Abstract
It is well known that estrogens are closely involved in the growth of human breast carcinoma, and that the great majority of breast carcinomas express estrogen receptor. Recent studies have demonstrated that estrogens are locally produced in breast carcinoma by several enzymes. Among these, aromatase is generally considered the most important enzyme, and aromatase inhibitors are currently used in the treatment of breast carcinoma in postmenopausal women as an estrogen deprivation therapy. Therefore, in this review, we summarize the results of recent studies on aromatase in breast carcinoma, and we discuss its biological and/or clinical significance. Aromatase was expressed in various cell types in breast carcinoma, such as carcinoma cells, intratumoral stromal cells and adipocytes adjacent to the carcinoma, and the aromatase expression was regulated by various factors, including carcinoma cell–stromal cell interactions, cytokines and nuclear receptors, depending on the cell types. Aromatase was involved not only in local estrogen production but also the inhibition of intratumoral androgen synthesis in breast carcinoma. Finally, tissue concentrations of sex steroids were significantly higher in noninvasive breast carcinoma, regarded as a precursor lesion to invasive carcinoma, than in non-neoplastic breast tissue, and various sex steroid-producing enzymes (including aromatase) were abundantly expressed in noninvasive breast carcinoma tissue. Therefore, sex steroids are locally produced in noninvasive breast carcinoma as well as invasive carcinoma, and endocrine therapies may be clinically effective in a select group of noninvasive breast carcinoma patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction: aromatase as a potent intratumoral estrogen-producing enzyme in breast carcinoma
Estrogens contribute immensely to the development of hormone-dependent human breast carcinoma by binding with the estrogen receptor (ER). Circulating estrogens are mainly secreted from the ovaries in premenopausal women, but the majority of breast carcinomas arise after menopause, when the ovaries have ceased to be functional. Previous investigations have demonstrated that tissue concentrations of bioactive estrogen, estradiol, are more than ten times higher in breast carcinoma tissue than in plasma, and human breast neoplasms can produce estradiol in vitro [1]. Tissue concentrations of estradiol are approximately two times higher in breast carcinoma tissue than in areas considered morphologically normal [2]. Intratumoral concentrations of estradiol are not significantly different for premenopausal and postmenopausal breast carcinomas, but the estradiol/estrone ratio was significantly higher in postmenopausal breast carcinoma [3]. Therefore, to date, a large proportion (approximately 75% before menopause and close to 100% after menopause) of the biologically active estrogen is considered to be produced locally in the breast carcinoma [4]. Considering that breast carcinoma occurring after menopause frequently expresses ER, intratumoral production of estrogens plays an important role in the proliferation of breast carcinoma cells, especially in postmenopausal women.
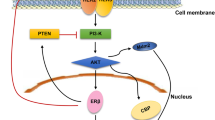
Figure 1 summarizes representative pathways of the intratumoral production of sex steroids in breast carcinoma. High concentrations of circulating inactive steroids, such as androstenedione and estrone sulfate, are major precursor substrates to intratumoral estrogen production. Aromatase catalyzes the conversion of androgens (androstenedione and testosterone) to estrogens (estrone and estradiol, respectively), while steroid sulfatase (STS) hydrolyzes estrone sulfate to estrone. Estrone is subsequently converted to estradiol by 17β-hydroxysteroid dehydrogenase type 1 (17βHSD1), and locally acts on the breast carcinoma cells through ER.
Scheme representing the intratumoral production of sex steroids in breast carcinoma. High concentrations of circulating inactive steroids, such as androstenedione and estrone sulfate, are precursor substrates to the intratumoral production of estrogens and/or androgens in breast carcinoma. Biologically active sex steroids, such as estradiol and 5α-dihydrotestosterone (DHT), are produced and act on the breast carcinoma cells through estrogen (ER) and androgen (AR) receptors, respectively. STS, steroid sulfatase; 17βHSD, 17β-hydroxysteroid dehydrogenase
Among these pathways, aromatase is considered the most important enzyme in estrogen biosynthesis, and the inhibition of aromatase is clinically useful for reducing the progression of breast carcinomas in postmenopausal women. Two types of aromatase inhibitors have been developed, and these have different mechanisms of action (Table 1). Agents that interfere with the substrate-binding sites of aromatase are androgen analogs known as steroidal (or “type 1”) aromatase inhibitors, while agents that block the electron transfer chain via the cytochrome P450 prosthetic group of aromatase are termed nonsteroidal (or “type 2”) inhibitors [5]. Third-generation aromatase inhibitors, such as anastrozole, letrozole and exemestane, are currently available, and these have been shown to efficiently suppress estrogen levels in plasma [6] and breast carcinoma tissue [7]. Results from large multicenter trials, such as the ATAC trial for anastrozole, the NCIC MA-17 trial for letrozole and the Intergroup exemestane study, have all demonstrated that aromatase inhibitors are significantly associated with improved disease-free survival and good tolerability in breast carcinoma patients [8–11].
Expression and localization of aromatase in breast carcinoma
It is very important to obtain a better understanding of aromatase expression in breast carcinoma in order to improve the clinical effects of aromatase inhibitors in breast carcinoma patients, because previous studies have demonstrated an association between aromatase activity in breast carcinoma and response to treatment with aromatase inhibitors [12].
Approximately 70% of breast carcinoma specimens have aromatase activities that are comparable with or that are greater than those found in other tissues [12], and the aromatase mRNA level was significantly increased in breast carcinoma compared to that in nonmalignant tissue [13]. In our study, the expression level of aromatase mRNA was significantly higher in breast carcinoma and adipose tissue adjacent to the carcinoma than in non-neoplastic breast tissue (P < 0.05, respectively) (Fig. 2a). When we further examined the localization of aromatase mRNA in breast carcinoma by laser capture microdissection (LCM)/real-time polymerase chain reaction (real-time PCR), aromatase mRNA was detected in both carcinoma and intratumoral stromal cells in breast carcinoma tissues (Fig. 2b), and the aromatase mRNA level was significantly (P < 0.01) higher in intratumoral stromal cells than in carcinoma cells (Fig. 2c). Therefore, aromatase is expressed in various types of cells in breast carcinoma, such as carcinoma cells, intratumoral stromal cells, and adipose tissues adjacent to the carcinoma.
Cellular expression of aromatase mRNA in breast carcinoma. a Aromatase mRNA levels were significantly higher in breast carcinoma and adipose tissue adjacent to the carcinoma (P < 0.05, respectively) than in non-neoplastic breast tissue (n = 12 in each group). b Localization of aromatase mRNA in breast carcinoma, obtained by LCM/real-time PCR analysis. Aromatase mRNA was detected in both breast carcinoma cells and intratumoral stromal cells. Three representative cases of breast carcinoma (1–3) and two breast carcinoma cell lines (MCF-7 and T47D) are represented in this agarose gel photo. M molecular marker, P positive control (placental tissue), N negative control (no cDNA substrate). c Cellular expression of aromatase mRNA in breast carcinoma by LCM/real-time PCR analysis. The aromatase mRNA level was significantly (P < 0.01) higher in intratumoral stromal cells than in breast carcinoma cells (n = 12 in each group). a, c The aromatase mRNA level was summarized as a ratio with an internal standard (β-actin) and then evaluated as a ratio (%) with the positive control (placental tissue)
The immunolocalization of aromatase in breast carcinoma was examined by several groups, but the results reported by them were inconsistent. Previously, Sasano et al. [14] showed the immunolocalization of aromatase in stromal cells, such as intratumoral fibroblasts (Fig. 3a) and adipocytes, of breast carcinoma, and Santen et al. [15] also demonstrated that aromatase immunoreactivity occurred predominantly in stromal cells. On the other hand, Esteban et al. [16] and Brodie et al. [17] reported the immunolocalization of aromatase in breast carcinoma cells. Recently, Sasano et al. [18] validated several aromatase antibodies that had been newly developed for immunohistochemistry, and demonstrated that the immunoreactivity of a monoclonal antibody for aromatase (#677) was detected in various types of cells, such as intratumoral stromal cells, carcinoma cells (Fig. 3b) and normal duct epithelial cells, which is in good agreement with the localization of aromatase mRNA described above. In our study, the aromatase immunoreactivity obtained by #677 antibody was significantly associated with the aromatase mRNA level in a carcinoma cell component (Fig. 3c), but not that in an intratumoral stromal cell component (data not shown), and Sasano et al. [18] reported that aromatase activity in breast carcinoma tissue was positively associated with aromatase immunoreactivity (#677) in a carcinoma cell component, but not that in a stromal cell component. However, further examinations are required to clarify the clinical and biological significance of aromatase in relation to cell types in breast carcinoma.
Immunohistochemistry for aromatase in breast carcinoma. a Aromatase immunoreactivity was detected in intratumoral stromal cells (St.), but not in carcinoma cells (Ca.), in invasive breast carcinoma, when we used the same rabbit polyclonal antibody as used in [14]. b On the other hand, aromatase immunoreactivity was detected in carcinoma cells in invasive breast carcinoma when we used the same mouse monoclonal antibody as used in [18] (#677). c Aromatase immunoreactivity (#677) was significantly (P < 0.05) correlated with the mRNA level in a carcinoma cell component in invasive breast carcinoma (n = 18). The aromatase mRNA level was evaluated by LCM/real-time PCR. d Aromatase immunoreactivity was mainly detected in carcinoma cells in noninvasive breast carcinoma. Bar represents 50 μm
Aromatase immunoreactivity was mainly detected in carcinoma cells in noninvasive breast carcinoma (Fig. 3d), regarded as a precursor lesion to invasive carcinoma [19].
The differences between the results for aromatase immunolocalization obtained in previous studies are possibly due to the different natures of the aromatase antibodies employed. Immunohistochemistry for aromatase is generally expected to be the most attractive method of evaluating aromatase expression, considering the great success that diagnostic laboratories have had in detecting ER, progesterone receptor (PR) and HER2 in breast carcinoma tissues. Therefore, further examinations are required to establish a standardized approach, including the determination of aromatase antibody, the immunohistochemical procedure and the evaluation system.
Regulatory factors of aromatase expression in breast carcinoma
The mechanism by which mechanism aromatase expression is increased in various types of cells in breast carcinoma remains largely unclear. When we examined the expression of aromatase mRNA in breast carcinoma tissues by real-time PCR analysis, the aromatase mRNA level was highest in invasive breast carcinoma, modest in noninvasive breast carcinoma, and lowest in the non-neoplastic breast tissue (Fig. 4a), and LCM/real-time PCR analysis revealed that the aromatase mRNA level was significantly higher in invasive breast carcinoma than in noninvasive breast carcinoma in both carcinoma cell and intratumoral stromal cell components [19]. Subsequent co-culture experiments demonstrated that aromatase activity was significantly increased when co-culturing with MCF-7 breast carcinoma cells and intratumoral stromal cells isolated from breast carcinoma tissue compared to the aromatase activities observed during each single culture (Fig. 4b, c) [20]. Previous in vitro studies have demonstrated that breast carcinoma cells secrete various factors that induce aromatase expression in adipose fibroblasts, including prostaglandin E2, interleukin (IL)-1, IL-6, IL-11 and tumor necrosis factor α [21, 22]. On the other hand, it has been also reported that exogenous growth factors such as epidermal growth factor, transforming growth factor and keratinocyte growth factor stimulate aromatase activity in MCF-7 cells [20]. Therefore, aromatase expression may be, partially at least, regulated by tumor–stromal interactions in breast carcinoma, which may be promoted by the invasion of carcinoma cells into stroma.
Aromatase expression in noninvasive and invasive breast carcinoma. a Aromatase mRNA expression was significantly (P < 0.05) higher in noninvasive breast carcinoma (n = 12) than in non-neoplastic breast tissue (n = 8). Aromatase mRNA level was also significantly (P < 0.05) higher in invasive breast carcinoma (n = 12) than in noninvasive carcinoma (n = 12). The aromatase mRNA level was summarized as a ratio (%) with that of an internal standard (ribosomal protein L 13a). b, c Effects of co-culturing on aromatase activity in breast carcinoma cells and intratumoral stromal cells. b Aromatase activity of MCF-7 cells was significantly (P < 0.05) increased when co-culturing with intratumoral stromal cells isolated from breast carcinoma tissue compared to the single culture. c Similarly, the aromatase activity of the intratumoral stromal cells was also significantly (P < 0.05) elevated when these cells were co-cultured with MCF-7 cells
Previous studies have also demonstrated the regulation of aromatase expression by various transcriptional factors. Transcription of aromatase is activated by steroidogenic factor 1/adrenal 4 binding protein (SF1; designated NR5A1) in the ovaries, which binds to a nuclear receptor half site within their promoter regions to mediate basal transcription and in part cAMP-induced transcription. However, SF1 is not expressed in breast carcinoma. Clyne et al. [23] and Zhou et al. [24] examined various orphan nuclear receptors known to bind to such a nuclear receptor half site in 3T3-L1 preadipocytes, and reported the induction of aromatase expression by liver receptor homolog-1 (LRH-1; NR5A2) in adipose stromal cells in breast carcinoma. LRH-1 was immunolocalized in adipocytes adjacent to the carcinoma and carcinoma cells [25]. LRH-1 expression was positively associated with aromatase in the adipose tissues adjacent to the carcinoma [24], but not in the breast carcinoma cells [25]. Therefore, LRH-1 may regulate aromatase expression mainly in the adipocytes adjacent to the breast carcinoma.
On the other hand, estrogen-related receptor α (ERRα; NR3B1) has a positive regulatory effect on aromatase in SK-BR-3 breast carcinoma cells [26], but not in 3T3-L1 preadipocytes [23]. ERRα was mainly immunolocalized in breast carcinoma cells, but not in intratumoral stromal cells or adipocytes [27], and the expression level of ERRα mRNA in carcinoma cells was positively associated with that of aromatase mRNA in breast carcinoma [20]. Thus, aromatase expression is regulated by various factors in breast carcinoma, and key regulators may differ according to the cell types that express the aromatase.
Expression of other estrogen-producing enzymes in breast carcinoma
STS
A major circulating form of plasma estrogens is estrone sulfate, a biologically inactive form of estrogen, in postmenopausal women. Estrone sulfate has a long half-life in peripheral blood, and the level of estrone sulfate is approximately ten times higher than that of unconjugated estrogens such as estrone, estradiol and estriol during the menstrual cycle and in postmenopausal women [28]. STS is a single enzyme that hydrolyzes estrone sulfate to estrone (Fig. 1). The enzymatic activity of STS is detected in the great majority of breast carcinomas, and is considerably higher than the aromatase activity in breast tumors [12]. STS immunoreactivity was detected in carcinoma cells in approximately 70% of breast carcinoma cases [29, 30], and STS immunoreactivity was significantly associated with an increased risk of recurrence in breast carcinoma patients [31]. STS mRNA expression was also reported to be higher in breast carcinoma tissue than in the normal tissue, and it was significantly associated with poor clinical patient outcome [31, 32]. STS inhibitors are currently being developed by several groups, and the results of a phase I study suggest that an STS inhibitor may provide effective treatment for hormone-dependent breast carcinomas, including those which progress upon treatment with aromatase inhibitors [33].
17βHSD1
17βHSD catalyzes an interconversion of estrogens or androgens. Thirteen isozymes of 17βHSD have been cloned [34], and 17β-reduction (17βHSD1, 3, 5, 7, etc.) or oxidation (17βHSD2, 4, 6, etc.) of estrogens and/or androgens is catalyzed by different 17βHSD isozymes. Among these isozymes, 17βHSD1 enzyme uses NADPH as a cofactor and mainly catalyzes the reduction of estrone to estradiol (Fig. 1). Oxidative 17βHSD activity is the preferential direction in normal breast tissues, but the reductive 17βHSD pathway is dominant in breast carcinoma [12]. Miyoshi et al. [3] reported that 17βHSD1 mRNA levels and the intratumoral estradiol/estrone ratio was significantly higher in postmenopausal than in premenopausal breast carcinoma. 17βHSD1 immunoreactivity was detected in carcinoma cells in approximately 60% of the breast carcinomas, and it was correlated with ER and PR [35]. In addition, breast carcinoma patients with high levels of 17βHSD1 mRNA were associated with increased risk of developing late relapses of breast carcinoma [36]. Therefore, 17βHSD1 is suggested to be responsible for regulating the process leading to the accumulation of estradiol in the breast carcinoma, and the majority of the estradiol synthesized by 17βHSD1 in breast carcinoma cells may act directly on these cells.
Aromatase as a negative regulator of intratumoral androgen production in breast carcinoma
In contrast to estrogens, androgens are considered to predominantly exert antiproliferative effects via androgen receptor (AR) in breast carcinoma cells, although some divergent findings have been reported [12]. Tissue concentrations of androgens in breast carcinomas were investigated by several groups [37–39]. A potent androgen, 5α-dihydrotestosterone (DHT), was significantly higher in breast carcinoma than in plasma [38], and intratumoral production of DHT in breast carcinoma has also been proposed for the circulating inactive androgen androstenedione, like estrogens (Fig. 1). AR is expressed in the majority of human breast carcinoma tissues [40–42], suggesting important roles for androgens in breast carcinoma, as well as estrogenic actions.
The substrates of aromatase are androstenedione and testosterone, and these are precursors not only to estradiol synthesis but also to DHT production (Fig. 1). DHT itself is nonaromatizable. The intratumoral concentration of DHT was significantly associated with that of testosterone in breast carcinoma tissue [37, 38], which suggests that the DHT level in the breast carcinoma is greatly influenced by the amount of the precursor. Spinola et al. [43] showed that treatment with an aromatase inhibitor markedly elevated intratumoral testosterone concentrations in dimethylbenz(a)anthracene (DMBA)-induced rat mammary tumors, and Sonne-Hansen and Lykkesfeldt [44] reported that aromatase preferred testosterone as a substrate in MCF-7 cells. Very recently, we demonstrated that aromatase expression was inversely associated with intratumoral DHT concentration in breast carcinoma, and that aromatase suppressed DHT production from androstenedione in co-culture experiments with MCF-7 cells and intratumoral stromal cells isolated from breast carcinoma [39]. Therefore, aromatase is suggested to act as a negative regulator for intratumoral DHT production in breast carcinoma, possibly by reducing concentrations of the precursor testosterone.
Results of large multicenter trials have demonstrated the superior efficacy of aromatase inhibitors compared to the anti-estrogen tamoxifen [8–11]. Although this might be due to agonistic effects of tamoxifen in an estrogen-deprived environment [8], we can also speculate that aromatase inhibitors exert additional antiproliferative effects by increasing the local DHT concentration upon estrogen deprivation. Further examinations are required to clarify the clinical importance of androgenic actions in association with the response to aromatase inhibitors in breast cancer patients.
Intratumoral production of sex steroids in noninvasive breast carcinoma
Noninvasive breast carcinoma is regarded as a precursor lesion to invasive breast carcinoma. The great majority of noninvasive breast carcinomas are histologically diagnosed as ductal carcinomas in situ (DCIS), and the risk of invasive ductal carcinoma developing after a diagnosis of DCIS was reported to be 4–10 times higher than in normal women [45, 46]. The incidence of noninvasive breast carcinoma has markedly increased over the past two decades with advances in mammographic screening [47, 48], and it now comprises approximately 10–20% of all breast carcinomas diagnosed [49–51].
It is well known that sex-steroid receptors, such as ER, PR and AR, are frequently positive in noninvasive breast carcinoma [50, 52–54], suggesting important roles for sex steroids in noninvasive breast carcinoma, just as in invasive carcinoma. Tamoxifen was reported to inhibit the growth of premalignant mammary lesions and the progression to invasive carcinoma in a transplantable mouse model of noninvasive breast carcinoma [55]. The National Surgical Adjuvant Breast Project (NSABP) P-1 trial demonstrated that tamoxifen significantly reduced the risk of noninvasive breast carcinoma by 50% [56], and the results of the NSABP B-24 trial indicated that adjuvant tamoxifen therapy reduced the recurrence of noninvasive breast carcinoma by 30% [57]. However, information on sex steroids in noninvasive breast carcinoma is currently very limited compared to that on sex steroids in invasive carcinoma, as described above, and the clinical and/or biological significance of sex steroids in noninvasive carcinoma remains largely unclear.
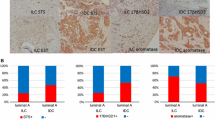
When we examined intratumoral concentrations of sex steroids in noninvasive breast carcinoma, both estradiol and DHT levels were significantly (P < 0.05, respectively) higher in noninvasive breast carcinoma than in non-neoplastic breast tissue (Fig. 5a, b) [19]. The results of the study also demonstrated that estrogen (aromatase, STS, and 17βHSD1) and androgen (17βHSD5 and 5α-reductase type 1) producing enzymes were abundantly expressed in noninvasive carcinoma tissues [19]. Therefore, it is suggested that both estrogens and androgens are locally produced in noninvasive breast carcinoma, as in invasive carcinoma, and that endocrine therapies may be clinically effective for a select group of noninvasive breast carcinoma patients. Further examinations are required to clarify the significance of sex steroids in noninvasive breast carcinoma.
Tissue concentrations of estradiol (a) and DHT (b) in noninvasive breast carcinoma. Both estradiol and DHT levels were significantly (P < 0.05, respectively) higher in noninvasive breast carcinoma (n = 12) than in non-neoplastic breast tissue (n = 8). In addition, the intratumoral concentration of DHT was significantly (P < 0.05) higher in noninvasive breast carcinoma than in invasive carcinoma (n = 12) in our study
References
Sasano H, Suzuki T, Nakata T, Moriya T. New development in intracrinology of breast carcinoma. Breast Cancer. 2006;13:129–36.
Chetrite GS, Cortes-Prieto J, Philippe JC, Wright F, Pasqualini JR. Comparison of estrogen concentrations, estrone sulfatase and aromatase activities in normal, and in cancerous, human breast tissues. J Steroid Biochem Mol Biol. 2000;72:23–7.
Miyoshi Y, Ando A, Shiba E, Taguchi T, Tamaki Y, Noguchi S. Involvement of up-regulation of 17β-hydroxysteroid dehydrogenase type 1 in maintenance of intratumoral high estradiol levels in postmenopausal breast cancers. Int J Cancer. 2001;94:685–9.
Labrie F, Luu-The V, Labrie C, Belanger A, Simard J, Lin SX, et al. Endocrine and intracrine sources of androgens in women: inhibition of breast cancer and other roles of androgens and their precursor dehydroepiandrosterone. Endocr Rev. 2003;24:152–82.
Miller WR, Dixon JM. Endocrine and clinical endpoints of exemestane as neoadjuvant therapy. Cancer Control. 2002;9:9–15.
Geisler J, Lonning PE. Endocrine effects of aromatase inhibitors and inactivators in vivo: review of data and method limitations. J Steroid Biochem Mol Biol. 2005;95:75–81.
Geisler J. Breast cancer tissue estrogens and their manipulation with aromatase inhibitors and inactivators. J Steroid Biochem Mol Biol. 2003;86:245–53.
Baum M. Current status of aromatase inhibitors in the management of breast cancer and critique of the NCIC MA-17 trial. Cancer Control. 2004;11:217–21.
Coombes RC, Hall E, Gibson LJ, Paridaens R, Jassem J, Delozier T, et al. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med. 2004;350:1081–92.
Howell A, Cuzick J, Baum M, Buzdar A, Dowsett M, Forbes JF, et al. Results of the ATAC (Arimidex, Tamoxifen, alone or in combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet. 2005;365:60–2.
Utsumi T, Kobayashi N, Hanada H. Recent perspectives of endocrine therapy for breast cancer. Breast Cancer. 2007;14:194–9.
Suzuki T, Miki Y, Nakamura Y, Moriya T, Ito K, Ohuchi N, et al. Sex steroid-producing enzymes in human breast cancer. Endocr Relat Cancer. 2005;12:701–20.
Utsumi T, Harada N, Maruta M, Takagi Y. Presence of alternatively spliced transcripts of aromatase gene in human breast cancer. J Clin Endocrinol Metab. 1996;81:2344–9.
Sasano H, Nagura H, Harada N, Goukon Y, Kimura M. Immunolocalization of aromatase and other steroidogenic enzymes in human breast disorders. Hum Pathol. 1994;5:530–3.
Santen RJ, Martel J, Hoagland M, Naftolin F, Roa L, Harada N, et al. Stromal spindle cells contain aromatase in human breast tumors. J Clin Endocrinol Metab. 1994;79:627–32.
Esteban JM, Warsi Z, Haniu M, Hall P, Shively JE, Chen S. Detection of intratumoral aromatase in breast carcinomas. An immunohistochemical study with clinicopathologic correlation. Am J Pathol. 1992;140:337–43.
Brodie AM, Lu Q, Long BJ, Fulton A, Chen T, Macpherson N, et al. Aromatase and COX-2 expression in human breast cancers. J Steroid Biochem Mol Biol. 2001;79:41–7.
Sasano H, Anderson TJ, Silverberg SG, Santen RJ, Conway M, Edwards DP, et al. The validation of new aromatase monoclonal antibodies for immunohistochemistry—a correlation with biochemical activities in 46 cases of breast cancer. J Steroid Biochem Mol Biol. 2005;95:35–9.
Shibuya R, Suzuki T, Miki Y, Yoshida K, Moriya T, Katsuhiko Ono K, et al. Intratumoral concentration of sex steroids and expression of sex steroid-producing enzymes in ductal carcinoma in situ of human breast. Endocr Relat Cancer. 2008;15:113–24.
Miki Y, Suzuki T, Tazawa C, Yamaguchi Y, Kitada K, Honma S, et al. Aromatase localization in human breast cancer tissues: possible interactions between intratumoral stromal and parenchymal cells. Cancer Res. 2007;67:3945–54.
Reed MJ, Purohit A, Woo LW, Newman SP, Potter BV. Steroid sulfatase: molecular biology, regulation, and inhibition. Endocr Rev. 2005;26:171–202.
Yamaguchi Y. Microenvironmental regulation of estrogen signals in breast cancer. Breast Cancer. 2007;14:175–81.
Clyne CD, Speed CJ, Zhou J, Simpson ER. Liver receptor homologue-1 (LRH-1) regulates expression of aromatase in preadipocytes. J Biol Chem. 2002;277:20591–7.
Zhou J, Suzuki T, Kovacic A, Saito R, Miki Y, Ishida T, et al. Interactions between prostaglandin E(2), liver receptor homologue-1, and aromatase in breast cancer. Cancer Res. 2005;65:657–63.
Miki Y, Clyne CD, Suzuki T, Moriya T, Nakamura Y, Ishida T, et al. Immunolocalization of liver receptor homologue-1 (LRH-1) in human breast carcinoma: possible regulator of in situ steroidogenesis. Cancer Lett. 2006;244:24–33.
Yang C, Zhou D, Chen S. Modulation of aromatase expression in the breast tissue by ERR α-1 orphan receptor. Cancer Res. 1998;58:5695–700.
Suzuki T, Miki Y, Moriya T, Shimada N, Ishida T, Hirakawa H, et al. Estrogen-related receptor α in human breast carcinoma as a potent prognostic factor. Cancer Res. 2004;64:4670–6.
Pasqualini JR. The selective estrogen enzyme modulators in breast cancer: a review. Biochim Biophys Acta. 2004;1654:123–43.
Saeki T, Takashima S, Sasaki H, Hanai N, Salomon DS. Localization of estrone sulfatase in human breast carcinomas. Breast Cancer. 1999;6:331–7.
Suzuki T, Nakata T, Miki Y, Kaneko C, Moriya T, Ishida T, et al. Estrogen sulfotransferase and steroid sulfatase in human breast carcinoma. Cancer Res. 2003;63:2762–70.
Utsumi T, Yoshimura N, Takeuchi S, Ando J, Maruta M, Maeda K, et al. Steroid sulfatase expression is an independent predictor of recurrence in human breast cancer. Cancer Res. 1999;59:377–81.
Miyoshi Y, Ando A, Hasegawa S, Ishitobi M, Taguchi T, Tamaki Y, et al. High expression of steroid sulfatase mRNA predicts poor prognosis in patients with estrogen receptor-positive breast cancer. Clin Cancer Res. 2003;9:2288–93.
Stanway SJ, Purohit A, Woo LW, Sufi S, Vigushin D, Ward R, et al. Phase I study of STX 64 (667 Coumate) in breast cancer patients: the first study of a steroid sulfatase inhibitor. Clin Cancer Res. 2006;12:1585–9.
Horiguchi Y, Araki M, Motojima K. 17beta-Hydroxysteroid dehydrogenase type 13 is a liver-specific lipid droplet-associated protein. Biochem Biophys Res Commun. 2008;370:235–8.
Suzuki T, Moriya T, Ariga N, Kaneko C, Kanazawa M, Sasano H. 17β-hydroxysteroid dehydrogenase type 1 and type 2 in human breast carcinoma: a correlation to clinicopathological parameters. Br J Cancer. 2000;82:518–23.
Gunnarsson C, Olsson BM, Stal O, Southeast Sweden Breast Cancer Group. Abnormal expression of 17β-hydroxysteroid dehydrogenases in breast cancer predicts late recurrence. Cancer Res. 2001;61:8448–51.
Mistry P, Griffiths K, Maynard PV. Endogenous C19-steroids and oestradiol levels in human primary breast tumour tissues and their correlation with androgen and oestrogen receptors. J Steroid Biochem. 1986;24:1117–25.
Recchione C, Venturelli E, Manzari A, Cavalleri A, Martinetti A, Secreto G. Testosterone, dihydrotestosterone and oestradiol levels in postmenopausal breast cancer tissues. J Steroid Biochem Mol Biol. 1995;52:541–6.
Suzuki T, Miki Y, Moriya T, Akahira J, Ishida T, Hirakawa H, et al. 5α-reductase type 1 and aromatase in breast carcinoma as regulators of in situ androgen production. Int J Cancer. 2007;20:285–91.
Isola JJ. Immunohistochemical demonstration of androgen receptor in breast cancer and its relationship to other prognostic factors. J Pathol. 1993;170:31–5.
Suzuki T, Darnel AD, Akahira JI, Ariga N, Ogawa S, Kaneko C, et al. 5α-reductases in human breast carcinoma: possible modulator of in situ androgenic actions. J Clin Endocrinol Metab. 2001;86:2250–7.
Moinfar F, Okcu M, Tsybrovskyy O, Regitnig P, Lax SF, Weybora W, et al. Androgen receptors frequently are expressed in breast carcinomas: potential relevance to new therapeutic strategies. Cancer. 2003;98:703–11.
Spinola PG, Marchetti B, Merand Y, Belanger A, Labrie F. Effects of the aromatase inhibitor 4-hydroxyandrostenedione and the antiandrogen flutamide on growth and steroid levels in DMBA-induced rat mammary tumors. Breast Cancer Res Treat. 1988;12:287–96.
Sonne-Hansen K, Lykkesfeldt AE. Endogenous aromatization of testosterone results in growth stimulation of the human MCF-7 breast cancer cell line. J Steroid Biochem Mol Biol. 2005;93:25–34.
Franceschi S, Levi F, La VC, Randimbison L, Te VC. Second cancers following in situ carcinoma of the breast. Int J Cancer. 1998;77:392–5.
Warnberg F, Yuen J, Holmberg L. Risk of subsequent invasive breast cancer after breast carcinoma in situ. Lancet. 2000;355:724–5.
Kusama R, Takayama F, Tsuchiya S. MRI of the breast: comparison of MRI signals and histological characteristics of the same slices. Med Mol Morphol. 2005;38:204–15.
Li CI, Daling JR, Malone KE. Age specific incidence rates of in situ breast carcinomas by histologic type, 1980 to 2001. Cancer Epidemiol Biomarkers Prev. 2005;14:1008–11.
Moriya T, Hirakawa H, Suzuki T, Sasano H, Ohuchi N. Ductal carcinoma in situ and related lesions of the breast: recent advances in pathology practice. Breast Cancer. 2004;11:325–33.
Kepple J, Henry-Tillman RS, Klimberg VS, Layeeque R, Siegel E, Westbrook K, et al. The receptor expression pattern in ductal carcinoma in situ predicts recurrence. Am J Surg. 2006;192:68–71.
Tsikitis VL, Chung MA. Biology of ductal carcinoma in situ classification based on biologic potential. Am J Clin Oncol. 2006;29:305–10.
Selim AG, El-Ayat G, Wells CA. Androgen receptor expression in ductal carcinoma in situ of the breast: relation to oestrogen and progesterone receptors. J Clin Pathol. 2002;55:14–6.
Barnes NL, Boland GP, Davenport A, Knox WF, Bundred NJ. Relationship between hormone receptor status and tumour size, grade and comedo necrosis in ductal carcinoma in situ. Br J Surg. 2005;92:429–34.
Rody A, Diallo R, Poremba C, Wuelfing P, Kissler S, Solbach C, et al. Androgen receptor expression in ductal carcinoma in situ of the breast: not a helpful marker for classification such as estrogen receptor alpha and progesterone receptor. Appl Immunohistochem Mol Morphol. 2005;13:25–9.
Namba R, Young LJ, Maglione JE, McGoldrick ET, Liu S, Wurz GT, et al. Selective estrogen receptor modulators inhibit growth and progression of premalignant lesions in a mouse model of ductal carcinoma in situ. Breast Cancer Res. 2005;7:R881–9.
Dunn BK, Wickerham DL, Ford LG. Prevention of hormone-related cancers: breast cancer. J Clin Oncol. 2005;23:357–67.
Cuzick J. Treatment of DCIS—results from clinical trials. Surg Oncol. 2003;12:213–9.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Suzuki, T., Miki, Y., Ohuchi, N. et al. Intratumoral estrogen production in breast carcinoma: significance of aromatase. Breast Cancer 15, 270–277 (2008). https://doi.org/10.1007/s12282-008-0062-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-008-0062-z