Abstract
Opportunistic microbes are able to exist as commensals or pathogens depending on local environmental conditions. The bacterial microbiome at mucosal sites (gut, oral and vaginal) has been well characterized but there has been less focus on the fungal component of the microbiome, the “mycobiome”, especially of the oral mucosa. Genomic characterization studies have shown that Candida species are the most prevalent fungal species in the mycobiomes of the murine gut and human oral cavity, with C. albicans being the most abundant fungal species in the oral cavity. In this review, we outline recent advances in the characterization of the oral mycobiome, how different Candida species colonize, invade and infect the oral cavity, and how epithelial surfaces play a key role in antifungal activity and discriminate between commensal and pathogenic Candida.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
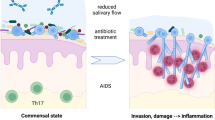
The first step of any mucosal infection is ‘colonization’, whereby a microbe ‘attaches’ to a host cell or moiety. Depending on the microbe, this can lead to either a degree of mutualism in the case of commensal microbes, or invasion of the epithelial barrier followed by disease and immune activation in the case of pathogenic microbes. In commensalism, the microbe and host live in a mutually beneficial relationship, whereby the microbe obtains all necessary nutrients to survive and grow on the mucosal surface without causing harm, while the host reaps the beneficial effects in ways that are often less well understood (e.g. utilizing microbial secondary metabolites and vitamins). This kind of commensal relationship is well documented for colonizing bacteria in the gut [1]. These commensal bacteria are important in general health since perturbations in the microbial gut flora can lead to multiple downstream effects, including aberrant immune responses and initiation of gut diseases such as inflammatory bowel disease (e.g. Crohn’s disease and ulcerative colitis) [2]. The consensus of opinion now is that there is a close relationship between microbiota and health at multiple mucosal sites [3].
Microbiomes, Fungi and the Oral Cavity
The interaction between a host and its microbiome is essential to health. Until recently, little information was available regarding the composition of the microbiota on mucosal surfaces and even less information with regard to identifying resident commensal microbes that could turn ‘pathogenic’ under suitably predisposing conditions. However, technological advances have permitted the ‘microbiomes’ of these sites to be studied in detail, providing a better understanding of the full microbial composition at various mucosal sites. These studies have focused heavily on bacterial gut microbiomes [3–6], although other mucosal microbiomes are now being investigated. Together, these studies demonstrate that the gut microbiome is the most diverse, followed by the oral microbiome, and the vaginal microbiome being least diverse [6]. Although each microbiome is diverse, it is also stable between individuals for the respective mucosal site [5]. Recently, there has been increased interest in the oral microbiome, which comprises viruses, bacteria, fungi, protozoa and archaea, as the bacteria present in this microbiome are responsible for the two most common diseases of humans: dental caries (tooth decay) and periodontal (gum) disease [7]. Unfortunately, although the presence of a fungal community on mucosal surfaces is well known, the fungal component of these microbiomes, the ‘mycobiome’, has largely been overlooked, despite fungi having the potential to be highly detrimental to human health.
Recently, the genomic characterization of the murine gut mycobiome has demonstrated that, although not homogeneous, surprisingly over 97 % of the population comprises just ten fungal species [8••]. Remarkably, a single fungus, Candida tropicalis, constituted over 65 % of the entire population in one mouse colony, demonstrating that Candida species can be a major part of the mammalian gut mycobiome. Notably, however, C. albicans and C. parapsilosis are the major fungal constituents in humans [9•]. Therefore, it would appear that differences exist between the mouse and human gut mycobiomes, and although the underlying reasons for this are unclear, these differences may provide clues as to why C. albicans is not a natural colonizer of mice. To this end, new murine models are continually being developed to specifically understand Candida colonization rather than infection [10–13].
Only one study has characterized the human oral mycobiome, undertaken in 20 healthy individuals using a multitag pyrosequencing approach and the panfungal internal transcribed spacer primers [14••]. The work identified 74 culturable and 11 nonculturable fungal genera. Of the culturable genera, Candida species were the most frequently observed (isolated from 75 % of participants), followed by Cladosporium (65 %), Aureobasidium, Saccharomycetales (50 %), Aspergillus (35 %), Fusarium (30 %) and Cryptococcus (20 %). Four of these genera (Candida, Aspergillus, Fusarium and Cryptococcus) are known to be pathogenic in humans. Notably, 36 % of fungi belonged to the nonculturable category. In total, 101 fungal species were identified. While the abundance of Candida in these individuals was clearly expected, the presence of Aspergillus, Fusarium and Cryptococcus in the healthy oral cavity is rather surprising, since these fungi are not generally recognized as oral colonizers. However, although the incidence of Cryptococcus and Aspergillus oral mycoses is rare, they have been reported in both immunocompromised and immunocompetent patients (e.g. in diabetes mellitus), though their occurrences are usually secondary to a more serious systemic infection [15]. One could postulate that low-level oral colonization of these fungi may provide a reservoir from which these species can migrate to drive disease under suitable predisposing conditions. Despite these data and speculations, the clinical relevance for the presence of a diverse population of fungal species in the oral cavity is still unclear [14••].
Candida and the Oral Cavity
By far the most common fungi endogenous to humans are Candida species. Although Candida species usually cause no pathology, these fungi can become pathogenic if there are alterations in the local environment, such as modifications in the normal microbiota or compromised immune defences [16]. As such, Candida species are the most common fungal pathogens in humans, giving rise to severe morbidity in millions of individuals worldwide. Oropharyngeal and vaginal infections are the most common manifestation and predisposing factors include antibiotic, glucocorticosteroid and hormone therapies, as well as diabetes mellitus and infections such as HIV and AIDS [17, 18]. Although C. albicans causes most of the Candida infections, non-C. albicans species such as C. glabrata, C. tropicalis, C. parapsilosis, C. guilliermondii and C. krusei are also pathogenic to humans and have emerged as important opportunistic pathogens in the oral mucosa [16]. In immunocompromised patients (e.g. transplant, cancer and intensive care patients), Candida species cause systemic infections with approximately 40 % mortality and are now the fourth most common hospital-acquired bloodstream infection [19]. Given that the majority of systemic Candida infections are acquired across the mucosae (predominantly gut), it is of paramount importance that we understand the basic biological mechanisms that normally restrict Candida species to mucosal surfaces.
The most abundant Candida species in the oral cavity is C. albicans, a polymorphic fungus that resides as a commensal in approximately 40–80 % of individuals, and is represented by a mixed strain population [20]. Notably, in the mycobiome study described above C. albicans was found in 40 % of individuals, followed by C. parapsilosis (15 %), C. tropicalis (15 %), C. khmerensis (5 %) and C. metapsilosis (5 %) [14••]. Surprisingly, C. glabrata was not found, despite being commonly isolated from the healthy oral cavity [21] and being the second leading species causing hospital-acquired Candida bloodstream infection [22]. Further, although C. metapsilosis is known to be an oral colonizer [23], C. khmerensis has not previously been described as a colonizer of any mucosal surface. Whether any interdependent relationships exist between these Candida species or other members of the oral mycobiome is not known, but future studies investigating potential interactions may shed light on the pathogenicity of these organisms [14••]. Given that C. albicans is the most common human fungal pathogen, this review predominantly targets the mechanisms utilized by C. albicans to promote colonization and infection of oral mucosal surfaces.
C. albicans Colonization and Infection of the Oral Cavity
Although C. albicans is normally a harmless commensal, the specific attributes required by this fungus to promote colonization rather than infection have rarely been studied [24]. However, in all likelihood, the same attributes are probably required for both colonization and infection, which in turn will be determined by the physiological and immune status of the host. It should also be noted that epithelial cells appear to possess innate antifungal activity (via annexin-A1) to restrict C. albicans growth [25], which may potentially help maintain C. albicans in the commensal state during health. However, when local environmental and/or immune conditions are perturbed, C. albicans is able to override these inhibitory mechanisms to cause local superficial mucosal infections, which require ‘virulence’ factors to promote pathogenicity. C. albicans possesses many putative virulence attributes that contribute to general survival, fitness and persistence within the host as well as specific factors associated with epithelial adhesion, biofilm formation, invasion, cell damage and induction/evasion of host responses [26–29]. The host defences include mechanical barriers to microbial penetration (such as epithelial cells), antimicrobial factors, and innate and cellular immune mechanisms [27, 30–33].
Epithelial cells at mucosal surfaces are the first point of contact with C. albicans and are therefor the first line of defence against the fungus. A prerequisite for colonization is the ability of C. albicans to adhere to epithelial cells, while invasion into and damage of epithelial cells are generally regarded as infection-specific qualities. Since the surface composition of the C. albicans cell wall is continually altering, especially during the yeast-to-hypha switch, the precise nature of C. albicans adhesion to epithelial cells is a complex and multifactorial process that involves several types of adhesins [34]. Notably, the interaction of the C. albicans yeast cell with epithelial cells is a potent stimulator of hypha formation [35, 36], and since hyphae are considerably more adherent than yeast forms, this indicates that specific hyphal proteins promote epithelial adhesion. This is supported by studies showing that wild-type C. albicans strains unable to produce true hyphae have a reduced ability to adhere to epithelial cells [37].
C. albicans Proteins that Promote Colonization and Infection of the Oral Cavity
Recent studies have implicated a number of fungal moieties as being important for epithelial adhesion (Table 1). A key adhesin family is the Als (agglutinin-like sequence) family, particularly Als3 which is a hypha-specific protein [38]. Als3 is one of eight Als proteins linked to glycosylphosphatidylinositol (GPI) that mediate adhesion to numerous host cells in which the N-terminal region is required for substrate binding [39, 40]. During in vitro oral epithelial cell infection, ALS3 gene expression is upregulated [35] and both gene disruption and Als3 protein overexpression studies have demonstrated a direct role for Als3 in adherence [40, 41]. Roles for other members of the Als family in epithelial adherence are somewhat contradictory. However, high-resolution structures of N-terminal Als1 and Als9 adhesins demonstrate that ligand recognition relies on a motif capable of binding flexible C-termini of peptides in an extended conformation. Central to this mechanism is an invariant lysine that recognizes the C-terminal carboxylate of ligands at the end of a deep-binding cavity [42•]. Additional structural studies of the Als3 adhesin will no doubt provide highly informative data regarding host–Candida interactions.
Hwp1 (hyphal wall protein 1) is another GPI-linked hypha-associated protein with a major role in epithelial adhesion. The N-terminal domain of this protein acts as a substrate for epithelial transglutaminases, enabling strong covalent links with other epithelial proteins [43•]. Like ALS3, HWP1 expression is upregulated during in vitro infection of oral epithelial cells [35] and is expressed in vivo in individuals colonized and orally infected with C. albicans [44]. HWP1 is required for mucosal pathogenicity as a hwp1∆/∆ mutant poorly adhered to murine oral epithelial cells and was significantly attenuated in virulence in a murine oropharyngeal candidosis model [45]. A huge number of hypha-associated genes exist, including well-known genes such as HYR1 and ECE1. Their precise roles in epithelial adhesion/interactions are still unclear, although Hyr1 may be required for pathogenicity in a murine oral biofilm model [46].
A number of other morphology-independent genes and cell wall-associated proteins play roles in epithelial cell adherence and mucosal pathogenicity. These include: surface GPI-proteins such as Iff4, Eap1, Utr2 and Ecm33; noncovalent wall-associated proteins such as Mp65, Phr1, Sun41 and Pra1; the integrin-like Int1 protein; secreted and cell-surface-associated proteases such as Sap1–Sap3, Sap9 and Sap10; internal endoplasmic reticulum (cell wall-processing) proteins such as Big1; and protein trafficking and vesicle transport proteins such as Vps11 [27]. However, the contribution of many of these proteins to epithelial adhesion and infection is likely to be indirect given that they possess complex, multifactorial functions that contribute to cell wall integrity and hypha formation.
C. albicans Invasion and Damage to Epithelial Cells
In commensalism, C. albicans normally resides on mucosal surfaces without causing disease or activating host defence mechanisms. However, if the correct environmental conditions arise, C. albicans can proliferate to cause superficial infections even in health (e.g. vaginitis). In these circumstances C. albicans invades the apical epithelial cells and may cause local damage, thereby eliciting host immune defences that ultimately reduce burdens to commensal levels or clear the fungus. These processes of invasion and subsequent damage induction are considered pathogenic rather than commensal attributes. C. albicans utilizes two invasion mechanisms for epithelial cell entry: induced endocytosis and active penetration [29, 36, 47]. The entry mechanism used is dependent on the epithelial cell lineage and appears to be triggered by hypha-associated factors [47, 48•]. The direct link between hypha formation and epithelial cell invasion is evident when screening C. albicans hypha-deficient mutants, which often exhibit adhesion and invasion defects [35]. Notably, the hypha-associated factors that promote adhesion, epithelial cell invasion and subsequent damage appear to be controlled by the fungal cAMP/PKA/Efg1 signalling pathway but not the MAPK pathways [35].
Induced endocytosis is mediated by the epithelial cell and occurs early during epithelial–C. albicans interactions, usually within 4 h [49]. Two C. albicans ‘invasins’, Als3 [48•] and Ssa1 [50], have been shown to induce endocytosis by oral epithelial cells (Table 1), which appears to be mediated via epithelial E-cadherin, through an actin-dependent mechanism requiring clathrin [51]. Once endocytosed, C. albicans hyphae enter the vacuolar endosomal compartments [52]. C. albicans hyphae-containing endosomes transiently express the early endosomal marker EEA1 but show marked defects in expression of the late endosomal marker LAMP1 and the lysosomal marker cathepsin D. Thus, C. albicans hyphae may prevent maturation of the endolysosome, potentially promoting intracellular fungal growth [52]. This survival strategy may allow C. albicans to survive and develop for extended periods within the epithelial cell, enabling the hypha to emerge out of one epithelial cell to infect a neighbouring cell. This will result in cell damage and immune activation, thus leading towards an infection phenotype. Similar survival strategy mechanisms might be employed by C. albicans to evade killing by macrophages [53, 54]. However, the direct role of Als3-mediated induced endocytosis in the induction of damage and immune activation of epithelial cells may be limited [55].
Active penetration occurs at later time points than induced endocytosis and requires the fungus to be viable. This process results in hypha invasion in between or through epithelial cells [35, 47] rather than being taken up by the cell. It is still unclear which C. albicans factors contribute to active penetration but roles for the secreted aspartic proteinases (Sap), lipases (Lip) and phospholipases (Plb) have been proposed [56–58] (Table 1). Of note, Sap5 activity appears to be required for E-cadherin degradation [59], which is present at high concentrations at interepithelial junctions. E-cadherin degradation may disrupt epithelial barrier function enabling hyphae to penetrate mucosal tissues. C. albicans hyphae that have directly invaded epithelial cells or the submucosal layers will induce tissue damage via necrosis and/or apoptosis [27, 35, 49]. Although apoptosis can be beneficial to the host, necrosis is nearly always detrimental. The precise contribution of each mechanism to damage induction is still unclear but maintenance of hypha formation appears critical for eliciting damage via necrosis [35, 36, 60], whilst cell surface N- and O-mannans may promote damage via apoptosis [61].
Non-C. albicans Colonization and Infection of the Oral Cavity
Historically, C. albicans accounts for approximately 80 % of all human candidoses [62], but over the past few decades non-C. albicans species, namely C. glabrata, C. tropicalis and C. parapsilosis, have emerged as important human pathogens [63]. C. glabrata is the second most prevalent human yeast pathogen after C. albicans but has distinguishing features, in that C. glabrata is haploid rather than diploid, does not form hyphae, and has an innate resistance to azole antifungals. Likewise, C. parapsilosis and C. tropicalis, the third and fourth most prevalent Candida pathogens, are diploid but do not form true hyphae. All three species fail to filament on epithelial surfaces and this probably explains their poor ability to infect epithelial cells compared with C. albicans [60, 64–66].
A key virulence factor of C. glabrata appears to be a major group of adhesins encoded by a large family of EPA (epithelial adhesin) genes [67], which have homology to the ALS genes of C. albicans. Although few studies have been performed on the C. glabrata Epa proteins, it appears that Epa1p is the main member of this family required for epithelial adhesion through its binding to N-acetyl lactosamine-containing glycoconjugates [68•]. Likewise, both C. parapsilosis [69] and C. tropicalis [70] possess multiple Als-like cell wall proteins, but limited data currently exist regarding their role in epithelial adhesion, colonization or mucosal infection. C. glabrata, C. tropicalis and C. parapsilosis all exhibit hydrolytic enzyme activity (thought to promote adhesion and invasion in C. albicans) to some degree, with all three species possessing SAP-like genes [71••, 72–74]. As with C. albicans [75–78], SAP gene expression and activity in C. parapsilosis [65] and C. tropicalis [64] is present at epithelial surfaces, where it is thought to contribute to mucosal colonization and infection. It should be noted, however, that the role of the C. glabrata SAP-like genes remains unclear [79]. C. glabrata, C. tropicalis and C. parapsilosis also exhibit Plb activity [63, 80, 81], but again their roles in mucosal colonization and infection are undetermined. Finally, unlike C. glabrata, C. parapsilosis possesses Lip activity, which appears to contribute to epithelial colonization/infection [82], and although the C. tropicalis genome contains LIP genes [71••], the role of these proteins in C. tropicalis colonization/infection is currently unknown.
Epithelial Discrimination Between Commensal and Pathogenic Candida
Given the apparent importance of hypha formation in epithelial invasion and pathogenicity, determining how epithelial cells are activated by the yeast and hyphal form of C. albicans might provide valuable information regarding how mucosal tissues discriminate between the commensal and pathogenic states of this fungus [83, 84]. Recent advances in this area have demonstrated that in oral epithelial cells C. albicans appears to activate two main signalling pathways, the mitogen-activated protein kinase (MAPK) and the nuclear factor-kappaB (NF-κB) pathways [85••]. The first, immediate but transient epithelial recognition event is independent of morphology and appears to constitute recognition of the fungal cell wall polysaccharides (mannans and β-glucans) via the NF-κB and all three MAPK pathways including p38, JNK (c-Jun N-terminal kinase) and ERK1/2 (extracellular signal-regulated protein kinase) [85••, 86]. This results in the activation of the p65/p50 transcription factor via NF-κB and the c-Jun transcription factor via JNK and ERK1/2. However, this is insufficient to fully activate the epithelial cells as proinflammatory cytokines are not induced. This recognition event may also occur during mucosal C. albicans colonization (commensalism). This lack of activation by C. albicans cell wall polysaccharides has also recently been demonstrated for skin keratinocytes [87], suggesting that these polysaccharides play a limited role in inducing epithelial/keratinocyte immune responses to fungal cells.
The second recognition event only occurs when C. albicans hyphae (but not yeast) are present at higher burdens. In these circumstances, there is strong activation of NF-κB and all three MAPK pathways which results in the activation of the c-Fos transcription factor (via p38) rather than c-Jun (via ERK1/2 and JNK). The combination of c-Fos and NF-κB activation appears to be sufficient for full activation of epithelial cells resulting in the induction of proinflammatory cytokines [85••]. This recognition event may occur during C. albicans invasion (pathogenicity). Thus, it has been proposed that epithelial MAPK-p38/c-Fos activation may constitute a ‘danger response’ mechanism that is kept in check to permit immune quiescence in the presence of low C. albicans burdens but immune activation when the C. albicans burden increases and becomes hyphal [27, 83, 84]. Interestingly, recent work has also demonstrated that tissue macrophages also respond selectively to C. albicans hyphae, although recognition in macrophages appears to occur through hyphal wall-specific β-glucan moieties [88]. Importantly, the MAPK-p38/c-Fos pathway is activated only by hypha-forming Candida species (C. albicans and C. dubliniensis) but not by C. tropicalis, C. glabrata, C. parapsilosis or C. krusei [60], and is also present in human vaginal epithelial cells [89].
Therefore, this MAPK-p38/c-Fos mechanism may enable different mucosal tissues to recognize invading Candida hyphae, thereby potentially identifying when this commensal fungus has become pathogenic. Together with similar findings in Caenorhabditis elegans (nematode worm) with C. albicans [90] and in murine intestinal epithelial cells with bacterial pathogens (Citrobacter rodentium) [91•], the data suggest that MAPK-p38 signalling may be required for epithelial recognition of ‘pathogenic’ microbes and to initiate inflammatory responses. The epithelial receptors mediating hyphal recognition via p38 are currently unknown but do not seem to involve Toll-like receptors or C-type lectins [85••], even though they are expressed in epithelial cells [92•, 93]. Therefore, epithelial cells appear to utilize sensing mechanisms for immune activation that are different from those in myeloid cells [94], possibly as they target alternative fungal moieties. This field of epithelial pattern recognition of Candida will no doubt be an active area of future research.
Conclusion
Over the past few years our understanding of the mechanistic events involved in host mucosal responses to Candida pathogens has grown significantly. Originally, the role of epithelial cells in these events was considered to be relatively unimportant, but it has become increasingly apparent that they are critical in fungal recognition and host defence. For C. albicans, hypha formation appears key to the host–pathogen interaction event at mucosal surfaces, since it is required by the fungus to promote adhesion, colonization and invasion, and is targeted by the epithelial cell to potentially discriminate between commensal and pathogenic states of the fungus. These advances provide important insights into the complex mechanisms by which appropriate host immune responses may be initiated and how this affects fungal colonization and infection.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Schluter J, Foster KR. The evolution of mutualism in gut microbiota via host epithelial selection. PLoS Biol. 2012;10:e1001424.
Willing BP, Dicksved J, Halfvarson J, et al. A pyrosequencing study in twins shows that gastrointestinal microbial profiles vary with inflammatory bowel disease phenotypes. Gastroenterology. 2010;139:1844–54.
Claesson MJ, Jeffery IB, Conde S, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488:178–84.
Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–7.
The Human Microbiome Project C. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–14.
Li K, Bihan M, Yooseph S, Methe BA. Analyses of the microbial diversity across the human microbiome. PLoS One. 2012;7:e32118.
Wade WG. The oral microbiome in health and disease. Pharmacol Res. 2013;69:137–43.
•• Iliev ID, Funari VA, Taylor KD, et al. Interactions between commensal fungi and the C-type lectin receptor Dectin-1 influence colitis. Science. 2012;336:1314–7. First characterization of the complex interaction between a murine host and the gut mycobiome that can affect health and disease.
• Scanlan PD, Marchesi JR. Micro-eukaryotic diversity of the human distal gut microbiota: qualitative assessment using culture-dependent and -independent analysis of faeces. ISME J. 2008;2:1183–93. First characterization of the human gut mycobiome.
Rahman D, Mistry M, Thavaraj S, et al. Murine model of concurrent oral and vaginal Candida albicans colonization to study epithelial host-pathogen interactions. Microbes Infect. 2007;9:615–22.
Samonis G, Galanakis E, Ntaoukakis M, et al. Effects of carbapenems and their combination with amikacin on murine gut colonisation by Candida albicans. Mycoses. 2012. doi: 10.1111/j.1439-0507.2012.02212.x.
Matsubara VH, Silva EG, Paula CR, et al. Treatment with probiotics in experimental oral colonization by Candida albicans in murine model (DBA/2). Oral Dis. 2012;18:260–4.
Kanaguchi N, Narisawa N, Ito T, et al. Effects of salivary protein flow and indigenous microorganisms on initial colonization of Candida albicans in an in vivo model. BMC Oral Health. 2012;12:36.
•• Ghannoum MA, Jurevic RJ, Mukherjee PK, et al. Characterization of the oral fungal microbiome (mycobiome) in healthy individuals. PLoS Pathog. 2010;6:e1000713. First description of the healthy human oral mycobiome, demonstrating that multiple fungal species exist in the oral cavity.
Iatta R, Napoli C, Borghi E, Montagna MT. Rare mycoses of the oral cavity: a literature epidemiologic review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:647–55.
Samaranayake LP, Keung Leung W, Jin L. Oral mucosal fungal infections. Periodontol 2000. 2009;49:39–59.
Samaranayake LP, Fidel PL, Naglik JR, et al. Fungal infections associated with HIV infection. Oral Dis. 2002;8 Suppl 2:151–60.
Fidel PL. History and update on host defense against vaginal candidiasis. Am J Reprod Immunol. 2007;57:2–12.
Wisplinghoff H, Bischoff T, Tallent SM, et al. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–17.
Jacobsen MD, Duncan AD, Bain J, et al. Mixed Candida albicans strain populations in colonized and infected mucosal tissues. FEMS Yeast Res. 2008;8:1334–8.
Merenstein D, Hu H, Wang C, Hamilton P, et al. Colonization by Candida species of the oral and vaginal mucosa in HIV-infected and noninfected women. AIDS Res Hum Retroviruses. 2013;29:30–4.
Pfaller MA, Diekema DJ. Epidemiology of invasive mycoses in North America. Crit Rev Microbiol. 2010;36:1–53.
Moris DV, Melhem MS, Martins MA, et al. Prevalence and antifungal susceptibility of Candida parapsilosis complex isolates collected from oral cavities of HIV-infected individuals. J Med Microbiol. 2012;61:1758–65.
Southern P, Horbul J, Maher D, Davis DA. C. albicans colonization of human mucosal surfaces. PLoS One. 2008;3:e2067.
Lilly EA, Yano J, Fidel Jr PL. Annexin-A1 identified as the oral epithelial cell anti-Candida effector moiety. Mol Oral Microbiol. 2010;25:293–304.
Fanning S, Mitchell AP. Fungal biofilms. PLoS Pathog. 2012;8:e1002585.
Naglik JR, Moyes DL, Wachtler B, Hube B. Candida albicans interactions with epithelial cells and mucosal immunity. Microbes Infect. 2011;13:963–76.
Hiller E, Zavrel M, Hauser N, et al. Adaptation, adhesion and invasion during interaction of Candida albicans with the host – focus on the function of cell wall proteins. Int J Med Microbiol. 2011;301:384–9.
Zhu W, Filler SG. Interactions of Candida albicans with epithelial cells. Cell Microbiol. 2010;12:273–82.
Weinberg A, Naglik JR, Kohli A, et al. Innate immunity including epithelial and nonspecific host factors: workshop 1B. Adv Dent Res. 2011;23:122–9.
Yano J, Lilly E, Barousse M, Fidel Jr PL. Epithelial cell-derived S100 calcium-binding proteins as key mediators in the hallmark acute neutrophil response during Candida vaginitis. Infect Immun. 2010;78:5126–37.
Dongari-Bagtzoglou A, Fidel Jr PL. The host cytokine responses and protective immunity in oropharyngeal candidiasis. J Dent Res. 2005;84:966–77.
Sweet SP, Challacombe SJ, Naglik JR. Whole and parotid saliva IgA and IgA-subclass responses to Candida albicans in HIV infection. Adv Exp Med Biol. 1995;371B:1031–4.
Chaffin WL. Candida albicans cell wall proteins. Microbiol Mol Biol Rev. 2008;72:495–544.
Wachtler B, Wilson D, Haedicke K, et al. From attachment to damage: defined genes of Candida albicans mediate adhesion, invasion and damage during interaction with oral epithelial cells. PLoS One. 2011;6:e17046.
Zakikhany K, Naglik JR, Schmidt-Westhausen A, et al. In vivo transcript profiling of Candida albicans identifies a gene essential for interepithelial dissemination. Cell Microbiol. 2007;9:2938–54.
Villar CC, Kashleva H, Dongari-Bagtzoglou A. Role of Candida albicans polymorphism in interactions with oral epithelial cells. Oral Microbiol Immunol. 2004;19:262–9.
Hoyer LL, Payne TL, Bell M, et al. Candida albicans ALS3 and insights into the nature of the ALS gene family. Curr Genet. 1998;33:451–9.
Liu Y, Filler SG. Candida albicans Als3, a multifunctional adhesin and invasin. Eukaryot Cell. 2011;10:168–73.
Hoyer LL, Green CB, Oh SH, Zhao X. Discovering the secrets of the Candida albicans agglutinin-like sequence (ALS) gene family – a sticky pursuit. Med Mycol. 2008;46:1–15.
Zhao X, Oh SH, Cheng G, et al. ALS3 and ALS8 represent a single locus that encodes a Candida albicans adhesin; functional comparisons between Als3p and Als1p. Microbiology. 2004;150:2415–28.
• Salgado PS, Yan R, Taylor JD, et al. Structural basis for the broad specificity to host-cell ligands by the pathogenic fungus Candida albicans. Proc Natl Acad Sci U S A. 2011;108:15775–9. High-resolution NMR analysis of N-terminal Als adhesins identifying a mechanism by which this adhesin family may adhere to host ligands.
• Staab JF, Bradway SD, Fidel Jr P, Sundstrom P. Adhesive and mammalian transglutaminase substrate properties of Candida albicans Hwp1. Science. 1999;283:1535–8. Identification of the first C. albicans hyphal protein that contributes to epithelial adhesion and mucosal infections.
Naglik JR, Fostira F, Ruprai J, et al. Candida albicans HWP1 gene expression and host antibody responses in colonization and disease. J Med Microbiol. 2006;55:1323–7.
Sundstrom P, Balish E, Allen CM. Essential role of the Candida albicans transglutaminase substrate, hyphal wall protein 1, in lethal oroesophageal candidiasis in immunodeficient mice. J Infect Dis. 2002;185:521–30.
Dwivedi P, Thompson A, Xie Z, et al. Role of Bcr1-activated genes Hwp1 and Hyr1 in Candida albicans oral mucosal biofilms and neutrophil evasion. PLoS One. 2011;6:e16218.
Dalle F, Wachtler B, L'Ollivier C, et al. Cellular interactions of Candida albicans with human oral epithelial cells and enterocytes. Cell Microbiol. 2010;12:248–71.
• Phan QT, Myers CL, Fu Y, et al. Als3 Is a Candida albicans invasin that binds to cadherins and induces endocytosis by host cells. PLoS Biol. 2007;5:e64. Identification of the first C. albicans invasin and provides evidence that Als3 is a molecular mimic of human cadherins.
Villar CC, Zhao XR. Candida albicans induces early apoptosis followed by secondary necrosis in oral epithelial cells. Mol Oral Microbiol. 2010;25:215–25.
Sun JN, Solis NV, Phan QT, et al. Host cell invasion and virulence mediated by Candida albicans Ssa1. PLoS Pathog. 2010;6:e1001181.
Moreno-Ruiz E, Galan-Diez M, Zhu W, et al. Candida albicans internalization by host cells is mediated by a clathrin-dependent mechanism. Cell Microbiol. 2009;11:1179–89.
Zhao XR, Villar CC. Trafficking of Candida albicans through oral epithelial endocytic compartments. Med Mycol. 2011;49:212–7.
Diez-Orejas R, Fernandez-Arenas E. Candida albicans-macrophage interactions: genomic and proteomic insights. Future Microbiol. 2008;3:661–81.
Tavanti A, Campa D, Bertozzi A, et al. Candida albicans isolates with different genomic backgrounds display a differential response to macrophage infection. Microbes Infect. 2006;8:791–800.
Murciano C, Moyes DL, Runglall M, et al. Evaluation of the role of Candida albicans agglutinin-like sequence (Als) proteins in human oral epithelial cell interactions. PLoS One. 2012;7:e33362.
Naglik J, Albrecht A, Bader O, Hube B. Candida albicans proteinases and host/pathogen interactions. Cell Microbiol. 2004;6:915–26.
Naglik JR, Challacombe SJ, Hube B. Candida albicans secreted aspartyl proteinases in virulence and pathogenesis. Microbiol Mol Biol Rev. 2003;67:400–28.
Hube B, Naglik JR. Extracellular hydrolases. In: Calderone RA, editor. Candida and candidiasis. Washington DC: ASM Press; 2002. p. 107–22.
Villar CC, Kashleva H, Nobile CJ, et al. Mucosal tissue invasion by Candida albicans is associated with E-cadherin degradation, mediated by transcription factor Rim101p and protease Sap5p. Infect Immun. 2007;75:2126–35.
Moyes DL, Murciano C, Runglall M, et al. Activation of MAPK/c-Fos induced responses in oral epithelial cells is specific to Candida albicans and Candida dubliniensis hyphae. Med Microbiol Immunol. 2012;201:93–101.
Wagener J, Weindl G, de Groot PWJ, et al. Glycosylation of Candida albicans cell wall proteins is critical for induction of innate immune responses and apoptosis of epithelial cells. PLoS One. 2012;7:e50518.
Calderone RA. Candida and candidiasis, vol. 2. Washington DC: ASM Press; 2002.
Silva S, Negri M, Henriques M, et al. Candida glabrata, Candida parapsilosis and Candida tropicalis: biology, epidemiology, pathogenicity and antifungal resistance. FEMS Microbiol Rev. 2012;36:288–305.
Silva S, Hooper SJ, Henriques M, et al. The role of secreted aspartyl proteinases in Candida tropicalis invasion and damage of oral mucosa. Clin Microbiol Infect. 2011;17:264–72.
Silva S, Henriques M, Oliveira R, et al. Characterization of Candida parapsilosis infection of an in vitro reconstituted human oral epithelium. Eur J Oral Sci. 2009;117:669–75.
Gacser A, Schafer W, Nosanchuk JS, et al. Virulence of Candida parapsilosis, Candida orthopsilosis, and Candida metapsilosis in reconstituted human tissue models. Fungal Genet Biol. 2007;44:1336–41.
De Las PA, Pan SJ, Castano I, et al. Virulence-related surface glycoproteins in the yeast pathogen Candida glabrata are encoded in subtelomeric clusters and subject to RAP1- and SIR-dependent transcriptional silencing. Genes Dev. 2003;17:2245–58.
Cormack BP, Ghori N, Falkow S. An adhesin of the yeast pathogen Candida glabrata mediating adherence to human epithelial cells. Science. 1999;285:578–82. Identification of the first C. glabrata adhesin required for binding to human epithelial cells.
Jackson AP, Gamble JA, Yeomans T, et al. Comparative genomics of the fungal pathogens Candida dubliniensis and Candida albicans. Genome Res. 2009;19:2231–44.
Hoyer LL, Fundyga R, Hecht JE, et al. Characterization of agglutinin-like sequence genes from non-albicans Candida and phylogenetic analysis of the ALS family. Genetics. 2001;157:1555–67.
•• Butler G, Rasmussen MD, Lin MF, et al. Evolution of pathogenicity and sexual reproduction in eight Candida genomes. Nature. 2009;459(7247):657–62. Report of the genome sequences of six Candida species enabling post-genomic analysis for pathogenicity studies.
Merkerova M, Dostal J, Hradilek M, et al. Cloning and characterization of Sapp2p, the second aspartic proteinase isoenzyme from Candida parapsilosis. FEMS Yeast Res. 2006;6:1018–26.
Kantarcioglu AS, Yucel A. Phospholipase and protease activities in clinical Candida isolates with reference to the sources of strains. Mycoses. 2002;45:160–5.
Zaugg C, Borg-von Zepelin M, et al. Secreted aspartic proteinase family of Candida tropicalis. Infect Immun. 2001;69:405–12.
Naglik JR, Moyes D, Makwana J, et al. Quantitative expression of the Candida albicans secreted aspartyl proteinase gene family in human oral and vaginal candidiasis. Microbiology. 2008;154:3266–80.
Lermann U, Morschhauser J. Secreted aspartic proteases are not required for invasion of reconstituted human epithelia by Candida albicans. Microbiology. 2008;154:3281–95.
Naglik JR, Rodgers CA, Shirlaw PJ, et al. Differential expression of Candida albicans secreted aspartyl proteinase and phospholipase B genes in humans correlates with active oral and vaginal infections. J Infect Dis. 2003;188:469–79.
Naglik JR, Newport G, White TC, et al. In vivo analysis of secreted aspartyl proteinase expression in human oral candidiasis. Infect Immun. 1999;67:2482–90.
Gelani V, Fernandes AP, Gasparoto TH, et al. The role of toll-like receptor 2 in the recognition of Aggregatibacter actinomycetemcomitans. J Periodontol. 2009;80:2010–9.
Kumar CP, Kumar SS, Menon T. Phospholipase and proteinase activities of clinical isolates of Candida from immunocompromised patients. Mycopathologia. 2006;161:213–8.
Ghannoum MA. Potential role of phospholipases in virulence and fungal pathogenesis. Clin Microbiol Rev. 2000;13:122–43.
Gacser A, Trofa D, Schafer W, Nosanchuk JD. Targeted gene deletion in Candida parapsilosis demonstrates the role of secreted lipase in virulence. J Clin Invest. 2007;117:3049–58.
Naglik JR, Moyes D. Epithelial cell innate response to Candida albicans. Adv Dent Res. 2011;23:50–5.
Moyes DL, Naglik JR. Mucosal immunity and Candida albicans infection. Clin Dev Immunol. 2011;2011:346307.
•• Moyes DL, Runglall M, Murciano C, et al. A biphasic innate immune MAPK response discriminates between the yeast and hyphal forms of Candida albicans in epithelial cells. Cell Host Microbe. 2010;8:225–35. Identification of an epithelial signalling mechanism that enables discrimination between yeast and hyphal forms of C. albicans, potentially representing a “danger response” pathway critical in discriminating between the commensal and pathogenic forms of this fungus.
Murciano C, Moyes DL, Runglall M, et al. Candida albicans cell wall glycosylation may be indirectly required for activation of epithelial cell proinflammatory responses. Infect Immun. 2011;79:4902–11.
de Koning HD, Rodijk-Olthuis D, van Vlijmen-Willems IM, et al. A comprehensive analysis of pattern recognition receptors in normal and inflamed human epidermis: upregulation of dectin-1 in psoriasis. J Invest Dermatol. 2010;130:2611–20.
Cheng SC, van de Veerdonk FL, Lenardon M, et al. The dectin-1/inflammasome pathway is responsible for the induction of protective T-helper 17 responses that discriminate between yeasts and hyphae of Candida albicans. J Leuk Biol. 2011;90(2):357–66.
Moyes DL, Murciano C, Runglall M, et al. Candida albicans yeast and hyphae are discriminated by MAPK signaling in vaginal epithelial cells. PLoS One. 2011;6:e26580.
Pukkila-Worley R, Ausubel FM, Mylonakis E. Candida albicans infection of Caenorhabditis elegans induces antifungal immune defenses. PLoS Pathog. 2011;7:e1002074.
• Guma M, Stepniak D, Shaked H, et al. Constitutive intestinal NF-kappaB does not trigger destructive inflammation unless accompanied by MAPK activation. J Exp Med. 2011;208:1889–900. Demonstrates that MAPK-p38 signalling may be required for epithelial recognition of ‘pathogenic’ microbes and to initiate inflammatory responses.
• Weindl G, Naglik JR, Kaesler S, et al. Human epithelial cells establish direct antifungal defense through TLR4-mediated signalling. J Clin Invest. 2007;117:3664–72. The first description of a PMN-dependent, TLR4-mediated protective mechanism at epithelial surfaces that may provide significant insights into how Candida infections are managed and controlled in the oral mucosa.
Hornef MW, Bogdan C. The role of epithelial Toll-like receptor expression in host defense and microbial tolerance. J Endotoxin Res. 2005;11:124–8.
Netea MG, Brown GD, Kullberg BJ, Gow NAR. An integrated model of the recognition of Candida albicans by the innate immune system. Nat Rev Microbiol. 2008;6:67–78.
Hoyer LL, Scherer S, Shatzman AR, Livi GP. Candida albicans ALS1: domains related to a Saccharomyces cerevisiae sexual agglutinin separated by a repeating motif. Mol Microbiol. 1995;15:39–54.
Zhu W, Phan QT, Boontheung P, et al. EGFR and HER2 receptor kinase signaling mediate epithelial cell invasion by Candida albicans during oropharyngeal infection. Proc Natl Acad Sci U S A. 2012;109:14194–9.
Fu Y, Luo G, Spellberg BJ, et al. Gene overexpression/suppression analysis of candidate virulence factors of Candida albicans. Eukaryot Cell. 2008;7:483–92.
Gale CA, Bendel CM, McClellan M, et al. Linkage of adhesion, filamentous growth, and virulence in Candida albicans to a single gene, INT1. Science. 1998;279:1355–8.
Mukherjee PK, Seshan KR, Leidich SD, et al. Reintroduction of the PLB1 gene into Candida albicans restores virulence in vivo. Microbiology. 2001;147:2585–97.
Schofield DA, Westwater C, Warner T, Balish E. Differential Candida albicans lipase gene expression during alimentary tract colonization and infection. FEMS Microbiol Lett. 2005;244:359–65.
Conflict of Interest
J.R. Naglik, S.X. Tang and D.L. Moyes have received grants from the Medical Research Council and the Biotechnology and Biological Science Research Council
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Naglik, J.R., Tang, S.X. & Moyes, D.L. Oral Colonization of Fungi. Curr Fungal Infect Rep 7, 152–159 (2013). https://doi.org/10.1007/s12281-013-0129-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12281-013-0129-y