]. A report [8] from the M.D. Anderson Cancer Center on a subset of 30 women with the malignant histological subtype estimated that 5- and 10-year overall survival rates were 79% and 42%, respectively. Recently, Macdonald et al. [9] reviewed data on primary nonmetastatic malignant phyllodes tumors (n = 821) obtained from the National Cancer Institute’s (NCI) Surveillance, Epidemiology, and End Results program and reported that predicted cause-specific survival rates were 91%, 89%, and 89%, at 5, 10, and 15 years, respectively, with median follow-up of 5.7 years. Another recent report [10] noted that the relative cumulative survival of malignant phyllodes patients was 87.4% at 10 years. In our study, the overall survival rates were 65%, 60%, and 52%, at 5, 10, and 20 years.
Previously reports [9] in the literature have suggested that stromal overgrowth, tumor size, surgical margin status, and types of surgery were predictive of local or distant recurrence after primary surgery. Patients with stromal overgrowth, tumor size >5 cm [8], and positive margins of excision [11] were found to have a high rate of distant failure. In the present series, the outcome of cases with history of fibroadenoma was significantly better than that of those without history of fibroadenoma. Multivariate analysis using the Cox model was used for stepwise regression technique to select the best subset of predictors, and revealed that size, SIZE/DOS, surgical treatment, and malignancy were effective predictors of survival. Nonmalignant transformation, large size, rapid growth, and mastectomy were significantly correlated with poor survival.
The course from fibroadenoma to phyllodes tumor was slow, but these tumors became histologically more malignant with every local recurrence. Recurrence was perhaps due to residual tumor secondary to inadequate excision of initial fibroadenoma, which can progress to phyllodes tumor. Chen et al. [12] reported that 22 of 172 phyllodes tumors patients had previously undergone fibroadenoma excisions, but that none of them had metastases. All 19 of these 22 patients had a first local recurrence of benign phyllodes tumor. According to his study of recurrent phyllodes tumors, the majority of recurrent tumors were histologically similar to the initial tumors; however, seven patients (19%) developed a malignant recurrence from an initially benign or borderline tumor [13]. Moreover, preoperative diagnosis of phyllodes tumors is difficult. Rapid growth and/or large size of apparent fibroadenomas may be the only imaging finding suggestive of phyllodes tumor. Whole-breast ultrasound showed that nearly one-third of women with phyllodes tumors had concurrent fibroadenoma [14]. It is important to examine most fibroadenomas with ultrasound, and to assess their rate of growth, if any. Rapid tumor growth or sudden increase in size is the most important clinical characteristic for prediction of progression. It is, however, difficult to assess the reliability of this observation, since no objective measurements of tumor growth rate were performed [1, 9]. Some reports notes that phyllodes tumors begin as fibroadenoma, and that subsequently a single stromal cell undergoes mutation and develops into a phyllodes tumor composed mainly of monoclonal stromal cells and partially of monoclonal epithelial cells. The results of monoclonal analysis of our excisional biopsy samples would thus be of great interest.
To our knowledge, this is the first report on the frequency and prognosis of malignant transformation from fibroadenoma to malignant phyllodes tumor. About 20–30% of cases of malignant phyllodes tumors begin as fibroadenomas, and these have better prognosis than those that do not.
Conclusions
The prognosis of malignant phyllodes tumor arising from a preexisting fibroadenoma is relatively good. Patients with malignant phyllodes tumors but without prior history of malignant transformation who exhibit rapid growth within 6 months require aggressive treatment.
References
Anderson BO, Lawton TJ, Lehman CD, Moe RE. Phyllodes tumors. In: Harris JR, Lippman ME, Morrow M, Osborne CK, editors. Disease of the breast. 3rd ed. Philadelphia: Lippincott Williams & Wilkins publishers; 2004. p. 991–1006.
McDivitt RW, Urban JA, Farrow JH. Cystosarcoma phyllodes. Johns Hopkins Med J. 1967;120:33–45.
Noguchi S, Yokouchi H, Aihara T, Motomura K, Inaji H, Imaoka S, et al. Progression of fibroadenoma to phyllodes tumor demonstrated by clonal analysis. Cancer. 1995;10:1779–85.
Kuijper A, Buerger H, Simon R, Schaefer KL, Croonen A, Boecker W, et al. Analysis of the progression of fibroepithelial tumors of the breast by PCR-based clonality assay. J Pathol. 2002;197:575–81.
Valdes EK, Boolbol SK, Cohen J-M, Feldman SM. Malignant transformation of a breast fibroadenoma to cystsarcoma phyllodes: case report and review of the literature. Am Surg. 2005;71:348–53.
Pietruszka M, Barnes L. Cystsarcoma phyllodes: clinicopathologic analysis of 42 cases. Cancer. 1978;41:1974–83.
Peto R, Peto J. Asymptotically efficient rank invariant test procedures (with discussion). J R Stat Soc Ser A Stat Soc. 1972;135:185–206.
Chaney AW, Pollack A, Mcneese MD, Zagars GK, Pister PWT, Pollock RE, et al. Primary treatment of cystsarcoma phyllodes of the breast. Cancer. 2000;89:1502–11.
Macdonald OK, Lee CM, Tward JD, Chappel CD, Gaffney DK. Malignant phyllodes tumors of the female breast. Association of primary therapy with cause-specific survival from the surveillance, epidemiology, and end results (SEER) program. Cancer. 2006;107:2127–33.
Asoglu O, Ugurlu MM, Blanchard K, Grant CS, Reynolds C, Cha SS, et al. Risk factors for recurrence and death after primary surgical treatment of malignant phyllodes tumors. Ann Surg Oncol. 2004;11:1011–7.
Fou A, Schnabel FR, Hamele-Bena D, Wei XJ, Cheng B, Tamer ME, et al. Long-term outcomes of malignant phyllodes tumors patients: an institutional experience. Am J Surg. 2006;192:492–5.
Chen W-H, Cheng S-P, Tzen C-Y, Yang T-L, Jeng K-S, Liu C-L, et al. Surgical treatment of phyllodes tumors of the breast: retrospective review of 172 cases. J Surg Oncol. 2005;91:185–94.
Tan EY, Hoon TP, Yong WS, Wong HB, Hui HG, Yeo AWY, et al. Recurrent phyllodes tumors of the breast: pathological features and clinical implications. ANZ J Surg. 2006;76:476–80.
Foxcroft LM, Evans EB, Porter AJ. Difficulties in the pre-operative diagnosis of phyllodes tumors of the breast: a study of 84 cases. Breast. 2006;27:27–37.
Acknowledgments
We are grateful for the secretarial assistance provided by Ms. Rie Gokan.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Abe, M., Miyata, S., Nishimura, S. et al. Malignant transformation of breast fibroadenoma to malignant phyllodes tumor: long-term outcome of 36 malignant phyllodes tumors. Breast Cancer 18, 268–272 (2011). https://doi.org/10.1007/s12282-009-0185-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-009-0185-x