Abstract
Azole resistance in Aspergillus fumigatus has been increasingly reported particularly over the last decade. Two routes of acquisition are described: selection of resistance during long term azole therapy in the clinical setting, and primary acquisition of resistant isolates from the environment due to the considerable use of azole fungicides in agriculture and for material preservation. Three specific resistance genotypes have been found in azole naïve patients. Two of these have also been found in the environment and are characterized by a tandem repeat in the promoter region of the target gene coupled with point mutation(s) in CYP51A (TR34/L98H and TR46/Y121F/T289A). In the third a single target enzyme alteration (G432S) is found. These resistant “environmental” strains have been detected in many West-European countries as well as in the Asia-Pacifics. Noticeably, these two continents account for the highest fungicide use in the global perspective (37 % and 24 %, respectively). Among the 25 azole fungicides, five have been associated with the potential to select for the TR34/L98H genotype; three of these are among those most frequently used. Although the number of antifungal fungicide compounds and classes available is impressive compared to the armamentarium in human medicine, azoles will remain the most important group in agriculture due to superior field performance and significant resistance in fungal pathogens to other compounds. Hence, further spread of environmental resistant Aspergillus genotypes may occur and will depend on the fitness of each resistant phenotype and the pattern of azole fungicide use.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aspergillus fumigatus is a ubiquitous saprophytic fungus associated with a variety of diseases including allergic manifestations and chronic infections in the immunocompetent population and acute invasive localized or disseminated aspergillosis in the immunocompromised host. The estimated burden of disease is 3–500,000 for the acute invasive infections, 3 million with chronic pulmonary aspergillosis and 4 million with allergic bronchopulmonary aspergillosis [1]. Azole antifungal drugs are the cornerstone in the antifungal treatment of aspergillosis due to the clinical superiority of voriconazole for invasive infections and the fact that this group is the only oral option for patients with allergic or chronic forms of aspergillosis treated outside the hospital [2–5]. Itraconazole is often the primary choice for the allergic and chronic aspergillosis, voriconazole is the first line agent for invasive infection and posaconazole is licensed for prophylaxis and salvage treatment in the immunocompromised host. Second line options are amphotericin B formulations and echinocandins (Fig. 1) [4, 5].
Azole resistance in Aspergillus spp. may be intrinsic or acquired. Intrinsic resistance is characteristic for some of the sibling species of the A. fumigatus that are not easily identified in the routine laboratory (e.g. A. lentulus and A. udagawae) and acquired resistance is seen in A. fumigatus isolates at rates most commonly not exceeding 5 % but with significant variation (zero to 61 %) depending on geographical location, case mix and method for resistance detection [6, 7••, 8].
Early and appropriate antifungal treatment is associated with lower failure rates in patients with acute invasive aspergillosis and not surprisingly azole resistance has been associated with a poorer outcome [2, 3, 9, 10, 11•]. Often the diagnosis of azole resistant aspergillosis is difficult or delayed. This is in part due to cultures having low diagnostic sensitivity and hence, in many cases no fungal isolate is available for susceptibility testing. However, even if the culture is positive, susceptibility testing is unfortunately not routinely performed at many centres despite recommendations to do so and despite the fact that azole breakpoints have recently been established [6, 12•, 13, 14••]. Therefore, understanding of the clinical relevance of azole resistance and when it should be suspected and tested for is of utmost importance and the fundamental basis for improving management of this disease. In this review we attempt to address azole resistance in Aspergillus with particular focus on the link between the azole use in agriculture and the risk for acquiring azole resistant Aspergillus disease in humans.
Azole Resistance in Aspergillus
Azoles inhibit the ergosterol biosynthetic pathway by binding to its target enzyme lanosterol 14-α demethylase encoded by the CYP51A gene. This enzyme belongs to the cytochrome P450 family and is required for converting lanosterol to ergosterol, an essential component of the fungal cell membrane (Fig. 1). This results in the accumulation of 14-α methyl sterols and impaired cell membrane integrity [15, 16]. Multiple mechanisms of acquired azole resistance in A. fumigatus have been suggested and include: 1) target gene mutations, 2) target gene up-regulation, 3) up-regulation of efflux pumps, 4) reduced membrane permeability and 5) other mechanisms (Table 1).
Azole Resistance in Aspergillus Isolates from Azole Exposed Patients Only
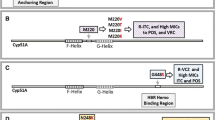
A number of cyp51A mutations have been detected in isolates with wild type azole susceptibility phenotype, whereas others are associated with mono- or multi-azole resistance (“hot spot mutations” [13]; Tables 1 and 2)). cyp51A knock-out mutant data, heterologous transformation analysis, molecular dynamic simulations and studies on site-directed mutagenesis, protein folding and homology modelling have assisted in establishing a role for the gene in azole resistance [17•, 18, 19, 20•, 21•], and identifying, confirming and predicting mutations conferring (cross-)resistance [20•, 21•, 22, 23]. Mutational cyp51A hot spot codons such as G54, G138, M220, Y431, and G448 are considered definitely involved in azole resistance and have been reported frequently from multiple centres (Table 2).
None of these mutations, however, are consistently present in clinically resistant isolates, and azole resistant isolates do not always exhibit cyp51A mutations [20•, 24, 25, 26•]. Relatively consistent azole susceptibility profiles have been described for isolates with hot spot mutations (Table 2). Most mutations confer itraconazole-resistance (Table 2), whereas pan-azole resistance typically has been reported in isolates with G138C or M220K alterations (Table 2). Acquired multi- or pan-azole resistance, where hot spot mutations have emerged during azole therapy over a period of less than a year, has been described [11•, 27–30].
Azole-Resistant A. Fumigatus Found in Azole Naïve Patients
Azole resistant A. fumigatus has been found in azole naïve patients and has been shown to harbour one of three different resistance mechanisms: 1) a 34 bp tandem repeat (TR34) in the CYP51A promoter region coupled with a L98H substitution in the CYP51A gene, 2) a 46 bp tandem repeat (TR46) coupled with Y121F/T289A substitutions in the CYP51A gene, or 3) a G432S substitution in the CYP51A gene. Of particular interest is the TR34/L98H genotype, which was initially detected at a Dutch centre in 12/13 itraconazole-resistant patient isolates [31••]; this mutation was subsequently found in the vast majority of itraconazole-resistant isolates from other Dutch centres [20•, 32] and associated with an up-regulation of the target enzyme level (mediated by the tandem repeat) and by decreased affinity for azoles (mediated by the substitution) in combination leading to the pan azole resistant phenotype [21•]. Using site-directed mutagenesis, Snelders et al. [21•] showed that the multi-azole resistant phenotype could not be induced exclusively by introducing either the TR34 or L98H mutation, indicating that the multi-azole resistant phenotype associated with L98H is dependent on the TR34 in the promoter region.
Link to the Agricultural Use of Azoles
Dominance of a single resistance mechanism as the one observed in the Netherlands is difficult to explain by resistance development in individual azole-treated patients, since a wide array of different resistance mechanisms is normally found in patients with resistance after long term treatment [11•, 26•, 33••]. Apart from being associated with multi-azole resistance in clinical isolates from azole naïve as well as exposed patients [31••, 33••, 34•, 35•], TR34/L98H has also been found in azole resistant, environmental isolates in Denmark and The Netherlands [32, 36•]. This raised the hypothesis that azole resistant patient isolates may not only result from longstanding azole therapy resulting in selection for resistant mutants, but be acquired directly from the environment [23]. Three additional observations support this notion. First, A. fumigatus isolates of identical microsatellite short tandem repeat (STR) genotype with and without azole resistance have been found in individual patients suggesting selection in vivo. But notably, although patients harbouring a TR34/L98H as well as a susceptible isolate have been described, these isolates have never had the same STR genotype, suggesting “double infection” with unrelated A. fumigatus isolates and not in vivo selection of resistance [33••]. Second, five specific azole fungicides (propiconazole, bromuconazole, epoxiconazole, difenoconazole and tebuconazole) show a molecular structure very similar to the medical triazoles, adopt similar poses while docking the target enzyme, have activity against wild type A. fumigatus but not against azole-resistant TR34/L98H-positive isolates and have all been introduced in The Netherlands between 1990 and 1996 directly preceding the isolation of the first TR34/L98H in 1998 [23, 37••]. And third, tebuconazole has been shown to be able to induce tandem repeats in the promoter region of CYP51A under laboratory conditions [37••].
In order to fully understand the nature and development of antifungal resistance, it is necessary to bear the following factors in mind [38]: Antifungal drug resistance (ADR) is not transmitted from person to person, 2) ADR is not conveyed by lateral gene transfer (as seen among bacteria via plasmids), 3) ADR has developed rapidly over the past few years, 4) ADR development under azole pressure (e.g. during therapy) presumably occurs during asexual reproduction, which is much more likely to occur in patients with chronic aspergillosis and aspergillomas than in patients with acute invasive aspergillosis, and 5), insertion of a tandem repeat acting as a transcriptional enhancer under azole pressure might be introduced more often during sexual reproduction requiring the presence of two opposite mating types which occurs mainly in the environment in A. fumigatus (teleomorph: Neosartorya fumigate) [39]. These factors have major implications for the interpretation on reports on cyp51A mutations, and may also to some extent predict the pattern of mutations seen in particular cohorts of patients in particular geographic areas. Hence, the finding of TR34/L98H mutations in azole resistant clinical isolates in patients with invasive aspergillosis in a country where this combination of mutations occurs in the environment may not be surprising. On the other hand, azole resistant isolates from patients with chronic aspergillosis and azole therapy may harbour either different, sporadic cyp51A mutations or the TR34/L98H genotype, depending on exposure and relative fitness of competing mutants. Conspicuously, TR34/L98H was recently found in 55.1 % of culture-negative, PCR positive sputum samples from patients with allergic bronchopulmonary/chronic pulmonary aspergillosis [7••]. If TR34/L98H mutants are less fit than wild type isolates and non-TR34/L98H mutants and thus more difficult to culture, this could explain why they were detectable only by PCR [7••]. This raises concern, since susceptibility testing is dependent on cultured isolates. On the other hand, the TR34/L98H genotype appears to be at least as fit in the environment as suggested by its clonal expansion across the Netherlands [40] and virulence studies in the animal model have failed to detect loss of virulence [41].
Recent reports of additional cyp51A mutants, namely TR46/Y121F/T289A, found in both clinical and environmental isolates in the Netherlands [42••], and a G432S mutant in an azole-naïve patient in France [43••] add support to the hypothesis that azole resistance acquired in the environment is often but not exclusively associated with upregulation of CYP51A induced by the presence of a tandem repeat in the promoter region. As described below this is also the case in fungal plant pathogens. Interestingly, both the Y121 and the G432 codons in these two resistance genotypes are also recognised as hot spot codons involved in azole fungicide resistance in the fungal plant pathogen Mycospherella graminicola (corresponding to the G460 and Y137 codons in this organism) [44].
Azole-Resistance in Other Aspergillus Spp.
All Aspergillus spp. are intrinsically resistant to fluconazole [45]. Whereas wild-type A. fumigatus sensu stricto is susceptible to other azoles, intrinsic multi/pan-azole resistance may operate in some morphologically similar species in the section Fumigati [46]. Thus, azole resistance has been reported in A. lentulus, which is characterised by primary CYP51A dependent resistance, and in A. fumigatiaffinis, Neosartorya pseudofischeri, and A. viridinutans [36•, 46–48]. However, recognition of these cryptic species happened only recently, and their respective roles in clinical aspergillosis and potential contribution to resistance problems remain to be further clarified.
Acquired resistance in other Aspergillus spp. has only been sporadically investigated and reported. However, since some of these species (e.g. A. terreus, A. flavus and A. nidulans) exhibit reduced susceptibility to amphotericin B [49–56], the importance of azole susceptibility surveillance of such species should not be underestimated. We recently demonstrated elevated azole MICs for two A. terreus isolates [57, 58•], one of which had a cyp51A M217I mutation (equivalent to M220I in A. fumigatus) [58•]. An S240A alteration in CYP51C was recently associated with clinical voriconazole-resistance in A. flavus [59•]. Itraconazole resistance in species belonging to the A. niger complex is not unusual [60, 61] but has not so far been linked to any particular cyp51A gene mutation [61]. The A. ustus complex includes A. calidoustus, which has been detected in transplant patients and appears to be intrinsically pan-azole resistant [49]. Resistance profiles of other Aspergillus species rarely reported as causes of clinical aspergillosis were recently reviewed by van der Linden et al. [62]. Noticeably, resistance conferred by cyp51A mutations coupled to tandem repeats or in azole naïve patients have so far only been demonstrated for A. fumigatus isolates, indicating that this type of resistance acquired in the environment may not yet be a significant issue except in A. fumigatus.
Control Practice in Agriculture
Fungal plant pathogens cause disease in many agricultural and horticultural crops compromising yield and quality [63]. Yield losses in the range of 10 % to 30 % are not uncommon. The approach for infection control varies significantly between countries and over the seasons as many plant pathogens are crop and climate associated, often with the most severe attacks in wet seasons. Effective fungicides have been available for more than 30 years and fungicides are today commonly used for many crops. Depending on the crop and local risks for attack the number of treatments may vary between 5 and 15 times/year in orchard crops and potatoes to 0–4 in cereal crops (Fig. 2). Fungicide use in European cereal crops and in wheat in particular is the largest market for fungicides worldwide [64] (Figs. 2 and 3).
Several classes of fungicides are available for plant protection including triazoles, strobilurins, morpholines, SDHIs and chloronitriles (Figs. 1 and 2). Fungicide resistance has been reported for the majority of fungicides although less commonly for the multisite inhibitors. Concerns related to human health specifically for the azoles have included the risk of endocrine side effects following exposure of farmers and green house workers from preparing spray mixtures or handling azole treated plants. Recently documented selection for azole resistance in human pathogenic fungi adds to this concern.
Triazoles in Agriculture and for Material Preservation
Azole fungicides constitute the most widely used class of antifungal agents for the control of fungal plant diseases (Fig. 2) [64] (personal communication Phillips McDougall, 2010). In agriculture, the first azoles (triadimefon and imazalil) were introduced in 1973 [65], and triazoles have been widely used since the beginning of 1980. In comparison with several other groups of fungicides the field performances of azoles have been relatively stable, suggesting that emergence of acquired resistance in fungal plant pathogens has been limited (www.FRAC.info; [66]). Triazoles are also commonly used for material preservation, but no official statistical information is available to verify to what extent. Examples are tebuconazole and propiconazole which are both used to protect the surface of materials or objects such as paints, plastics, sealants, wall adhesives, binders, papers, art works, wood, and for the preservation of fibrous or polymerised materials, such as leather, rubber, paper and textile products. Additionally tebuconazole is used for preservation and remedial treatment of masonry or other construction materials (EU regulation).
A total of 25 different triazoles/imidazoles have been developed for agricultural crops [65]. The products are applied either as a seed treatment or as foliar applications (sprayed on growing plants), the latter potentially applying a greater selection pressure on other fungal pathogens. In addition to protecting against fungal plant pathogens, triazole compounds may offer plant growth-regulating properties [67] and the ability to protect plants against various environmental stresses [68, 69]. Each new triazole often offered some new advantages in basic activity, spectrum, persistency or mobility in the crop. Initially triadimenol followed by propiconazole and prochloraz were most commonly used, whereas today these have largely been replaced by more potent triazoles including tebuconazole, metconazole, epoxiconazole and prothioconazole. Three of these are among the five azole fungicides which have been associated with a high potential for selecting the TR34/L98H A. fumigatus genotype as described above [37••].
Even though other groups of chemicals e.g. strobilurins and SDHI fungicides (Fig. 1) [70], have been made available, problems related to resistance have been so significant that they are no longer appropriate for control of major diseases in many crops [71–73]. Hence, azoles alone or combinations of several agents (typically including at least one azole) are used in order to limit further selection of resistance [71, 74, 75].
Azole Resistance Mechanism in Fungal Plant Pathogens
Triazole resistance has over the years appeared in several plant pathogenic fungi. Field resistance was first reported for the cucumber pathogen Sphaerotheca fuliginea [76], and subsequently in several other pathogens like Penicillium digitatum [77], Blumeria graminis f.sp. hordei [78], Venturia inaequalis [79], Rhynchosporium secalis [80], and Mycosphaerella graminicola [71].
In several European countries a 10–100 times loss of susceptibility in vitro of M. graminicola populations has been reported over the last 20 years [71, 73, 81, 82]. Four azole resistance mechanisms have been found, most of which are identical to those described for A. fumigatus above: 1) point mutations in CYP51, 2) upregulation of target gene production, 3) efflux pumps, and 4) altered sterol biosynthesis; the latter has been found only in laboratory selected mutants and thus will not be dealt with any further here.
Point Mutations in Target Gene CYP51
A variety of different point mutations has been found in the CYP51 gene in plant pathogens but at a relatively low prevalence. Initially, Blumeria graminis f.sp. hordei and Uncinula necator with an Y136F alteration were associated with triazole resistance [83]. Since then, a total of 22 different alterations have been verified in M. graminicola [75]. Amino acid sequence alignment of CYP51 from M. graminicola and Candida albicans showed eight identical alterations in azole resistant isolates of the two organisms, while other eight unique alterations were found specifically for M. graminicola [75]. Moreover, alterations often accumulate in a single isolate leading to a stepwise shift in resistance which is also typical for C. albicans but apparently not for A. fumigatus [71, 84]. For example the alterations Y459D/C, G460D, and Y461H have each been linked to low level resistance, whereas I381V in combination with one of these resulted in a significantly higher level of resistance to all azoles [85]. Sequence alignment for M. graminicola and A. fumigatus identifies three codons found in azole resistant isolates of both species, namely Y137, Y459 and G460 in M. graminicola and Y121, Y431 and G432 in A. fumigatus, respectively [42••, 43••, 44]. Notably, two of these have been involved in azole resistance in azole naïve patients as described above [42••, 43••], whereas the third (Y431) was found in a patient with chronic aspergillosis and bilateral aspergilloma, and was shown to have been selected for in vivo [11•] (Fig. 4). The latter observation suggests that some alterations can be induced by fungicide as well as human azole use.
Alignment of amino acid (AA) sequences of Cyp51 from A. fumigatus (Genbank accession no. AAF32372) and M. graminicola (Genbank accession no. ACI29117) where alterations have been associated with azole resistance. References: Leroux and Walker, Pest Management Sci (2011) and as in Table 2.  = Codons associated with azole resistance in azole exposed patients.
= Codons associated with azole resistance in azole exposed patients.  = Codons associated with azole resistance in azole naïve patients or in M. graminicola; * associated with a tandem repeat in the promoter region of A. fumigatus
= Codons associated with azole resistance in azole naïve patients or in M. graminicola; * associated with a tandem repeat in the promoter region of A. fumigatus
The European population of M. graminicola is currently dominated by two molecular types, one being tebuconazole susceptible (V136A) and another being tebuconazole resistant (A379G, I381V and ΔY459/G460) [71, 85–87]. This has had major impact on the field performances of tebuconazole but less impact on other azoles such as epoxiconazole and prothioconazole [75].
Over Expression of the CYP51 Gene
Over expression linked to insertions or duplications in the promoter region of CYP51 resulting in elevated intracellular levels of the target enzyme has been detected in different plant pathogens with reduced azole susceptibility [88, 89]. Specifically for M. graminicola an insertion in the promoter of CYP51 was found coupled with a cyp51 alteration I381V in several isolates, but the increase in cyp51 expression remains to be demonstrated experimentally [44].
Role of Efflux Pumps
The simultaneous resistance to a variety of structurally unrelated toxic compounds is most commonly caused by upregulation of efflux pumps and was initially described in plant pathogens by De Ward et al. [90]. This has been studied for azoles in Botrytis cinerea [91], Pyrenophora tritici repentis [92] and M. graminicola [84]. Pump inhibitors like e.g. promazine slightly increase the susceptibility to azoles [87] and the biological potential for efflux resistance exists in the population of M. graminicola, but the genes identified have not so far been identified in field isolates [75].
In France, isolates of M. graminicola cross resistant to azoles, thiolcarbamates and SDHI’s have been reported, suggesting a combination of mutations in CYP51 and overexpression of drug efflux transporters to be involved (Figs. 1 and 2) [44]. Azole resistant A. fumigatus without CYP51A mutations have been found in up to 40 % of the resistant isolates in the clinical setting [26•]. To what extent efflux pumps may operate in these isolates and if so to what extent such may be induced by human azole medication or environmental use remains to be understood.
Future Prospects for the Control of Fungal Plant Pathogens
Although the number of fungicide classes from the perspectives of human medicine appears impressive, many are not true options for use as single agents either due to resistance having already emerged or because the risk when used as single agent is too high. Hence, azoles will remain the most commonly used class in cereal crops for the foreseeable future in agriculture. Various initiatives have been undertaken by European authorities as well as by the industrial community (Fungicide Resistance Action Committee, FRAC) to promote practises that reduce risk of selection of resistance although consensus as to how has not been established [82, 93–95]. How this will proceed and to which extent it may influence selection of resistance in human pathogens is yet to be seen.
Conclusions
Substantial data today support that azole resistance in A. fumigatus has been induced during long term azole treatment in individual patients but also occurs in naïve patients due to the selection for resistant mutants in the environment. The first azole resistant environmental strain (the TR34/L98H genotype) has spread throughout The Netherlands since 1998 and now accounts for between 6 % and 12.8 % of clinical A. fumigatus in this country illustrating the fitness and competitiveness of this genotype [96]. Subsequently, TR34/L98H has also been detected in many other West-European countries including clinical isolates from Denmark [33••], Norway [32], the UK [7••, 11•], Belgium [97], France [97, 98, 99•], and Spain [22], and in the Asia-Pacific region including India [35•] and China [34•]. Noticeably, West-Europe and Asia-Pacific represent the regions with the highest and second-highest fungicide use in a global perspective, respectively (Fig. 3) [8, 33••, 35•, 36•]. Additionally, two more resistant genotypes have recently been reported in azole naïve patients in France and The Netherlands, and thus again in West-Europe accounting for 37 % of the global fungicide market [42••, 43••]. Importantly, susceptibility testing is not routinely performed in many centres and the resistance rates reported may therefore very well represent the tip of the iceberg. We have yet to see if resistance emerges in S-America where the fungicide use has increased over the recent years and to which extent the resistance rates increase further in the parts of the world where it is already present. Important players are the fitness of the resistant phenotypes and the pattern of azole fungicide use where not only amounts but also choice of individual compounds plays an important role.
Obviously, these changes have clinical implications. Whereas acquired resistance in clinical practise may be expected after long term treatment, it is important to realise that azole susceptibility is not obligate in the azole naïve patients with aspergillosis. This suggests susceptibility testing should be performed in all patients with Aspergillus infection requiring antifungal therapy and highlights the need for better diagnostics improving the culture positivity rate and establishing alternative options for culture negative cases like direct detection of prevalent environmental mutants by PCR [100••]. Moreover, initial combination therapy may be considered in areas with higher prevalence of environmental azole resistant isolates for patients with severe infection. And finally, surveillance studies in both the clinical setting and the environment should be conducted in order to provide updated local data on susceptibility rates.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Denning DW, Perlin DS. Azole resistance in Aspergillus: a growing public health menace. Future Microbiol. 2011;6:1229–32.
Herbrecht R, Denning DW, Patterson TF, et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002;347:408–15.
Lortholary O, Gangneux JP, Sitbon K, et al. Epidemiological trends in invasive aspergillosis in France: the SAIF network (2005–2007). Clin Microbiol Infect. 2011;17:1882–9.
Walsh TJ, Anaissie EJ, Denning DW, et al. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:327–60.
Maertens J, Marchetti O, Herbrecht R, et al. European guidelines for antifungal management in leukemia and hematopoietic stem cell transplant recipients: summary of the ECIL 3–2009 update. Bone Marrow Transplant. 2011;46:709–18.
Snelders E, Melchers WJ, Verweij PE. Azole resistance in Aspergillus fumigatus: a new challenge in the management of invasive aspergillosis? Future Microbiol. 2011;6:335–47.
•• Denning DW, Park S, Lass-Florl C, et al. High-frequency triazole resistance found in nonculturable Aspergillus fumigatus from lungs of patients with chronic fungal disease. Clin Infect Dis. 2011;52:1123–9. Study comparing diagnostic sensitivity by PCR and culture of Aspergillus in patients with IPA (22 BAL samples), ABPA (19 sputum samples) and CPA (42 sputum samples). Significantly more patients are found positive by PCR, however, on the cost of a positive rate of a third among 11 BAL samples from healthy volunteers. Remarkably, using a very sensitive nested PCR format amplifying the CYP51A gene, as many as 55 % were found to harbor resistance mutations. If confirmed this raises obvious concerns for current diagnostic procedures.
van der Linden J, Arendrup M, Verweij P, SCARE-Network: Prospective International Surveillance of Azole Resistance (AR) in Aspergillus fumigatus (Af) (SCARE-Network). 51th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC). Chicago, USA, 2011: Abstract M-490.
Greene RE, Schlamm HT, Oestmann JW, et al. Imaging findings in acute invasive pulmonary aspergillosis: clinical significance of the halo sign. Clin Infect Dis. 2007;44:373–9.
van der Linden JW, Jansen RR, Bresters D, et al. Azole-resistant central nervous system aspergillosis. Clin Infect Dis. 2009;48:1111–3.
• Howard SJ, Cerar D, Anderson MJ, et al. Frequency and evolution of Azole resistance in Aspergillus fumigatus associated with treatment failure. Emerg Infect Dis. 2009;15:1068–76. Thorough study demonstrating the huge variety of different mutations and other mechanisms present in a cohort of azole exposed patients with chronic forms of aspergillosis in Manchester, UK.
• Arendrup MC, Bille J, Dannaoui E, Ruhnke M, Heussel CP, Kibbler C. ECIL-3 classical diagnostic procedures for the diagnosis of invasive fungal diseases in patients with leukaemia. Bone Marrow Transplant. In Press; doi:10.1038/bmt.2011.246. Official ELIC document offering detailed guidelines on how and when to perform and interpret culture, susceptibility testing and imaging diagnostics approached for patients at risk of fungal infections.
Howard SJ, Arendrup MC. Acquired antifungal drug resistance in Aspergillus fumigatus: epidemiology and detection. Med Mycol. 2011;49 Suppl 1:S90–5.
•• Arendrup MC, Cuenca-Estrella M, Lass-Flörl C, Hope WW, The European Committee on Antimicrobial Susceptibilty Testing Subcommittee on Antifungal Testing (EUCAST-AFST). EUCAST technical note on Aspergillus and amphotericin B, itraconazole, and posaconazole. Clin Microbiol Infect. In Press; doi:10.1111/j.1469-0691.2012.03890.x. First official breakpoints for Aspergillus species.
Lamb D, Kelly D, Kelly S. Molecular aspects of azole antifungal action and resistance. Drug Resist Updat. 1999;2:390–402.
Lupetti A, Danesi R, Campa M, Del Tacca M, Kelly S. Molecular basis of resistance to azole antifungals. Trends Mol Med. 2002;8:76–81.
• Albarrag AM, Anderson MJ, Howard SJ, et al. Interrogation of related clinical pan-azole-resistant Aspergillus fumigatus strains: G138C, Y431C, and G434C single nucleotide polymorphisms in cyp51A, upregulation of cyp51A, and integration and activation of transposon Atf1 in the cyp51A promoter. Antimicrob Agents Chemother. 2011;55:5113–21. Elegant study examining the underlying resistance mechanisms in isolates from two azole exposed patients. Various point mutations, a transposon inserted in the promoter in an isolate with up-regulated target enzyme level and efflux mediated resistance is found illustrating the multiple ways by which Aspergillus may escape azole treatment.
Warrilow AG, Melo N, Martel CM, et al. Expression, purification, and characterization of Aspergillus fumigatus sterol 14-alpha demethylase (CYP51) isoenzymes A and B. Antimicrob Agents Chemother. 2010;54:4225–34.
Hu W, Sillaots S, Lemieux S, et al. Essential gene identification and drug target prioritization in Aspergillus fumigatus. PLoS Pathog. 2007;3:e24.
• Snelders E, Karawajczyk A, Schaftenaar G, Verweij PE, Melchers WJ. Azole resistance profile of amino acid changes in Aspergillus fumigatus CYP51A based on protein homology modeling. Antimicrob Agents Chemother. 2010;54:2425–30. This study includes almost 3000 A. fumigatus isolates collected in NL between 1994–2008. Results include a) dominance of the TR 34 /L98H resistant genotype among resistant isolates, b) demonstration of a variety of other non-synonomous mutations, and c) utility of a Cyp51A protein homology model to correlate individual mutations to their potential role for azole susceptibility.
• Snelders E, Karawajczyk A, Verhoeven RJ, et al. The structure-function relationship of the Aspergillus fumigatus cyp51A L98H conversion by site-directed mutagenesis: the mechanism of L98H azole resistance. Fungal Genet Biol. 2011;48:1062–70. Molecular dynamics simulations combined with site-directed mutagenesis of amino acid substitutions in the CYP51A gene demonstrate that both the L98H substitution and the 34 bp TR are needed to obtain the multi-azole resistant phenotype.
Mellado E, Garcia-Effron G, Alcázar-Fuoli L, et al. A new Aspergillus fumigatus resistance mechanism conferring in vitro cross-resistance to azole antifungals involves a combination of cyp51A alterations. Antimicrob Agents Chemother. 2007;51:1897–904.
Snelders E, Veld RA Huis In’t, Rijs AJ, Kema GH, Melchers WJ, Verweij PE. Possible environmental origin of resistance of Aspergillus fumigatus to medical triazoles. Appl Environ Microbiol. 2009;75:4053–7.
Arendrup MC, Mavridou E, Mortensen KL, et al. Development of azole resistance in Aspergillus fumigatus during azole therapy associated with change in virulence. PLoS One. 2010;5:e10080.
Tashiro M, Izumikawa K, Minematsu A, et al. Antifungal susceptibilities of Aspergillus fumigatus clinical isolates obtained in Nagasaki, Japan. Antimicrob Agents Chemother. 2012;56:584–7.
• Bueid A, Howard SJ, Moore CB, et al. Azole antifungal resistance in Aspergillus fumigatus: 2008 and 2009. J Antimicrob Chemother. 2010;65:2116–8. Follow up study on the Manchester cohort of patients with chronic forms of aspergillosis, demonstrating an increasing resistance rate and as many as 40 % due to other mechanisms than target gene mutations illustrating the complexity of resistance in the azole exposed patient population with respect to treatment and challenges for future molecular resistance detection in this particular setting.
Camps SM, van der Linden JW, Li Y, et al. Rapid induction of multiple resistance mechanisms in Aspergillus fumigatus during azole therapy: a case study and review of the literature. Antimicrob Agents Chemother. 2012;56:10–6.
Chen J, Li H, Li R, Bu D, Wan Z. Mutations in the cyp51A gene and susceptibility to itraconazole in Aspergillus fumigatus serially isolated from a patient with lung aspergilloma. J Antimicrob Chemother. 2005;55:31–7.
Dannaoui E, Borel E, Monier MF, Piens MA, Picot S, Persat F. Acquired itraconazole resistance in Aspergillus fumigatus. J Antimicrob Chemother. 2001;47:333–40.
Bellete B, Raberin H, Morel J, Flori P, Hafid J, Manhsung RT. Acquired resistance to voriconazole and itraconazole in a patient with pulmonary aspergilloma. Med Mycol. 2010;48:197–200.
•• Verweij PE, Mellado E, Melchers WJ. Multiple-triazole-resistant aspergillosis. N Engl J Med. 2007;356:1481–3. First report on the TR 34 /L98H genotype.
Snelders E, van der Lee HA, Kuijpers J, et al. Emergence of azole resistance in Aspergillus fumigatus and spread of a single resistance mechanism. PLoS Med. 2008;5:e219.
•• Mortensen KL, Jensen RH, Johansen HK, et al. Aspergillus species and other molds in respiratory samples from patients with cystic fibrosis: a laboratory-based study with focus on Aspergillus fumigatus azole resistance. J Clin Microbiol. 2011;49:2243–51. First report on TR 34 /L98H in CF patients. Genotyping suggested selection for resistance in the patient as well as resistance being achieved in the environment as 1) susceptible and resistant isolates (not involving TR 34 /L98H isolates) with identical or very closely related genotypes (two patients), and 2) two related susceptible isolates and a third unrelated resistant isolate with a unique genotype and the TR/L98H resistance combination (one patient) were found.
• Lockhart SR, Frade JP, Etienne KA, Pfaller MA, Diekema DJ, Balajee SA. Azole resistance in Aspergillus fumigatus isolates from the ARTEMIS global surveillance study is primarily due to the TR/L98H mutation in the cyp51A gene. Antimicrob Agents Chemother. 2011;55:4465–8. First report on TR 34 /L98H in China.
• Chowdhary A, Kathuria S, Randhawa HS, Gaur SN, Klaassen CH, Meis JF. Isolation of multiple-triazole-resistant Aspergillus fumigatus strains carrying the TR/L98H mutations in the cyp51A gene in India. J Antimicrob Chemother. 2012;67:362–6. First report on TR 34 /L98H in India.
• Mortensen KL, Mellado E, Lass-Flörl C, Rodriguez-Tudela JL, Johansen HK, Arendrup MC. Environmental study of azole-resistant Aspergillus fumigatus and other aspergilli in Austria, Denmark, and Spain. Antimicrob Agents Chemother. 2010;54:4545–9. First report demonstrating the TR 34 /L98H genotype in the environment outside the Netherlands.
•• Snelders E, Camps SM, Karawajczyk A, et al. Triazole fungicides can induce cross-resistance to medical triazoles in Aspergillus fumigatus. PLoS One. 2012;7:e31801. This study provides data suggesting five specific agricultural fungicides are the most important candidates responsible for selecting azole resistance in Aspergillus. Furthermore, one of these are shown to be able to induce a tandem repeat in the promoter region of the target gene thus adding further support to the link between agricultural fungicides and resistance in Aspergillus.
Verweij PE, Snelders E, Kema GH, Mellado E, Melchers WJ. Azole resistance in Aspergillus fumigatus: a side-effect of environmental fungicide use? Lancet Infect Dis. 2009;9:789–95.
O’Gorman CM, Fuller HT, Dyer PS. Discovery of a sexual cycle in the opportunistic fungal pathogen Aspergillus fumigatus. Nature. 2009;457:471–4.
Klaassen CH, Gibbons JG, Fedorova ND, Meis JF, Rokas A. Evidence for genetic differentiation and variable recombination rates among Dutch populations of the opportunistic human pathogen Aspergillus fumigatus. Mol Ecol. 2012;21:57–70.
Mavridou E, Meletiadis J, Jancura P, et al. Association of in vitro growth parameters with in vivo virulence of Aspergillus fumigatus based on a novel virulence index. 21st European Congress of Clinical Microbiology and Infectious Diseases (ECCMID) and 27th International Congress of Chemotherapy (ICC). Milan, Italy, 2011. Abstract P1768.
•• Kuipers S, Brüggemann RJ, de Sévaux RG, et al. Failure of posaconazole therapy in a renal transplant patient with invasive aspergillosis due to Aspergillus fumigatus with attenuated susceptibility to posaconazole. Antimicrob Agents Chemother. 2011;55:3564–6. First and so far the only report on the second environmental azole resistant A. fumigatus with a combination of a tandem repeat and point mutations (TR 46 /Y121F/T289A).
•• Alanio A, Cordonnier C, Bretagne S. Low prevalence of resistance to azoles in Aspergillus fumigatus in a French cohort of patients treated for haematological malignancies-authors’ response. J Antimicrob Chemother. 2011;66:955–5. First report of the recovery of the azole resistant G432S genotype in an azole naïve patient suggesting this genotype may also derive from the environment.
Leroux P, Walker AS. Multiple mechanisms account for resistance to sterol 14 alpha-demethylation inhibitors in field isolates of Mycosphaerella graminicola. Pest Manag Sci. 2011;67:44–59.
Willger SD, Puttikamonkul S, Kim KH, et al. A sterol-regulatory element binding protein is required for cell polarity, hypoxia adaptation, azole drug resistance, and virulence in Aspergillus fumigatus. PLoS Pathog. 2008;4:e1000200.
Alcazar-Fuoli L, Mellado E, Alastruey-Izquierdo A, Cuenca-Estrella M, Rodriguez-Tudela JL. Aspergillus section Fumigati: antifungal susceptibility patterns and sequence-based identification. Antimicrob Agents Chemother. 2008;52:1244–51.
Mellado E, Alcazar-Fuoli L, Cuenca-Estrella M, Rodriguez-Tudela JL. Role of Aspergillus lentulus 14-α sterol demethylase (Cyp51A) in azole drug susceptibility. Antimicrob Agents Chemother. 2011;55:5459–68.
Alhambra A, Catalán M, Moragues MD, et al. Isolation of Aspergillus lentulus in Spain from a critically ill patient with chronic obstructive pulmonary disease. Rev Iberoam Micol. 2008;25:246–9.
Baddley JW, Marr KA, Andes DR, et al. Patterns of susceptibility of Aspergillus isolates recovered from patients enrolled in the Transplant-Associated Infection Surveillance Network. J Clin Microbiol. 2009;47:3271–5.
Steinbach WJ, Benjamin DK, Kontoyiannis DP, et al. Infections due to Aspergillus terreus: a multicenter retrospective analysis of 83 cases. Clin Infect Dis. 2004;39:192–8.
Walsh TJ, Petraitis V, Petraitiene R, et al. Experimental pulmonary aspergillosis due to Aspergillus terreus: pathogenesis and treatment of an emerging fungal pathogen resistant to amphotericin B. J Infect Dis. 2003;188:305–19.
Koss T, Bagheri B, Zeana C, Romagnoli MF, Grossman ME. Amphotericin B-resistant Aspergillus flavus infection successfully treated with caspofungin, a novel antifungal agent. J Am Acad Dermatol. 2002;46:945–7.
Seo K, Akiyoshi H, Ohnishi Y. Alteration of cell wall composition leads to amphotericin B resistance in Aspergillus flavus. Microbiol Immunol. 1999;43:1017–25.
Hsueh PR, Lau YJ, Chuang YC, et al. Antifungal susceptibilities of clinical isolates of Candida species, Cryptococcus neoformans, and Aspergillus species from Taiwan: surveillance of multicenter antimicrobial resistance in Taiwan program data from 2003. Antimicrob Agents Chemother. 2005;49:512–7.
Hachem RY, Kontoyiannis DP, Boktour MR, et al. Aspergillus terreus: an emerging amphotericin B-resistant opportunistic mold in patients with hematologic malignancies. Cancer. 2004;101:1594–600.
Kontoyiannis DP, Lewis RE, May GS, Osherov N, Rinaldi MG. Aspergillus nidulans is frequently resistant to amphotericin B. Mycoses. 2002;45:406–7.
Mortensen KL, Johansen HK, Fuursted K, et al. A prospective survey of Aspergillus spp. in respiratory tract samples: prevalence, clinical impact and antifungal susceptibility. Eur J Clin Microbiol Infect Dis. 2011;30:1355–63.
• Arendrup MC, Jensen RH, Grift K, et al. In vivo emergence of Aspergillus terreus with reduced azole susceptibility and a Cyp51a M217I alteration. J Infect Dis. In Press. First and so far only report on the role of CYP51A in acquired itraconazole resistance in A. terreus.
• Liu W, Sun Y, Chen W, Wan Z, Bu D, Li R. T778G mutation in cyp51C gene confers voriconazole-resistance in Aspergillus flavus causing aspergillosis. Antimicrob Agents Chemother. In Press; doi:10.1128/AAC.05477-11. First and so far only report on the role of CYP51C in acquired itraconazole resistance in A. flavus.
Alcazar-Fuoli L, Mellado E, Alastruey-Izquierdo A, Cuenca-Estrella M, Rodriguez-Tudela JL. Species identification and antifungal susceptibility patterns of species belonging to Aspergillus section Nigri. Antimicrob Agents Chemother. 2009;53:4514–7.
Howard SJ, Harrison E, Bowyer P, Varga J, Denning DW. Cryptic species and azole resistance in the Aspergillus niger complex. Antimicrob Agents Chemother. 2011;55:4802–9.
Van Der Linden JW, Warris A, Verweij PE. Aspergillus species intrinsically resistant to antifungal agents. Med Mycol. 2011;49 Suppl 1:S82–9.
Oerke EC. Crop losses to pests. J Agric Sci. 2006;144:31–43.
Kuck K-H, Leadbeater A, Gisi U. FRAC mode of action classification and resistance risk of fungicides. In: Modern crop protection compounds. Wiley-VCH Verlag GmbH & Co. KGaA; 2012. 539–57.
Russell PE. A century of fungicide evolution. J Agric Sci. 2005;143:11–25.
Brent K, Holloman D. Fungicide resistance in crop pathogens: how can it be managed? In: FRAC Monograph 1. 2nd Ed. 2007. http://www.frac.info/frac/publication/anhang/FRAC_Mono2_2007.pdf. Accessed 25 May 2012.
Child RD, Evans DE, Allen J, Arnold GM. Growth-responses in oilseed rape (Brassica napus L.) to combined applications of the triazole chemicals triapenthenol and tebuconazole and interactions with gibberellin. Plant Growth Regul. 1993;13:203–12.
Still JR, Pill WG. Growth and stress tolerance of tomato seedlings (Lycopersicon esculentum Mill.) in response to seed treatment with paclobutrazol. J Hortic Sci Biotechnol. 2004;79:197–203.
Jaleel CA, Gopi R, Manivannan P, Panneerselvam R. Responses of antioxidant defense system of Catharanthus roseus (L.) G. Don. to paclobutrazol treatment under salinity. Acta Physiol Plant. 2007;29:205–9.
Bartlett DW, Clough JM, Godwin JR, Hall AA, Hamer M, Parr-Dobrzanski B. The strobilurin fungicides. Pest Manag Sci. 2002;58:649–62.
Leroux P, Albertini C, Gautier A, Gredt M, Walker AS. Mutations in the CYP51 gene correlated with changes in sensitivity to sterol 14 alpha-demethylation inhibitors in field isolates of Mycosphaerelia graminicola. Pest Manag Sci. 2007;63:688–98.
Fraaije BA, Butters JA, Coelho JM, Jones DR, Hollomon DW. Following the dynamics of strobilurin resistance in Blumeria graminis f.sp. tritici using quantitative allele-specific real-time PCR measurements with the fluorescent dye SYBR Green I. Plant Pathol. 2002;51:45–54.
Jørgensen LN, Thygesen K. Fungicide resistance and its impact on recommendations to farmers – experiences from Denmark. Asp Appl Biol. 2006;78:65–70.
Avenot HF, Michailides TJ. Progress in understanding molecular mechanisms and evolution of resistance to succinate dehydrogenase inhibiting (SDHI) fungicides in phytopathogenic fungi. Crop Prot. 2010;29:643–51.
Cools H, Fraaije B. Resistance to azole fungicides in Mycosphaerella graminicola: mechanisms and management. In: Thind T, editor. Fungicide resistance in crop protection: risk and management. Cambridge: CABI; 2012. p. 64–77.
Schepers H. Fitness of isolates of Sphaerotheca fuliginea resistant or sensitive to fungicides which inhibit ergosterol biosynthesis. Neth J Plant Pathol. 1985;91:65–76.
Eckert JW. Penicillium digitatum biotypes with reduced sensitivity to imazalil. Phytopathology. 1987;77:1728–8.
Heaney S. Population dynamics of DMI fungicide sensitivity changes in barley powdery mildew. In: Delp CJ, editor. Fungicide resistance in North America. St. Paul: ARS Press, American Phytopathological Society; 1988. p. 89–92.
Hildebrand PD, Lockhart CL, Newbery RJ, Ross RG. Resistance of Venturia inaequalis to bitertanol and other demethylation-inhibiting fungicides. Can J Plant Pathol. 1988;10:311–6.
Kendall SJ, Hollomon DW, Cooke LR, Jones DR. Changes in sensitivity to DMI fungicides in Rhynchosporium secalis. Crop Prot. 1993;12:357–62.
Gisi U, Pavic L, Stanger C, Hugelshofer U, Sierotzki H. Dynamics of Mycosphaerella graminicola populations in response to selection by different fungicides. In: Dehne H, Gisi U, Kuck K, Russell P, Lyr H, editors. Modern fungicides and antifungal compounds IV. Alton: BCPC; 2005. p. 89–101.
Clark W. Septoria tritici and azole performance. Fungicide resistance: are we winning the battle but losing the war? Asp Appl Biol. 2006;78:127–32.
Delye C, Bousset L, Corio-Costet MF. PCR cloning and detection of point mutations in the eburicol 14alpha-demethylase (CYP51) gene from Erysiphe graminis f.sp. hordei, a “recalcitrant” fungus. Curr Genet. 1998;34:399–403.
Cools HJ, Fraaije BA. Are azole fungicides losing ground against Septoria wheat disease? Resistance mechanisms in Mycosphaerella graminicola. Pest Manag Sci. 2008;64:681–4.
Fraaije BA, Cools HJ, Kim SH, Motteram J, Clark WS, Lucas JA. A novel substitution I381V in the sterol 14 alpha-demethylase (CYP51) of Mycosphaerella graminicola is differentially selected by azole fungicides. Mol Plant Pathol. 2007;8:245–54.
Stammler G, Carstensen M, Koch A, Semar M, Strobel D, Schlehuber S. Frequency of different CYP51-haplotypes of Mycosphaerella graminicola and their impact on epoxiconazole-sensitivity and -field efficacy. Crop Prot. 2008;27:1448–56.
Chassot C, Hugelshofer U, Sierotzki H, Gisi U. Sensitivity of CYP51 genotypes to DMI fungicides in Mycosphaerella graminicola. In: Dehne H, Deising H, Gisi U, Kuck K, Russell P, Lyr H, editors. Modern fungicides and antifungals compounds V. Braunschweig: DPG-Selbstverlag; 2008. p. 129–36.
Hamamoto H, Hasegawa K, Nakaune R, et al. Tandem repeat of a transcriptional enhancer upstream of the sterol 14 alpha-demethylase gene (CYP51) in Penicillium digitatum. Appl Environ Microbiol. 2000;66:3421–6.
Schnabel G, Jones AL. The 14alpha-demethylasse (CYP51A1) gene is overexpressed in Venturia inaequalis strains resistant to myclobutanil. Phytopathology. 2001;91:102–10.
de Waard MA, Andrade AC, Hayashi K, Schoonbeek HJ, Stergiopoulos I, Zwiers LH. Impact of fungal drug transporters on fungicide sensitivity, multidrug resistance and virulence. Pest Manag Sci. 2006;62:195–207.
Hayashi K, Schoonbeek HJ, De Waard MA. Modulators of membrane drug transporters potentiate the activity of the DMI fungicide oxpoconazole against Botrytis cinerea. Pest Manag Sci. 2003;59:294–302.
Reimann S, Deising HB. Inhibition of efflux transporter-mediated fungicide resistance in Pyrenophora tritici-repentis by a derivative of 4 ′-hydroxyflavone and enhancement of fungicide activity. Appl Environ Microbiol. 2005;71:3269–75.
Shaw MW. A model of the evolution of polygenically controlled fungicide resistance. Plant Pathol. 1989;38:44–55.
Shaw M. Is there such a thing as a fungicide resistance strategy? A modeller’s perspective. Asp Appl Biol. 2006;78:37–43.
van den Bosch F, Paveley N, Shaw M, Hobbelen P, Oliver R. The dose rate debate: does the risk of fungicide resistance increase or decrease with dose? Plant Pathol. 2011;60:597–606.
Verweij PE, Camps SM, Kema GH, Melchers WJ. Comment on: low prevalence of resistance to azoles in Aspergillus fumigatus in a French cohort of patients treated for haematological malignancies. J Antimicrob Chemother. 2011;66:954–5.
Lagrou K, De Vleeschouwer J, Meerseman W, Dupont L, Verleden G, Melchers W, et al. Triazole resistance among 229 clinical Aspergillus fumigatus isolates. 3rd Advances Against Aspergillosis Conference. Miami, Florida, USA, 2008. Abstract 33.
Burgel PR, Baixench MT, Amsellem M, et al. High prevalence of azole-resistant Aspergillus fumigatus in adults with cystic fibrosis exposed to itraconazole. Antimicrob Agents Chemother. 2012;56:869–74.
• Morio F, Aubin GG, Danner-Boucer I, et al. High prevalence of triazole resistance in Aspergillus fumigatus, especially mediated by TR/L98H, in a French cohort of patients with cystic fibrosis. J Antimicrob Chemother. In Press; doi:10.1093/jac/dks160. This study demonstrates the clinical utility of the itraconazole screening agar for routine identification of potentially resistant isolates and furthermore detects a 6 % rate of TR 34 /L98H in a French cohort of CF patients.
•• Garcia-Effron G, Dilger A, Alcazar-Fuoli L, Park S, Mellado E, Perlin DS. Rapid detection of triazole antifungal resistance in Aspergillus fumigatus. J Clin Microbiol. 2008;46:1200–6. Description of a molecular assay for the detection of azole resistance due to the most commonly found alterations in the CYP51A gene (codon L98, M220 and G54 and G138).
Bodey GP. Azole antifungal agents. Clin Infect Dis. 1992;14 Suppl 1:S161–9.
Kelly SL, Lamb DC, Corran AJ, Baldwin BC, Kelly DE. Mode of action and resistance to azole antifungals associated with the formation of 14 alpha-methylergosta-8,24(28)-dien-3 beta,6 alpha-diol. Biochem Biophys Res Commun. 1995;207:910–5.
Hodiamont CJ, Dolman KM, Ten Berge IJ, Melchers WJ, Verweij PE, Pajkrt D. Multiple-azole-resistant Aspergillus fumigatus osteomyelitis in a patient with chronic granulomatous disease successfully treated with long-term oral posaconazole and surgery. Med Mycol. 2009;47:217–20.
Nascimento AM, Goldman GH, Park S, et al. Multiple resistance mechanisms among Aspergillus fumigatus mutants with high-level resistance to itraconazole. Antimicrob Agents Chemother. 2003;47:1719–26.
Slaven JW, Anderson MJ, Sanglard D, et al. Increased expression of a novel Aspergillus fumigatus ABC transporter gene, atrF, in the presence of itraconazole in an itraconazole resistant clinical isolate. Fungal Genet Biol. 2002;36:199–206.
da Silva Ferreira ME, Capellaro JL, dos Reis Marques E, et al. In vitro evolution of itraconazole resistance in Aspergillus fumigatus involves multiple mechanisms of resistance. Antimicrob Agents Chemother. 2004;48:4405–13.
Moore CB, Sayers N, Mosquera J, Slaven J, Denning DW. Antifungal drug resistance in Aspergillus. J Infect. 2000;41:203–20.
Cannon RD, Lamping E, Holmes AR, et al. Efflux-mediated antifungal drug resistance. Clin Microbiol Rev. 2009;22:291–321.
Manavathu EK, Vazquez JA, Chandrasekar PH. Reduced susceptibility in laboratory-selected mutants of Aspergillus fumigatus to itraconazole due to decreased intracellular accumulation of the antifungal agent. Int J Antimicrob Agents. 1999;12:213–9.
Xiong Q, Hassan SA, Wilson WK, et al. Cholesterol import by Aspergillus fumigatus and its influence on antifungal potency of sterol biosynthesis inhibitors. Antimicrob Agents Chemother. 2005;49:518–24.
Mowat E, Butcher J, Lang S, Williams C, Ramage G. Development of a simple model for studying the effects of antifungal agents on multicellular communities of Aspergillus fumigatus. J Med Microbiol. 2007;56:1205–12.
Müller FM, Seidler M, Beauvais A. Aspergillus fumigatus biofilms in the clinical setting. Med Mycol. 2011;49 Suppl 1:S96–S100.
Ramage G, Mowat E, Jones B, Williams C, Lopez-Ribot J. Our current understanding of fungal biofilms. Crit Rev Microbiol. 2009;35:340–55.
Richie DL, Hartl L, Aimanianda V, et al. A role for the unfolded protein response (UPR) in virulence and antifungal susceptibility in Aspergillus fumigatus. PLoS Pathog. 2009;5:e1000258.
Diaz-Guerra TM, Mellado E, Cuenca-Estrella M, Rodriguez-Tudela JL. A point mutation in the 14alpha-sterol demethylase gene cyp51A contributes to itraconazole resistance in Aspergillus fumigatus. Antimicrob Agents Chemother. 2003;47:1120–4.
Mann PA, Parmegiani RM, Wei SQ, et al. Mutations in Aspergillus fumigatus resulting in reduced susceptibility to posaconazole appear to be restricted to a single amino acid in the cytochrome P450 14alpha-demethylase. Antimicrob Agents Chemother. 2003;47:577–81.
Rodriguez-Tudela JL, Alcazar-Fuoli L, Mellado E, Alastruey-Izquierdo A, Monzon A, Cuenca-Estrella M. Epidemiological cutoffs and cross-resistance to azole drugs in Aspergillus fumigatus. Antimicrob Agents Chemother. 2008;52:2468–72.
Manavathu E, Baskaran I, Krishnan S, Alangaden G, Chandrasekar P. Cytochrome P450 14alpha-sterol demethylase mutation dependent triazole cross-resistance in Aspergillus fumigatus. 43rd interscience conference on antimicrobial agents and chemotherapy. Chicago, Illinois, USA. 2003: Abstract M-471.
Escribano P, Recio S, Peláez T, Bouza E, Guinea J. Aspergillus fumigatus strains with mutations in the cyp51A gene do not always show phenotypic resistance to itraconazole, voriconazole, or posaconazole. Antimicrob Agents Chemother. 2011;55:2460–2.
van der Linden JW, Snelders E, Kampinga GA, et al. Clinical implications of azole resistance in Aspergillus fumigatus, The Netherlands, 2007–2009. Emerg Infect Dis. 2011;17:1846–54.
Arendrup MC, Perkhofer S, Howard SJ, et al. Establishing in vitro-in vivo correlations for Aspergillus fumigatus: the challenge of azoles versus echinocandins. Antimicrob Agents Chemother. 2008;52:3504–11.
Mellado E, Garcia-Effron G, Alcazar-Fuoli L, Cuenca-Estrella M, Rodriguez-Tudela JL. Substitutions at methionine 220 in the 14alpha-sterol demethylase (Cyp51A) of Aspergillus fumigatus are responsible for resistance in vitro to azole antifungal drugs. Antimicrob Agents Chemother. 2004;48:2747–50.
Asano M, Kano R, Makimura K, Hasegawa A, Kamata H. Molecular typing and in-vitro activity of azoles against clinical isolates of Aspergillus fumigatus and A. niger in Japan. J Infect Chemother. 2011;17:483–6.
Escribano P, Recio S, Peláez T, González-Rivera M, Bouza E, Guinea J. In vitro acquisition of secondary azole resistance in Aspergillus fumigatus isolates after prolonged exposure to itraconazole: presence of heteroresistant populations. Antimicrob Agents Chemother. 2012;56:174–8.
Disclosure
Dr. M.C. Arendrup has received research grants from Astellas, Gilead, MSD and Pfizer, been advisor or consultant for Gilead, MSD and Pfizer and received speakers honorarium for talks from Astellas, Gilead, MSD and Pfizer.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stensvold, C.R., Jørgensen, L.N. & Arendrup, M.C. Azole-Resistant Invasive Aspergillosis: Relationship to Agriculture. Curr Fungal Infect Rep 6, 178–191 (2012). https://doi.org/10.1007/s12281-012-0097-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12281-012-0097-7