Abstract
The recent advances in nanotechnology have a great potential to improve the prevention, diagnosis, and treatment of human diseases. Nanomaterials for medical applications are expected to grasp pharmacokinetics and the toxicity for application to medical treatment on the aspect of safety of the nanomaterials and nanodevices. We describe a generation of CdSe nanoparticles [quantum dots (QDs)] conjugated with monoclonal anti-HER2 antibody (Trastuzumab), for single molecular in vivo imaging of breast cancer cells. We established a high-resolution in vivo 3D microscopic system for a novel imaging method at the molecular level. The cancer cells expressing HER2 protein were visualized by the nanoparticles in vivo at subcellular resolution, suggesting future utilization of the system in medical applications to improve drug-delivery systems to target the primary and metastatic tumors for made-to-order treatment. We also describe sentinel node navigation using fluorescent nanoparticles for breast cancer surgery in experimental model, which have shown the potential to be an alternative to existing tracers in the detection of the sentinel node if we select the appropriate particle size and wavelength. Future innovation in cancer imaging by nanotechnology and novel measurement technology will provide great improvement, not only in the clinical field but also in basic medical science for the development of medicine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tumor targeting anti-cancer therapeutics by conjugating tumor-specific antibodies is of great interest in oncology, pharmacology, and nanomedicine. This approach will allow to increase therapeutic efficacy and to decrease systemic adverse effect [1, 2]. Quantitative investigation of dynamics of such drug delivery in vivo is crucial to enable the development of more effective drug-delivery systems. One of the best ways to perform this is to apply new technology in biophysics that the positions of proteins are detected quantitatively at the single-molecule level with nanometer precision [3]. However, the specific delivery processes in vivo is not known at the single-particle level. Conventional imaging modalities such as computed tomography, magnetic resonance imaging, positron emission tomography, and organic fluorescence or luminescence imaging have insufficient resolution to analyze the pharmacokinetics of drugs at the single-particle level in vivo [4].
To address this issue, real-time single-particle tracking using quantum dots (Qdots) has been applied to the study of drug delivery. Qdots, fluorescent nanocrystals, were expected to be a good biomarker because of their intense brightness and stability, in contrast to existing organic dyes and GFP [5, 6]. In cultured cells, single-particle tracking has yielded invaluable information on the function of purified proteins [7, 8]. Recent work shows that the antibody-conjugated Qdots have allowed real-time tracking of single-receptor molecules on the surface of living cells [9]. However, no real-time single-particle tracking in live animals has been reported, and it is uncertain if the single particle of Qdots could be observed or tracked in live animals. We analyzed the movement of single functional Qdots in the tumors of mice from a capillary vessel to cancer cells by a highly sensitive measurement system.
The made-to-order treatment for disease is now strongly expected as well as the evidence-based medicine. In the area of surgery, sentinel node navigation surgery is a major method for performing made-to-order surgery for malignancies. Treatments of cancer with minimum invasive surgery without lymph node dissection based on sentinel lymph node (SN) navigation surgery has become a major concern of made-to-order and low-invasiveness medicine. Some radioisotopes and dyes are utilized for SN detection in the standard method, however, each detection method has advantages and disadvantages. To make up for the disadvantages, we aimed at developing a new noninvasive method using nanosized fluorescent beads of uniform size that could efficiently visualize SN from outside the body and perform experiments to determine the appropriate size and fluorescent wavelength and confirm that uniformly nanosized fluorescent beads have the potential to be an alternative to existing tracers [10, 11] in the detection of the SN in animal. These data should be useful in establishing clinical fluorescence measurement methods in the future.
Materials and methods
Single molecular imaging of breast cancer by quantum dots conjugated with anti-HER2 antibody
We made conjugations of Qdot (Quantum Dot Corporation, Hayward, CA) and trastuzumab (Chugai pharmaceutical Co., LTD, Tokyo, Japan) with a Qdot 800 Antibody Conjugation Kit (Quantum Dot Corporation, Hayward, CA) via poly ethylene glycol (M.W. 2000) and heterobifunctional cross-linker 4-(maleimidomethyl)-1-cyclohexanecarboxylic acid N-hydroxysuccinimide ester (SMCC).
The final concentration of Qdots and trastuzumab complexes (QT complexes) was determined by measuring the conjugate absorbance at 550 nm and using an extinction coefficient of 1,700,000 M−1 cm−1 at 550 nm.
The human breast cancer cell line KPL-4 [12], which overexpresses HER2 and is sensitive to trastuzumab, was kindly supplied from Dr. J. Kurebayashi (Kawasaki Medical School, Kurashiki, Japan). KPL-4 cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 5% fetal bovine serum. A suspension of KPL-4 was transplanted subcutaneously to the dorsal skin of female Balb/c nu/nu mice at 6–10 weeks of age (Charles River Japan, Yokohama, Japan). Mice bearing a tumor volume of 100–200 mm3 were selected for experiments. All operations on animals were in accordance with the institutional animal use and care regulations. This study was approved by Animal Care and Use Committee of Tohoku University.
QT complexes were injected into tail vein of mice. The mice were placed under anesthesia by the intraperitoneal injection of a ketamine and xylazine mixture at a dosage of 95 and 5 mg/kg, respectively. The temperature of mice was maintained at 37°C by a thermo-plate and objective lens heater. The modified dorsal skin fold chamber (DSFC) method [13] was used to fix the exposed mouse tumor on the stage of the microscope. Two sterilized polyvinyl chloride plates (0.5 mm thickness) containing a window were mounted so as to fix the extended double layer of dorsal skin including the tumor site. Skin between chambers sutured with 6-0 nylon around the window, and the tumor could be located in the center of the window and fixed without influence from the beating of the heart and breathing. The tumor was placed surface down on the neutral saline mounted cover slip on a viewing platform of an inverted microscope. The mouse was fixed to a metal plate on the stage designed to stabilize the chamber. Tumors can be visualized directly by means of this setup.
The mice were killed by CO2 overdose, after imaging. The tumors were removed and divided for histological and immunohistochemical examination. In the histological Qdot uptake study, tumors were frozen and cryosectioned 6 μm thickness, fixed with acetone at 0°C and examined with an imaging system. For immunohistological examination, tumors were fixed in 10% neutral-buffered formalin overnight and then transferred into ethanol before processing and paraffin embedding. Immunohistochemical analysis was performed on paraffin sections at 6-μm thickness using the HercepTest (Dako Cytomation, CA) to confirm HER2 expression.
As shown in Fig. 1, the optics system for 3D observation consisted primarily of an epi-fluorescent microscope (IX71, Olympus) with modifications, a Nipkow lens type confocal unit (CSU10, Yokogawa) and an electron multiplier type CCD camera (iXon 887, Andor). The confocal unit adopts multi-beam scanning using about a thousand beams that are simultaneously emitted through a pin-hole disk to facilitate high-speed scanning. The EMCCD has advantage that offering unsurpassed sensitivity performance, and has been shown to yield markedly improved SN ratio. The objective lens was moved by a piezo actuator with a feedback loop for stabilizing the position of the focus. A computer controlled the piezo actuator in synchronization with the image acquisitions in order that the objective lens remained within the exposure time of the CCD camera. An area of 30 × 30 μm2 was irradiated by a green laser (532 nm, Crystalaser).
The xy-position of the fluorescent spot was calculated by fitting to a 2D Gaussian curve. The single molecule could be identified by the fluorescence spot, and quantitative and qualitative information such as velocity, directionality, and transport mode was obtained using time-resolved trajectories of particles. The resolution of the position was determined from the position of immobile QT complexes in a chemically fixed tumor cell. The resolution of the x and y directions of images taken at an exposure time of 33 ms was 30 nm, taking into consideration the standard deviation.
Sentinel node navigation by nanosized fluorescent beads
We employed “FluoSpheres®” manufactured from high-quality polystyrene microspheres (Molecular Probe Inc, OR, USA) for sentinel node detection. We selected beads of sizes 20, 40, 100, and 200 nm, and fluorescent colors of yellow–green (YG) (excitation/emission maxima at 505/515 nm), dark red (DR) (660/680 nm), far red (FR) (690/720 nm) and infrared (IR) (715/755 nm). The size of beads is very homogeneous, 0.02 ± 0.004 μm in 20-nm size and 0.1 ± 0.005 μm in 100 nm. We designed a laser scanning fluorescence detection system that consists of three lasers, a resonant scanner (resonant frequency/200 Hz), a rotational pulse-stage and a CCD camera (Fig. 2). We used a diode pumped solid-state blue laser (473 nm, 7 mW) as the excitation source of YG fluorescent beads. For excitation of DR, FR, and IR fluorescent beads, we used a He-Ne laser (632.8 nm, 14.6 mW) or a laser diode (657 nm, 3.56 mW). The inguinal and femoral areas were continuously irradiated and scanned over an area of 30 x 50 mm. The fluorescence image was observed using a CCD camera (XC-EI50, Sony) with an optimum band-pass filter for each fluorescent bead.
In addition, the spectrum of scanned area was analyzed with a spectrometer. Under ether anesthesia, the hair of rats’ lower body was removed to avoid autofluorescence of it. Then 50 μl of FluoSpheres® 2% w/v suspension was subcutaneously injected at the footpad of the hind leg. Spectral analysis of fluorescence from rats injected with beads was performed to clarify the signal-to-noise ratio of fluorescence from beads and autofluorescence. After observation from outside the body (through the skin) for 30–180 min, we peeled back the skin at the subcutaneous layer and ascertained the area of lymph nodes with navigation of their specific fluorescence. Then, the lymph nodes were removed and fixed with formalin for histological observation to confirm that the tissue was a lymph node.
Results
Single molecular imaging of breast cancer by semiconductor quantum dots conjugated with anti-HER2 antibody
The molar ratio of trastuzumab to the Qdots of the QT complexes was approximately 3:1. KPL-4 cell membrane and QT complexes strongly bound 5 min after mixture of KPL-4 and 10-nM QT complexes. Six hours after the mixing, the signals from the QT complexes were found mainly in the cytosol neighboring the nucleus, suggesting that the QT complexes were endocytosed. Qdots without trastuzumab did not bind or accumulate in the KPL-4 cells. A control study using AH109A cells, a HER2 negative cell line, also showed no binding of the QT complexes, indicating that QT complexes selectively bind to the HER2 protein.
A tumor-bearing mice model was prepared with subcutaneous implantation of KPL-4. A single Qdot in the tumor-bearing mice was observed using a high-resolution intravital imaging system through the DSFC. This system captures images of single Qdots at a video rate of 33 ms per frame. The DSFC was firmly mounted on the microscope stage to minimize the distorting influence of the heartbeat and breathing. 3D confocal intravital images of single QT complexes were taken by moving an objective lens. 3D images of the tumor were taken by reconstructing 10–20 confocal images from the surface of the mice to a depth of 150 mm inside the tumor through the DSFC. Fluorescence micro-angiography was performed after injection of the QT complexes into the tail vein. The membranes of the KPL-4 tumor cells were clearly stained with QT complexes at 6 h after the injection. Up to 24 h after the injection, the QT complexes had been internalized into and increased in the tumor cells.
After in vivo tumor imaging of the mice, histological examination of the chemically fixed tumors was performed to confirm that QT complexes in the living mice exhibit activity on the KPL-4 cells. QT complexes observed under the 3D microscope were located at the cell membrane and near the nuclear membrane. The position of the objective was fixed and 300–3,000 sequential confocal 2D images (total 10–100 s) were taken at this fixed position. Within 30 s after the injection, the current of the QT complex in a vessel was observed. When we could observe a vessel and cells clearly, the single QT complex in the current of the tumor vessel was then analyzed. The fluorescence image of the circulating QT complexes was not a circle but an ellipse and sometimes a line at the video rate because QT complexes at times moved >1 mm in a single frame. The speed of the movement of the single particles was calculated from the positional changes of the centroid of the QT complex images. The average speed of each complex ranged from 100 to 600 μm/s, in agreement with a previous report by another method [14]. Each particle exhibited slow and fast movement in the bloodstream. Such fast and slow movement characteristics could be induced by the pulse and nonuniform current within a vessel such as the Hagen–Poiseuille current. The slow speed of the complexes inside a tumor vessel would be important to locate pores between the vessel cells and then moved out through these pores.
Focusing on the vessel walls, a movement was observed of the QT complex extravasated from the intravascular space. The edge of the vascular inner surface was not clear on a single-frame image. Therefore, all the images obtained were averaged to precisely determine the position of the edge. The complexes were positioned first on the vascular surface and then extravasated. This is the first example of video rate observation of extravasation of nanoparticles, such as Qdots, in a mouse model.
Two hours after the injection, many complexes had migrated into the tumor interstitial area close to the tumor vessels. Most of the movement of the complexes was random in both orientation and speed, indicating that complexes diffuse by the Brownian motion exerted by thermal energy. The average diffusion coefficient of the complexes was 0.0014 mm2/s, much smaller than that at free diffusion in solution (~10 mm2/s). Many complexes also moved randomly within a restricted small area of ~1 mm in diameter and then hopped by ~1 mm. The movement was restricted by a cage formed by the extracellular matrix.
Six hours after the injection, QT complexes had bound to the KPL-4 cell membrane on which the HER2 protein is located. We successfully captured specific images of the QT complexes bound to the cell membrane. Movements of a single QT complex are identified in single frames. To identify the positions of the tumor vessels and cells in living mice without further fluorescence staining, images were averaged. Many QT complexes bound to the cell membrane exhibited Brownian motion within a restricted region of ~500 nm in diameter. This region is significantly larger than the area of ~30 nm, which was drawn by position noise of the complexes fixed on a coverslip, indicating the movement is due to the anchor of the HER2 to a flexible component of the cytoskeleton such as an actin filament [15]. The QT complexes restricted to the small area initiated linear movement in one direction along the cell membrane with speed of 400–600 nm/s and traveled for several micrometers.
We also observed in pursuing the transport of QT complexes from the peripheral region of the cell to the perinuclear region. The QT complex in a given cell moved almost straight towards the cell membrane with a velocity of 100–300 nm/s, changed direction to parallel to the cell membrane, and moved toward the cell nucleus at a velocity of ~600 nm/s. Finally, the directional movement of the QT complex ceased and Brownian motion commenced within a small area, ~1 μm in a diameter, near the nucleus. The first two movements, straight towards and along the cell membrane, would most likely be produced by the transport of an acto-myosin system binding to vesicle containing QT complexes [16], because the actin filaments in cultured cells are highly concentrated in the peripheral region of cells. Movement towards the nucleus would most likely be on a microtubule transported by dynein [17] since there are almost no actin filaments near the nucleus, but rather, a high concentration of microtubules.
Sentinel node navigation by nanosized fluorescent beads
We performed the experiment on four types of beads with diameters of 20, 40, 100, and 200 nm. In the experiment using 20-nm beads, 22 ft from 13 rats were tested. SNs were detected in 10 ft of 22 (45%) by fluorescence contrast as shown in Fig. 3. The time of detection from injection was 0–6 min. The average time for detection was 2.5 min and the median time was 2 min. With the 40-nm beads, SNs were detected in 50 of 72 ft (69%). The time of detection from injection was 0 to 28 min. SNs in 42 ft were detected within 5 min. They were the most representative cases (84%). The average time was 4.6 min and the median time was 3 min. With 100-nm beads, SNs were detected in two of 10 ft (20%). The average and median times were both 56 min. With the 200-nm beads, SNs were detected in seven of 18 ft (39%). The average time was 127 min. The median time was 135 min. In the 40- and 20-nm experiments, there was a significant difference in both the positive rate and average time. In the same way, we compared 40 with 100- or 200-nm beads.
We investigated three excitation and emission wavelengths with the 40-nm beads, yellow–green (YG), dark red (DR) and infrared (IR). Beads of 40 nm in diameter were found to be the most suitable size in the previous experiment. In the experiment using YG, 10 ft from five rats were tested. SNs in three of 10 ft (30%) were observed by fluorescence measurement. With DR, SNs in 24 of 31 ft (77%) were observed and with IR, SNs in 23 of 31 ft (74%) were observed. DR and IR have advantage of positive rate of fluorescence detection as compared with YG.
In these experiments, four different fluorescent wavelengths 515, 680, 720, and 755 nm were studied. DR and IR were more sensitive than YG in the detection rate experiments with 40 nm. There was no significant difference in detection rate, but in average time between DR and IR. However, spectral analysis of DR and IR showed that IR has a higher signal-to-noise ratio compared to DR.
Discussion
In the study for single molecular imaging of cancer, we captured the specific delivery of single QT complexes in tumor vessels to the perinuclear region of tumor cells in live mice after QT complexes had been injected into the tail vein of mice. Six stages were detected (Fig. 4), (1) vessel circulation, (2) extravasation, (3) movement into the extracellular region, (4) binding to HER2 on the cell membrane, (5) movement from the cell membrane to the perinuclear region after endocytosis and (6) in the perinuclear region. The transport speed of QT complexes in each process was highly variable, even in the vessel circulation. The movement of the complexes in each process was also found to be “stop-and-go”, i.e., the complex remaining within a highly restricted area and then moving suddenly. This indicates that the movement was promoted by a motive power and constrained by both the 3D structure and protein-protein interactions. The motive power of the movements was produced by blood circulation (essential in processes 1 and 2), diffusion force driven by thermal energy (2, 3 and 4) and active transport by motor proteins (5). The cessation of movement is most likely induced by a structural barricade such as a matrix cage (2, 3, and 4) and/or specific interaction between proteins, e.g., an antibody and HER2 (4), motor proteins, and rail filaments such as actin filaments and microtubules (5).
The molecular mechanism underlying the movement and its cessation during delivery of nanoparticles in animal models is the fundamental basis of drug delivery. There have been many different approaches to tumor-targeting “nanocarriers” including anti-cancer drugs, for passive targeting such as Myocet [18], Doxil [19] and for active targeting such as MCC-465 [20], anti-HER2 immunoliposome [21]. There is still very little understanding of the biological behavior of nanocarriers, including such crucial features as their transport in the blood circulation, cellular recognition, translocation into the cytoplasm, and final fate in the target cell. These results suggest that the transport of nanocarriers would be quantitatively analyzable in the tumors of living animals by the present method. This approach thus should afford great potential new insight into particle behavior in complex biological environments. Such new insight in turn will allow rational improvements in particle design to increase the therapeutic index of the tumor targeting nanocarriers.
Nanocrystal semiconductor quantum dots conjugated with antibody may serve fundamentally as new controllable materials for medical purposes including cancer molecular imaging.
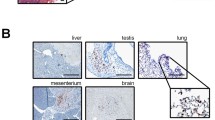
Homogeneous nanosized beads have shown an advantage for efficient SN detection compared to existing colloids agents of heterogeneous size in the fluorescent nanoparticle study for sentinel navigation surgery. Although the appropriate size for SN detection for human beings is predicted to be 500 nm, and the optimum size may be different between animal species, the appropriate size should be determined for humans with accurately measured nanosized beads of strictly same dimensions. There are two forms of transportation mechanism regarding a particle material to lymphatic system that is injected into tissue space. One is physical and active extracellular transportation; a particle passes through lymph capillaries. The other one is intracellular transportation of a particle. Foreign materials shift to the lymph capillaries after phagocytosis of particle. An investigation of in vivo dynamics of tracers is important in SN biopsy. It is reported that lymph node was detected by protected graft copolymer combined with Cy5.5, or methoxypolyethyleneglycol-poly-l-lysine combined with Cy5.5 as a tracer. The nanoparticle may distribute to reticuloendothelial system as described in previous reports [22, 23], or may be excreted from kidney for their hydrophilia. We histologically observed liver and kidney 2 weeks after subcutaneous injection of fluorescent beads. Consequently, we did not find beads trapped in liver or kidney by fluorescent microscopic observation, suggesting that the safety of these beads would be ensured when they are given in vivo.
The fluorescent beads that we used are mainly consisted of polystyrene. As polystyrene is the material often used for surgical strings in operation, it would be safe to gave fluorescent beads to living organisms. Accumulation and toxicity are under investigation.
The depth of targets is a serious problem in fluorescence measurement of living tissue. The local excitation illumination within tissue exponentially attenuates due to absorption and scattering from the surface to that depth. This problem of lack of transmission prevents us from detection in tissues deeper than 1 cm from the surface of the body at present [10]. We can detect the SN of small animals like rats, but may have difficulty in detection in larger animals because of the depth at which SNs. For example, lymph node in human is buried in fat and is located deeper than 1 cm. The detection technique to find SN up to 2 cm in depth is recommended. To solve this problem, we should select the appropriate wavelength of fluorescence and fluorescent materials, and also develop imaging techniques. Hemoglobin absorbs light in the range of visible light below 650 nm, and water absorbs light above 1,100 nm, but in the near-infrared range, between 650 and 1,100 nm, the absorption of light in living tissue is minimum. This range is called the optical window. In addition to collagen, NADH and FAD are substances that in vivo have the fluorescent wavelengths in the range of 400–500 nm. So, from this point of view, NIR range has the advantage for the fluorescence measurement. We are also investigating the application of semiconductor nanocrystal that has extremely stronger fluorescent intensity than usual fluorescent beads to increase the detection ability and fluorescence tomography based on acousto-optic modulation imaging [24].
Nanotechnology should be a great aid in improving tailor-made medicine by their hyper-sensitive and super-selective property for diagnoses. Advanced sensing technologies such as the single-molecule imaging technique and acousto-optic modulation imaging technique are also required to make the best use of the functional nanomaterials for achievement of hyper-sensitive and super-selective imaging. These novel products of advanced technologies may realize a revolution of medicine in near future.
This work was supported by Grants-in-aid for Research Projects, Promotion of Advanced Medical Technology (H14-Nano-010 and H18-Nano-001), from the Ministry of Health, Labor and Welfare, Japan.
References
Torchilin VP, Lukyanov AN, Gao Z, Papahadjopoulos-Sternberg B. Immunomicelles: targeted pharmaceutical carriers for poorly soluble drugs. Proc Natl Acad Sci USA. 2003;100:6039–44.
Krauss WC, Park JW, Kirpotin DB, Hong K, Benz CC. Emerging antibody-based HER2 (ErbB-2/neu) therapeutics. Breast Dis. 2000;11:113–24.
Lyons SK. Advances in imaging mouse tumour models in vivo. J Pathol. 2005;205:194–205.
Ishijima A, Kojima H, Funatsu T, Tokunaga M, Higuchi H, Tanaka H. Simultaneous observation of individual ATPase, mechanical events by a single myosin molecule during interaction with actin. Cell. 1998;92:161–71.
Wu X, Liu H, Liu J, Haley KN, Treadway JA, Larson JP. Immunofluorescent labeling of cancer marker Her2, other cellular targets with semiconductor quantum dots. Nat Biotechnol. 2003;21:41–6.
Gao X, Cui Y, Levenson RM, Chung LW, Nie S. In vivo cancer targeting, imaging with semiconductor quantum dots. Nat Biotechnol. 2004;22:969–76.
Yildiz A, Forkey JN, McKinney SA, Ha T, Goldman YE, Selvin PR. Myosin V walks hand-over-hand: single fluorophore imaging with 1.5-nm localization. Science. 2003;300:2061–5.
Dahan M, Levi S, Luccardini C, Rostaing P, Riveau B, Triller A. Diffusion dynamics of glycine receptors revealed by single-quantum dot tracking. Science. 2003;302:442–5.
Lidke DS, Nagy P, Heintzmann R, Arndt-Jovin DJ, Post JN, Grecco HE, Jares-Erijman EA, Jovin TM. Quantum dot ligands provide new insights into erbB/HER receptor-mediated signal transduction. Nat Biotechnol. 2004;22:198–203.
Krag DN, Weaver DL, Alex JC. Surgical resection and radio-localization of the sentinel node in breast cancer using a gamma probe. Surg Oncol. 1993;2:335–40.
Tafra L, Lannin DR, Swanson MS. Multicenter trial of sentinel node biopsy for breast cancer using both technetium sulfur colloid and isosulfan blue dye. Ann Surg. 2001;233:51–9.
Kurebayashi J, Otsuki T, Tang CK, Kurosumi M, Yamamoto S, Tanaka K, Mochizuki M, Nakamura H, Sonoo H. Isolation and characterization of a new human breast cancer cell line, KPL-4, expressing the Erb B family receptors and interleukin-6. Br J Cancer. 1999;79:707–17.
Leunig M, Yuan F, Menger MD, Boucher Y, Goetz AE, Messmer K, Jain RK. Angiogenesis, microvascular architecture, microhemodynamics, and interstitial fluid pressure during early growth of human adenocarcinoma LS174T in SCID mice. Cancer Res. 1992;52:6553–60.
Braun RD, Abbas A, Bukhari SO, Wilson W III. Hemodynamic parameters in blood vessels in choroidal melanoma xenografts and rat choroid. Invest Ophthalmol Vis Sci. 2002;43:3045–52.
Carraway CA, Carvajal ME, Carraway KL. Association of the Ras to mitogen-activated protein kinase signal transduction pathway with microfilaments. Evidence for a p185 (neu)-containing cell surface signal transduction particle linking the mitogenic pathway to a membrane-microfilament association site. J Biol Chem. 1999;274:25659–67.
Buss F, Arden SD, Lindsay M, Luzio JP, Kendrick-Jones J. Myosin VI isoform localized to clathrin-coated vesicles with a role in clathrin-mediated endocytosis. EMBO J. 2001;20:3676–84.
Kamal A, Goldstein LS. Connecting vesicle transport to the cytoskeleton. Curr Opin Cell Biol. 2000;12:503–8.
Mross K, Niemann B, Massing U, Drevs J, Unger C, Bhamra R, Swenson CE. Pharmacokinetics of liposomal doxorubicin (TLC-D99; Myocet) in patients with solid tumors: an open-label, single-dose study. Cancer Chemother Pharmacol. 2004;54:514–24.
O’Brien ME, Wigler N, Inbar M, Rosso R, Grischke E, Santoro A. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol. 2004;15:440–9.
Hamaguchi T, Matsumura Y, Nakanishi Y, Muro K, Yamada Y, Shimada Y. Antitumor effect of MCC-465, pegylated liposomal doxorubicin tagged with newly developed monoclonal antibody GAH, in colorectal cancer xenografts. Cancer Sci. 2004;95:608–13.
Park JW, Kirpotin DB, Hong K, Shalaby R, Shao Y, Nielsen UB. Tumor targeting using anti-her2 immunoliposomes. J Control Release. 2001;74:95–113.
Josephson L, Mahmood U, Wunderbaldinger P, Tang Y, Weissleder R. Pan and sentinel lymph node visualization using a near-infrared fluorescent probe. Mol Imaging. 2003;2:18–23.
Patrick W, Karl T, Chrstoph B. Near-infrared fluorescence imaging of lymph nodes using a new enzyme sensing activatable macromolecular optical probe. Eur Radiol. 2003;13:2206–11.
Kobayashi M, Mizumoto T, Shibuya Y, Takeda M, Enomoto M. Fluorescence tomography in turbid media based on acousto-optic modulation imaging. Appl Phys Lett. 2006;89:181102.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is based on a presentation delivered at the Presidential Symposium 1, “Breast cancer: individualized diagnosis for tailored treatment,” held on 29 June 2007 at the 15th Annual Meeting of the Japanese Breast Cancer Society in Yokohama.
About this article
Cite this article
Takeda, M., Tada, H., Higuchi, H. et al. In vivo single molecular imaging and sentinel node navigation by nanotechnology for molecular targeting drug-delivery systems and tailor-made medicine. Breast Cancer 15, 145–152 (2008). https://doi.org/10.1007/s12282-008-0037-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-008-0037-0