Abstract
Purpose of Review
This review summarizes the fungal literature currently available for tinea capitis (TC), as well as providing data for clinical utility.
Recent Findings
Available studies in TC are scarce; however, they provide important information about efficacy and outcome in clinical practice.
Summary
Treatment of TC is effective; however, it requires a minimum of 1 month. Systemic treatment is often required to favor enhance drug penetration into the deep part of the hair follicle. The newest oral antifungal has higher efficacy rates than conventional therapy, as well as much shorter duration of treatment but at higher costs. We perform a review of the literature including treatment schemes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tinea capitis (TC) is an infection or parasitization of the hair, scalp, and annexes (eyebrows and eyelashes). It is commonly seen on the scalp hair related to dermatophytic fungi (Trichophyton and Microsporum) and more frequently in children [1, 2•]. The worldwide epidemiology of TC is varied; Trichophyton tonsurans is the most frequent agent of TC in the Americas, Europe, and Africa, followed by Microsporum canis, which is a common agent in Mediterranean countries. The most important dermatophyte carriers are cats, dogs, and rabbits [3].
Epidemiology
Until now, the incidence of TC is unknown. It develops more often in children between 3 and 7 years old. It is rare among young adults, although it commonly affects women during menopause and elderly patients. In Europe, a prevalence of TC between 0.23 and 2.6% has been reported [1]. A similar trend is noted in the USA and Mexico [2•]. The main pathogens in many countries are anthropophilic species (90% of cases in North America). T. tonsurans is the most common cause of TC followed by M. canis [3]. M. canis is the most frequent agent in Mediterranean countries. In a recent study, Kallel et al. [4•], in 947 cases of TC in Tunisian children, reported that microsporic tinea was the most frequent cause (63.25%, Microsporum canis (67%)) followed by trichophytic tinea (29.78%, Trichophyton violaceum (31.68%)). This change in etiological agent is related to the increasing cohabit with cats in this population [5]. Besides cats, dogs as well as rabbits are the most important dermatophyte carriers [3]. There are reports of infections related to T. violaceum and M. audouinii in several not previously endemic countries (e.g., Brazil) for these organisms [6, 7, 8•]. Asymptomatic carrier status, usually in adults in the same patient’s family, is related to repeat infections in children [1]. T. schoenleinii develops a rare form of TC, often developed in youth and extending into adulthood [9].
Some factors are commonly associated with TC; these included socioeconomic status (rural and suburban areas), poor hygiene, and overcrowding, with schools being the site where the infection is most rapidly disseminated. Hair shaving is one of the habits most commonly related to TC. Immunosuppression is also a risk factor for TC, mostly on adult patients [10, 11••]. In Mexico, TC accounts for 4 to 10% of tineas, 90% corresponding to non-inflammatory type [12, 13].
Etiopathogeny
Dermatophytes are keratinophilic fungi including in three genera: Trichophyton, Microsporum, and Epidermophyton. Dermatophytes are classified, according to host preference and habitat, as anthropophilic, zoophilic, and geophilic. Many species of dermatophytes, mostly anthropophilic and zoophilic [14], are able to invade hair shafts; however, other agents like T. tonsurans, Trichophyton schoenleinii, and T. violaceum may develop this type of infection [2•, 14].
Fungus growth occurs inside the follicular sheath, generating spores without affecting the cuticle. When hyphae destroy the cuticle and invade the outer root sheath, the spores develop the ectothrix type. These infections develop parallel to the length of the hair [2•, 11••].
In favus, hyphae develop tunnels within the follicular sheath [14]. Endothrix and favus are less contagious than ectothrix anthropophilic infections, which rapidly spread [15].
The adhesion of the fungus to the keratinocyte favors the infection from one host to another, which eventually progresses an active infection [16]. Fungal proteolytic enzymes favor invasion even in hostile (acid or alkaline) media [17], and these protease genes are variably expressed with distinct enzyme patterns in infections or in vitro cultures [18].
The human skin has several mechanisms for preventing fungal infections. Antimicrobial peptides (e.g., human β-defensins, cathelicidins (LL-37), and dermicidin) [19, 20] are naturally produced as an innate defense against several infectious agents, including fungi (e.g., dermatophytes and Candida albicans).
IL-6 secretion, dependent on the activation of inflammasome and upregulated by several intracellular protein complexes, is commonly related to M. canis infection [21]. The presence of saturated fatty acids in sebum can inhibit fungal growth, although these mechanisms are diminished or absent among children, and thus increasing the frequency of TC in this age group [22]. In addition, a destructive process of dermatophytes through neutrophils and macrophages may be observed [23].
Th17 cells might stimulate neutrophils and exacerbate skin inflammation, exerting a protective effect against the fungus, although it can contribute to hair loss [24]. The exact mechanism remains unknown, although it may be due to stimulation of inflammation through a dectin-1-dependent mechanism, as reported in infections by M. canis [21].
Classification
According to the microscopic pattern of fungal invasion, TC has four types of hair invasion (two non-inflammatory and two inflammatory), settling different clinical pictures [11••]. In ectothrix, the hair shaft is invaded at the mid-follicle. Microsporum species are the commonest causes (M. gypseum and M. nanum as non-fluorescent agents and M. audouinii and M. canis as fluorescent agents); however, T. verrucosum (a non-fluorescent megasporated agent) can cause a form of ectothrix infection. Ectothrix infection usually develops a scaly lesion, often with inflammation and pruritus. The hairs often break developing partial hair loss, with hair stubs [2•, 11••].
Endothrix infection is commonly related to T. tonsurans, T. mentagrophytes (var. mentagrophytes), T. soudanense, and T. violaceum [2•, 11••].
Favus is related to T. schoenleinii, an anthropophilic fluorescent dermatophyte. Long tunnels within hair shafts are often seen as well as large clusters at the base of the hair, representing fungal hair invasion [2•].
Kerion celsi is severe, acute inflammatory process of the scalp, related to M. canis and M. gypseum [25], and probably to a T cell-mediated hypersensitivity process against dermatophytes [26] (Table 1). Recently, based on molecular methods performed by de Hoog et al. [27], two tinea capitis agents took another nomenclature: M. gypseum changed for Nannizzia gypsea and M. nanum for Nannizzia nana.
Clinical Manifestations
The clinical appearance of TC is determined through hair invasion by the pathogenic fungi, the host resistance, and the immune status. Clinical pictures range from an asymptomatic carrier to severe inflammatory presentations developing scarring alopecia. Most affected patients are children from 6 months to 12 years old. The common clinical pattern includes broken hairs with scaling; however, this picture has changed due to geographic distribution of the infectious species [28].
In M. audouinii infection, the most common picture is patching alopecia, often circular, showing several broken hairs with minimal inflammation and scaly skin. In Microsporum sp. infections, similar clinical patterns are seen when compared M. canis with M. audouinii; however, the latter are usually more inflammatory and pruriginous. In Trichophyton sp. infections, multiple patchy hair loss is often seen with minimal inflammation and scaling, also exhibiting swollen hair shafts (black dots).
Kerion celsi is a severe, painful, inflammatory process, discharging purulent exudate, commonly leading to scarring alopecia; lymphadenopathy is frequently seen. This reaction is usually related to zoophilic species (T. verrucosum or T. mentagrophytes (var. mentagrophytes)) and less often to anthropophilic species. This process is usually accompanied by secondary infection [2•, 28].
In inflammatory TC (kerion), there are one or multiple tender nodules with pustules, leading to scarring alopecia. It also presents with fever, adenopathy, and less often with a diffuse, morbilliform rash. Leukocytosis and increased erythrocyte sedimentation rate are also reported with a positive skin test to fungal antigen; this is in relation to the immune response associated to the inflammatory process [2•, 11••].
T. schoenleinii infection (favus) is still endemic in some countries like Ethiopia. Favus develops yellowish cup-shaped crusts called scutula, formed at the base of hair shafts. In longstanding cases, scarring alopecia and atrophy may develop [2•].
Carrier status is considered in infections not clinically obvious and asymptomatic. This is usually seen in children, becoming potential fungi reservoirs; this may be the cause of the increased prevalence of TC [2•, 11••, 14].
Diagnosis
Clinical diagnosis must be confirmed through potassium hydroxide (KOH) direct examination, followed by hair and scale cultures. Samples are obtained by scraping the scales through a disposable brush [11••].
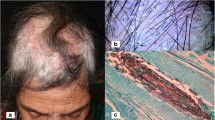
Wood’s light examination is a diagnostic support in schools where small TC epidemic can be seen. In ecthotrix (anthropophilic) cases (related to M. canis, M. audouinii, and M. distortum), Wood’s light may show a bright yellow-green fluorescence in affected hair [29]; however, Wood’s light examination is useless for screening and monitoring in endothrix infections related to T. tonsurans, T. mentagrophytes, and T. violaceum since those fungi do not fluoresce [29] (Fig. 1). Wood’s light effectiveness depends on the observer’s skills as well as on the quality of the sample, leading to 5–15% of false-negative results [30].
The incubation period at 20–30 °C ranges from 3 to 4 weeks; however, in cases related to T. verrucosum, T. violaceum, or T. soudanense, the incubation period may be prolonged to 2 or more weeks. Fungal identification is based on macroscopic (growth characteristics, pigment formation) as well as microscopic morphology (mostly macroconidia and microconidia). In atypical isolates, additional tests (e.g., urease and in vitro hair perforation test) may be required [30].
Robert et al. [30] suggested the use of Congo red (a β-d-glucans stain) or calcofluor-white 0.1% solution (a chitin-binding fluorochrome dye) through a fluorescence microscope eases fungal visualization.
Sabouraud’s agar and Mycobiotic agar containing antibiotics (chloramphenicol and cycloheximide) are the most commonly used media to suppress saprophytic bacterial growth representing sample contamination [1].
Rapid nucleic acid-based methods are also useful for dermatophyte identification [11••, 31]; however, their cost limits their extensive use [32].
Introducing molecular tools with sufficient specificity using different techniques, standard polymerase chain reaction, [33, 34] as well as the newest diagnostic methods like multiplex ligation-dependent probe amplification and rolling circle amplification [35, 36], develops faster and accurate results for identifying dermatophytes. Dermatoscopy for trichoscopy is a widely used diagnostic technique in dermatology [37, 38]. In infections by T. tonsurans, multiple comma-shaped hairs and corkscrew hairs can be seen; the latter is probably due to fungal-mediated internal hair damage, progressing to hair degradation and external resistance [39], whereas in M. canis infection, we can observe dystrophic and elbow-shaped hairs and different height levels of broken hair [40]. Campos et al. [41], in a prospective study, found that trichoscopy is also useful for monitoring response to treatment; they observed at 8 weeks after initiating treatment all evaluated dermatoscopic signs of TC decreased, and thus, at the end of the follow-up, no dystrophic hair was present. The authors concluded that the disappearance of dystrophic hair may be a marker of therapeutic success.
In a study led by Arenas et al. [42], which included 37 Latin patients mostly children, the following dermatoscopic patterns were confirmed: comma hairs (41%), corkscrew hairs (22%), short hairs (49%), and black dots (33%). The presence of scales (89%), peripilar casts (46%), alopecia (65%), pustules (8%), and meliceric crusts (16%) were also observed.
Histopathology is not usually required. It reveals spores within the hair follicles or stratum corneum. Arthroconidia and hyphae may be found on the surface of the hair (ectothrix) or inside the hair shaft (endothrix). In favus, the hyphae are located in the stratum corneum, in the hair shaft, and in the cup-shaped crusts. Follicular atrophy may occur. In kerion, there are several patterns of inflammation (perifolliculitis, suppurative folliculitis with or without suppurative, granulomatous dermatitis, as well as fibrosing dermatitis) [2•].
Trichophytin extracted from T. mentagrophytes has been used to evaluate the immune response to dermatophytes. Galactomannan is the antigenic determinant that cross-react with several fungi other than T. mentagrophytes. It produces two responses: an immediate type I response (related to polysaccharide fraction) and a delayed type IV response (related to peptide fraction) [2•].
Differential Diagnosis
It includes alopecia areata, bacterial folliculitis, atopic dermatitis, seborrheic dermatitis, pseudotinea amiantacea, trichotillomania, trichorrhexis nodosa, and psoriasis. Discoid lupus erythematosus and lichen planus are also differential diagnosis of TC. Inflammatory tinea needs to be distinguished from folliculitis decalvans and cellulitis dissecans [2•, 43].
Treatment and Follow-up
The most important goal of treatment is to obtain clinical and mycological cure, avoiding drugs adverse events, mostly in children, and without relapse. Relapses are frequent, especially in relation to the use of topical treatments, so in most cases, oral antifungal treatment is required, although the clinical appearances and itching improve shortly. Topical treatment (shampoo with 2% selenium sulfide or 2% ketoconazole) is recommended in breastfeeding patients. Clinical and mycological cure determines treatment duration [2•].
Terbinafine, itraconazole, griseofulvin, and fluconazole are the most commonly used oral antifungal [44]. Griseofulvin, the first systemic antifungal used in TC, continues to be an effective option in most cases, particularly for Microsporum-related cases. Griseofulvin is fungistatic and inhibits the synthesis of nucleic acids and interrupts cell division in metaphase, thereby preventing fungal cell wall synthesis; additionally, griseofulvin has immunomodulatory properties. The dose and duration of treatment at 10 to 20 mg/kg/day for 8 to 12 weeks is usually helpful; however, T. tonsurans may require longer periods (Table 2).
Terbinafine, an allylamine with fungicide action through acting on the fungal cell membrane and with a special affinity to keratin, is effective against all dermatophytes. The recommended dose ranges from 3 to 6 mg/kg/day; however, higher doses may be required in cases associated with Microsporum, although the efficacy of terbinafine against this infection is not clearly elucidated [11••].
Itraconazole, a fungistatic and fungicidal azole, acts depleting ergosterol in cell membrane and thus, leading to permeability detachment. A dose of 100 mg/day for approximately 4 weeks or 5 mg/kg/day is recommended for children [11••].
Similar to itraconazole, fluconazole is an effective drug against T. tonsurans, at 6 mg/kg/day for 20 days. There is a lack of studies pointing out the appropriate treatment length with these oral antifungal [2•, 11••].
In a Cochrane review about the efficacy of oral antifungal for TC [45], it stated that terbinafine, itraconazole, and fluconazole are comparable to the efficacy of griseofulvin for TC caused by Trichophyton sp. These options should be preferred for improving patient’s adherence to treatment. In a recent abridged Cochrane review, Chen et al. [46••] included 25 randomized controlled trials (4449 study subjects). They found that terbinafine and griseofulvin had similar effects for children with both microsporic and trichophytic infections. Terbinafine showed a higher rate of complete cure when compared to griseofulvin in T. tonsurans infections; however, griseofulvin was better for Microsporum sp. Itraconazole and fluconazole exhibit similar efficacy against Trichophyton infections than griseofulvin or terbinafine. Previously, Tey et al. [47] published similar data suggesting that terbinafine was a better choice for TC due to Trichophyton, while for Microsporum infections, griseofulvin was a better choice.
Oral steroids have been successfully used in the treatment of severe inflammatory TC such as kerion. Prednisone is generally used at a dose of 1 mg/kg/day for 1–2 weeks. For infections produced by zoophilic and anthropophilic species, it is strongly suggested to identify the source to reduce the potential transmission. Shemer et al. [48], in a comparative study in 90 patients with TC, found that “griseofulvin and fluconazole reduced the potential for disease transmission in children with TC, with griseofulvin being more effective for M. canis infections, although children with TC may be potentially contagious even after up to 3 weeks of treatment.”
Some individuals are not clinically cured at follow-up; this may be due to several factors, e.g., lack of adherence, suboptimal drug absorption, agent-related sensitivity, biofilm formation [49], and possible reinfection. If fungi can still be isolated at the end of treatment, but the clinical signs have improved, we continue the treatment for an additional month. If there has been no clinical response and signs persist at the end of the treatment period, we may increase the dose or duration of the treatment. Oral antifungal most commonly used safely during long periods, or even at higher doses include griseofulvin and terbinafine. In resistant cases, it should be considered to change the treatment for another oral antifungal, for example, in cases of lack of response to griseofulvin, an option may be terbinafine or itraconazole.
Fluconazole is used as an alternative therapy, particularly in cases unresponsive to conventional treatment [50]; it can be administered daily at doses of 5–6 mg/kg/day or at weekly doses of 8 mg/kg/day [13, 51].
The definitive end-point for adequate treatment is not clinical response but mycological cure; therefore, follow-up with repeat mycology sampling is recommended at the end of the standard treatment period and then monthly until mycological clearance is documented. Treatment should, therefore, be tailored for each individual patient [11••].
Complications
Secondary bacterial infection is not common even in kerion, where this may occur under large superficial crusts rather than in the form of folliculitis. Rarely, erythema nodosum has been described in association with TC. This reaction can be associated with the deposit of immune complexes and is known as an Id reaction. [52].
Conclusion
TC is still a common disease worldwide, with a wide variety of clinical presentations leading to numerous differential diagnoses. Control of TC is possible with current understanding of the immunology and host susceptibility. Although there are cases that can be treated with topical therapy, the use of oral antifungal medications for clinical and mycological cures is commonly required. Continuous surveillance in schools and in the patient’s family is necessary to identify asymptomatic carriers and reduce the risk of spreading infection among the population.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major Importance
Bennassar A, Grimalt R. Management of tinea capitis in childhood. Clin Cosmet Investig Dermatol. 2010;3:89–98.
• Rebollo N, López-Barcenas AP, Arenas R. Tinea capitis. Actas Dermosifiliogr. 2008;99(2):91–100. This paper is an overview of the disease.
Hay RJ, Robles W, Midgley G, Moore MK. European Confederation of Medical Mycology Working Party on Tinea Capitis. Tinea capitis in Europe: new perspective on an old problem. J Eur Acad Dermatol Venereol. 2001;15(3):229–33.
• Kallel A, Hdider A, Fakhfakh N, Belhadj S, Belhadj-Salah N, Bada N, et al. Tinea capitis: main mycosis child. Epidemiological study on 10 years. J Mycol Med. 2017;27(3):345–50. This paper showed the most common clinical manifestations of tinea capitis in children.
Ziegler W, Lempert S, Goebeler M, Kolb-Mäurer A. Tinea capitis: temporal shift in pathogens and epidemiology. J Dtsch Dermatol Ges. 2016;14(8):818–25. (Abstract)
Kieliger S, Glatz M, Cozzio A, Bosshard PP. Tinea capitis and tinea faciei in the Zurich area - an 8-year survey of trends in the epidemiology and treatment patterns. J Eur Acad Dermatol Venereol. 2015;29(8):1524–9.
Sei Y. 2011 epidemiological survey of dermatomycoses in Japan. Med Mycol J. 2015;56(4):J129–35.
• Brito-Santos F, Figueiredo-Carvalho MHG, Coelho RA, Sales A, Almeida-Paes R. Tinea capitis by Microsporum audouinii: case reports and review of published global literature 2000‑2016. Mycopathologia. 2017;182(11–12):1053–60. An important review of the disease mainly on therapeutic options
Niczyporuk W, Krajewska-Kułak E, Łukaszuk C. Tinea capitis favosa in Poland. Mycoses. 2004;47(5–6):257–60.
Khosravi AR, Shokri H, Vahedi G. Factors in etiology and predisposition of adult tinea capitis and review of published literature. Mycopathologia. 2016;181(5–6):371–8.
•• Hay RJ. Tinea capitis: current status. Mycopathologia. 2017;182(1–2):87–93. This paper gives important strategies for treating tinea capitis.
Martínez-Suárez H, Guevara-Cabrera N, Mena C, Valencia A, Araiza J, Bonifaz A. Tiña de la cabeza. Reporte de 122 casos. Derma CMQ. 2007;5(1):9–14.
Bonifaz A, Carrasco-Gerard E, Palacios-López C, Araiza J. Tratamiento de la cabeza con dosis intermitentes (semanales) de fluconazol. Derma Cosmet Med Quir. 2003;1:11–9.
Gupta AK, Summerbell RC. Tinea capitis. Med Mycol. 2000;38(4):255–87.
Ilkit M, Demirhindi H. Asymptomatic dermatophyte scalp carriage: laboratory diagnosis, epidemiology and management. Mycopathologia. 2008;165(2):61–71.
Zurita J, Hay RJ. Adherence of dermatophyte microconidia and arthroconidia to human keratinocytes in vitro. J Investig Dermatol. 1987;89(5):529–34.
Sriranganadane D, Waridel P, Salamin K, Feuermann M, Mignon B, Staib P, et al. Identification of novel secreted proteases during extracellular proteolysis by dermatophytes at acidic pH. Proteomics. 2011;11(22):4422–33.
Staib P, Zaugg C, Mignon B, Weber J, Grumbt M, Pradervand S, et al. Differential gene expression in the pathogenic dermatophyte Arthroderma benhamiae in vitro versus during infection. Microbiology. 2010;156(Pt3):884–95.
Niyonsaba F, Ogawa H. Protective roles of the skin against infection: implication of naturally occurring human antimicrobial agents beta-defensins, cathelicidin LL-37 and lysozyme. J Dermatol Sci. 2005;40(3):157–68.
Abdelaal NH, Rashed LA, Ibrahim SY, Abd El Halim MH, Ghoneim N, Saleh NA, et al. Cathelicidin (LL-37) level in the scalp hair of patients with tinea capitis. Med Mycol. 2017;55(7):733–6.
Mao L, Zhang L, Li H, Chen W, Wang H, Wu S, et al. Pathogenic fungus Microsporum canis activates the NLRP3 inflammasome. Infect Immun. 2014;82(2):882–92.
Wagner DK, Sohnle PG. Cutaneous defenses against dermatophytes and yeasts. Clin Microbiol Rev. 1995;8(3):317–35.
Calderon RA, Hay RJ. Fungicidal activity of human neutrophils and monocytes on dermatophyte fungi, Trichophyton quinckeanum and Trichophyton rubrum. Immunology. 1987;61(3):289–95.
Sakuragi Y, Sawada Y, Hara Y, Ohmori S, Omoto D, Haruyama S, et al. Increased circulating Th17 cell in a patient with tinea capitis caused by Microsporum canis. Allergol Int. 2016;65(2):215–6.
Gorgievska-Sukarovska B, Skerlev M, Žele-Starčević L, Husar K, Halasz M. Kerion celsi due to Microsporum canis with a dermatophytid reaction. Acta Dermatovenerol Croat. 2017;25(2):151–4.
Brissos J, Gouveia C, Neves C, Varandas L. Remember kerion celsi. BMJ Case Rep. 2013;2013
de Hoog GS, Dukik K, Monod M, Packeu A, Stubbe D, Hendrickx M, et al. Toward a novel multilocus phylogenetic taxonomy for the dermatophytes. Mycopathologia. 2017;182(1–2):5–31.
Farooqi M, Tabassum S, Rizvi DA, Rahman A, Rehanuddin, Awan S, et al. Clinical types of tinea capitis and species identification in children: an experience from tertiary care centres of Karachi, Pakistan. J Pak Med Assoc. 2014;64(3):304–8.
Kefalidou S, Odia S, Gruseck E, Schmidt T, Ring J, Abeck D. Wood’s light in Microsporum canis positive patients. Mycoses. 1997;40(11–12):461–3.
Robert R, Pihet M. Conventional methods for the diagnosis of dermatophytosis. Mycopathologia. 2008;166(5–6):295–306.
Symoens F, Jousson O, Planard C, Fratti M, Staib P, Mignon B, et al. Molecular analysis and mating behaviour of the Trichophyton mentagrophytes species complex. Int J Med Microbiol. 2011;301(3):260–6.
Kanbe T, Suzuki Y, Kamiya A, Mochizuki T, Kawasaki M, Fujihiro M, et al. Species-identification of dermatophytes Trichophyton, Microsporum and Epidermophyton by PCR and PCR-RFLP targeting of the DNA topoisomerase II genes. J Dermatol Sci. 2003;33(1):41–54.
Brillowska-Dabrowska A, Michałek E, Saunte DM, Nielsen SS, Arendrup MC. PCR test for Microsporum canis identification. Med Mycol. 2013;51(6):576–9.
Verrier J, Krähenbühl L, Bontems O, Fratti M, Salamin K, Monod M. Dermatophyte identification in skin and hair samples using a simple and reliable nested polymerase chain reaction assay. Br J Dermatol. 2013;168(2):295–301.
Deng S, Zhou Z, de Hoog GS, Wang X, Abliz P, Sun J, et al. Evaluation of two molecular techniques for rapid detection of the main dermatophytic agents of tinea capitis. Br J Dermatol. 2015;173(6):1494–500.
Deng S, de Hoog GS, Verweij PE, Zoll J, Ilkit M, Morsali F, et al. In vitro antifungal susceptibility of Trichophyton violaceum isolated from tinea capitis patients. J Antimicrob Chemother. 2015;70(4):1072–5.
Brasileiro A, Campos S, Cabete J, Galhardas C, Lencastre A, Serrão V. Trichoscopy as an additional tool for the differential diagnosis of tinea capitis: a prospective clinical study. Br J Dermatol. 2016;175(1):208–9.
Elghblawi E. Tinea capitis in children and trichoscopic criteria. Int J Trichology. 2017;9(2):47–9.
Lu M, Ran Y, Dai Y, Lei S, Zhang C, Zhuang K, et al. An ultrastructural study on corkscrew hairs and cigarette-ash-shaped hairs observed by dermoscopy of tinea capitis. Scanning. 2016;38(2):128–32.
Schechtman RC, Silva ND, Quaresma MV, Bernardes Filho F, Buçard AM, Sodré CT. Dermatoscopic findings as a complementary tool in the differential diagnosis of the etiological agent of tinea capitis. An Bras Dermatol. 2015;90(3 Suppl 1):13–5.
Campos S, Brasileiro A, Galhardas C, Apetato M, Cabete J, Serrão V, et al. Follow-up of tinea capitis with trichoscopy: a prospective clinical study. J Eur Acad Dermatol Venereol. 2017;31(11):e478–80.
Arrazola-Guerrero J, Isa-Isa R, Torres-Guerrero E, Arenas R. Tinea capitis. Dermoscopic findings in 37 patients. Rev Iberoam Micol. 2015;32(4):242–6.
Velho GD, Selores M, Amorim I, Lopes V. Guess what! Tinea capitis by Trichophyton schöenleinii. Eur J Dermatol. 2001;11(5):481–2.
Fuller LC, Barton RC, Mohd Mustapa MF, Proudfoot LE, Punjabi SP, Higgins EM. British Association of Dermatologists’ guidelines for the management of tinea capitis 2014. Br J Dermatol. 2014;171(3):454–63.
Chen X, Jiang X, Yang M, González U, Lin X, Hua X, et al. Systemic antifungal therapy for tinea capitis in children. Cochrane Database Syst Rev. 2016;5:CD004685.
•• Chen X, Jiang X, Yang M, Bennett C, González U, Lin X, et al. Systemic antifungal therapy for tinea capitis in children: an abridged Cochrane review. J Am Acad Dermatol. 2017;76(2):368–74. An important paper that summarizes the clinical trials available for tinea capitis
Tey HL, Tan AS, Chan YC. Meta-analysis of randomized, controlled trials comparing griseofulvin and terbinafine in the treatment of tinea capitis. J Am Acad Dermatol. 2011;64(4):663–70.
Shemer A, Grunwald MH, Gupta AK, Lyakhovitsky A, Daniel CR 3rd, Amichai B. Griseofulvin and fluconazole reduce transmission of tinea capitis in schoolchildren. Pediatr Dermatol. 2015;32(5):696–700.
Danielli LJ, Lopes W, Vainstein MH, Fuentefria AM, Apel MA. Biofilm formation by Microsporum canis. Clin Microbiol Infect. 2017;23(12):941–2.
Shemer A, Plotnik IB, Davidovici B, Grunwald MH, Magun R, Amichai B. Treatment of tinea capitis - griseofulvin versus fluconazole - a comparative study. J Dtsch Dermatol Ges. 2013;11(8):737–41–2. (Abstract)
Kakourou T, Uksal U, European Society for Pediatric Dermatology. Guidelines for the management of tinea capitis in children. Pediatr Dermatol. 2010;27(3):226–8.
Papaiordanou F, da Silveira BR, Abulafia LA. Hypersensitivity reaction to Sporothrix schenckii: erythema nodosum associated with sporotrichosis. Rev Soc Bras Med Trop. 2015;48(4):504.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Fungal Infections of Skin and Subcutaneous Tissue
Rights and permissions
About this article
Cite this article
Tirado-Sánchez, A., Bonifaz, A. Tinea Capitis: Current Review of the Literature. Curr Fungal Infect Rep 12, 120–126 (2018). https://doi.org/10.1007/s12281-018-0320-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12281-018-0320-2