Abstract
Understanding which fungal factors allow colonization and infection of a human host is critical to lowering the incidence of human mycoses and related mortalities. In the pathogen Aspergillus fumigatus, secondary metabolites, small bioactive molecules produced by many opportunistic fungal pathogens, have important roles in suppressing and providing protection from host defenses. Deletion of LaeA, a global regulator of secondary metabolism in fungi, significantly decreases A. fumigatus virulence, in part owing to loss of gliotoxin and hydrophobin production. In addition to gliotoxin, dihydroxynaphthalene (DHN) melanin and siderophores are other A. fumigatus virulence factors; all three metabolites are derived from hallmark secondary metabolite gene clusters. Many of the gene clusters producing toxin metabolites have yet to be deciphered, and the study of secondary metabolites and their role in the virulence of human pathogens is a nascent field.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Members of the Fungi kingdom, from biodegraders to devastating plant pathogens, play a critical role in diverse aspects of ecology. The first eukaryote to be sequenced was the yeast Saccharomyces cerevisiae, which serves as a model organism to elucidate mechanisms of cell biology. Many fungi are used in industry to produce food constituents, pharmaceuticals, and textile additives. A subset of fungi, the topic of this review, are critical human pathogens. Unfortunately, advances in medicine such as transplantation and therapies for malignancies, as well as the emergence of HIV/AIDS, have correlated with tremendous increases in fungal infections and related mortality. These mycoses are usually associated with a recurring set of pathogens (Aspergillus, Coccidioides, Candida, Cryptococcus, Histoplasma, Blastomyces, Paracoccidiodes, and other rare fungi) displaying both conserved and unique virulence factors involved in pathogenesis. An emerging area of concern is the role of fungal secondary metabolites in human mycoses.
Secondary metabolites have been loosely defined as those compounds “not generally included in the standard metabolic charts” [1]; their absence often does not result in observable phenotypes in the producing organism when grown in laboratory conditions. Nevertheless, these small-molecule bioactive compounds provide selective advantages to fungi, and loss of their production can be associated with dire ecologic consequences, as illustrated by insect preference for fungi that are free of secondary metabolites [2]. In certain plant-pathogen interactions, the virulence role of secondary metabolites is well established: for example, in Fusarium graminearum, a pathogen of cereal grains, disruption of trichothecene biosynthesis renders the fungus nonvirulent [3]. In contrast, although they are cytotoxic, the immunomodulatory and enzyme inhibitory effects of purified secondary metabolites from human fungal pathogens are well documented; a role for these compounds in virulence is just beginning to emerge. The secondary metabolites of Aspergillus fumigatus and their role in virulence are the primary focus of this review, with supporting examples from other human pathogenic fungi where relevant. Though consumption of fungal toxins (eg, aflatoxins, fumonisins, ochratoxin, or trichothecenes) in food products also leads to serious or fatal human illness, this discussion is limited to secondary metabolites actively produced by fungi during human ingress. Several excellent reviews address the topic of mycotoxicoses [4, 5].
Regulation of Secondary Metabolism
Fungal secondary metabolism (SM) is characterized by several hallmark features: the biosynthetic pathway genes are typically clustered together in the genome, the genes within clusters are coordinately regulated, and these pathways use characteristic enzymes (nonribosomal peptide synthases, polyketide synthases, terpene cyclases, or dimethylallyl tryptophan synthases) to initiate the carbon backbone of each metabolite. (Here the reader is referred to recent reviews focusing on the diverse chemistry of fungal secondary metabolites [6•, 7]). Adjacent to these synthases are genes encoding for “typical” secondary metabolite synthesis enzymes, such as esterases, methyltransferases, P450 mono-oxygenases, and, frequently, cluster-specific transcription factors [8]. Using these common features, a sequence analysis of the Aspergillus genomes, including A. fumigatus, revealed far more secondary metabolite gene clusters than the number of compounds previously known to be produced from these fungi [9]. This discovery highlights that many secondary metabolite gene clusters are “silent” when the fungus is grown in laboratory conditions, thus complicating investigations of any putative role of such metabolites in human mycoses (Table 1).
SM is an expensive cellular process, and several levels of genetic regulation act to govern when and where secondary metabolites will be produced. At the individual pathway level, clusters often contain a gene or genes encoding a pathway-specific transcription factor. Frequently, this is a C6 zinc binuclear transcription factor, which commonly binds palindromic sequences in promoters [10]. Well-described SM transcription factors include GliZ, regulating gliotoxin enzymatic gene expression [11]; AflR, regulating aflatoxin synthesis [12]; and Tri6, regulating trichothecene synthesis [13]. Activation of C6 zinc binuclear transcription factors can be sufficient to produce otherwise silent metabolites [6•]. Additionally, transcription factors involved in nitrogen (AreA), carbon (CreA), and pH (PacC) homeostasis often affect the expression of SM clusters [14].
An exciting advance was the discovery that a large percentage of SM can be manipulated by the modulation of a single gene, laeA. Targeted deletions of laeA in several ascomycete genera repress SM, including in the human pathogens A. fumigatus and Aspergillus flavus [15–18] and the industrial agent Penicillium chrysogenum [19]. This list is expected to grow larger as LaeA partners with another nuclear protein, VeA (discussed below). VeA regulates SM in several genera, including Fusarium and Acremonium, as well as Aspergillus [20, 21]. Several microarray studies have shown the global nature of LaeA regulation of SM clusters. For example, there are at least 30 predicted SM gene clusters in A. fumigatus [18] and a microarray analysis with a ΔlaeA strain revealed that 13 clusters were downregulated in one single experimental condition, including the known virulence determinant, gliotoxin [18, 22].
How LaeA functions to regulate SM is still unknown, but many insights have been generated using several in vivo techniques. Constitutively nuclear, LaeA forms part of the light-regulated heterotrimeric Velvet complex composed of partner proteins VeA and VelB [20]. In light conditions where sexual development and secondary metabolism are limited, VeA is diffused throughout the cell [23]. Upon a switch to dark conditions, both sexual development and SM are induced, and VeA accumulates in the nucleus, forming a complex with LaeA and VelB [24]. LaeA regulation is positional: moving genes into a LaeA-regulated cluster places them under LaeA control, whereas moving them out of a LaeA-regulated cluster releases them from LaeA control [24]. The sequence of LaeA is homologous to methyltransferase enzymes, and mutation of a conserved S-adenosyl methionine binding motif is functionally equivalent to deletion in vivo. Taken together, these genetic characterizations of LaeA suggest a role for this protein in chromatin remodeling. This hypothesis is supported by work showing that manipulation of chromatin-modifying enzymes can partially rescue ΔlaeA repression of SM, and that ΔlaeA strains result in increased concentration of the heterochromatic mark histone 3 lysine 9 methylation in the sterigmatocystin gene cluster [25, 26]. However, no substrates of LaeA have been reported, and the mechanism that connects the Velvet complex and LaeA to the regulated gene clusters remains to be determined.
Although the mechanism of its regulation of SM remains cryptic, LaeA has been shown by several laboratories to be a virulence determinant in immunocompromised mouse model systems and ex vivo cellular models [16, 27–29]. Cellular parameters regulated by LaeA that have been linked with virulence in A. fumigatus include production of several toxic secondary metabolites, including gliotoxin and reduction in the spore hydrophobic protein RodA [16, 27]. Consistent with the downregulation of gliotoxin—a known immunosuppressive mycotoxin reviewed below—culture filtrates of a ΔlaeA strain failed to suppress neutrophil reactive oxygen species or induce apoptosis of EL4 thymoma cells. Additionally, disruption of laeA impairs the ability to evade host defenses and rapidly colonize the host, as ΔlaeA conidia are taken up at a higher rate than wild-type conidia, and histopathology of mouse lungs reveals a delayed progression of induced pulmonary lesions and a decrease in necrosis [29]. RodA was recently identified as an inert compound that evades detection by the host immune system; decreased RodA protein results in increased phagocytosis of conidia [30•], an observation also applicable to ΔlaeA conidia [27]. Moreover, many of the genes regulated by LaeA are subtelomerically located and are associated with activation in initial murine lung colonization [31].
In conclusion, LaeA has been a critical player in illustrating the role of SM in mycoses. LaeA is a virulence factor in invasive aspergillosis [16, 27, 28], is conserved in ascomycetes, and governs virulence in A. flavus [21]. As will be illustrated below, LaeA provided a major conduit towards the definitive role of gliotoxin in invasive aspergillosis.
Secondary Metabolites
Gliotoxin
Gliotoxin production in A. fumigatus cultures was first observed as a contaminant in the preparation of another secondary metabolite, fumigacin [32]. In the decades since its initial discovery, a large body of evidence has accumulated implicating gliotoxin as a virulence component of invasive aspergillosis. Classified as an epipolythiodioxopiperazine (EPT) secondary metabolite, this compound is characterized by a disulphide bridged cyclic dipeptide (Fig. 1a) and has antibacterial, antifungal, antiviral, anticancer, enzyme inhibitory, and immunomodulatory properties. Toxic effects have prevented the clinical development of gliotoxin as a pharmaceutical or antimicrobial agent, so research has focused on the immunomodulatory properties of gliotoxin and its role in virulence.
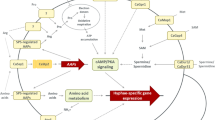
Virulence factor biosynthetic pathways of Aspergillus fumigatus. On these schematic representations of the three secondary metabolite biosynthetic pathways confirmed as virulence factors in animal models, the genes whose deletion results in a significant decrease in virulence are indicated in yellow squares and genes whose disruption are reported as not affecting virulence are indicated in red circles. The arrows represent the putative steps in each pathway. a, Gliotoxin biosynthesis. The initiating nonribosomal peptide synthetase (NRPS) GliP condenses phenylalanine and serine to a dipeptide. Disruption of gliP results in a decrease in virulence in cortisone acetate–immunosuppressed mouse models [41•]. b, DHN melanin biosynthetic pathway. The pathway initiates by the condensation of one acetyl-CoA with four malonyl-CoA molecules by pksP, which is a virulence factor in this pathway. The other reported virulence factor is arp1, which reduces scytalone to 1,3,8-trihydroxynaphthalene (1,3,8-THN). Disruption of abr2, a laccase-encoding enzyme, or arp2, a 1,3,6,8-THN reductase, is reported not to affect virulence or susceptibility to reactive oxygen species [48, 50]. (Adapted from Pihet et al. [63].) c, Siderophore biosynthetic pathway. L-ornithine is used as a common precursor of both external and internal siderophore biosynthetic pathways; the first enzyme in this pathway, sidA, is a reported virulence factor. Each pathway uses unique NRPS enzymes and both are virulence factors. In A. fumigatus, the final products of each pathway are extracellular triacetylfusarine C (TAFC) and intracellular hydroxyferricrocin (HFC) siderophores. R—acetyl. (Adapted from Schrettl et al. [52])
Gliotoxin biosynthesis is a classic fungal SM. The putative gliotoxin biosynthetic cluster was identified by searching the A. fumigatus genome for genes with homology to the genes encoding a similar structured EPT molecule, sirodesmin, produced by the fungus Leptosphaeria maculans. Twelve genes have been proposed to be included in the cluster, including the pathway-specific transcription factor gliZ [33]. The first enzymatic step in the biosynthetic pathway catalyzed by a nonribosomal peptide synthetase (NRPS), gliP, condenses serine and phenylalanine to a core dipeptide [34, 35]. The remaining modifications of the core dipeptide structure and the responsible biosynthetic enzymes remain to be correlated (Fig. 1a). Interestingly, both gliotoxin and sirodesmin possess antifungal properties, and it has been shown in L. maculans that an ABC transporter gene in the sirodesmin cluster affords self-protection to this fungus. Possibly both metabolites evolved to secure niches in the environment.
In vitro data suggesting a role for gliotoxin in assisting the colonizing A. fumigatus to disarm the host’s innate and adaptive immune system is supported by several observations. Gliotoxin is a redox active toxin and the mechanism of action is proposed to be via generation of reactive oxygen species and by formation of mixed disulphides with proteins having accessible thiol groups [36]. Apoptosis in macrophages and neutrophils is induced by gliotoxin, and the reactive oxygen burst of polymorphonuclear leukocytes is attenuated. The in vivo immunomodulatory role of gliotoxin was assessed in an interesting study using Listeria monocytogenes infection of mice as a model [37]. This bacterial infection model is well established for the study of the innate (and particularly the T-cell–mediated) adaptive immune response. At an early point, before an adaptive immune response was mounted, gliotoxin treatment increased the bacterial load in target organs. After an adaptive response was mounted, the bacterial load continued to increase only in gliotoxin-treated mice. Although this result clearly shows that gliotoxin is immunosuppressive in vitro, the data identifying gliotoxin as a bona fide virulence factor required supporting in vivo experiments. Five different groups generated gliotoxin-deficient A. fumigatus mutants in four different strains, and then evaluated them in two different immunosuppression regimens with different mice strains [11, 35, 38–40]. Gliotoxin mutants demonstrated wild-type virulence in neutropenic models but reduced virulence in non-neutropenic models. These findings highlighted the importance of the model system when interpreting virulence data. Gliotoxin may not be critical for disease progression in neutropenic patients, but it is relevant for patients that are non-neutropenic but are otherwise immunosuppressed [41•].
As mentioned above, LaeA regulates gliotoxin synthesis in A. fumigatus. Three separate studies compared the pathogenicity of laeA and gliotoxin mutants in mice, and all provided evidence that the loss of gliotoxin plays a role in ΔlaeA decreased virulence but that other factors—including other SMs—also account for the importance of LaeA in invasive aspergillosis [16, 28, 29]. The prominent tissue inflammation and ischemia associated with invasive aspergillosis stimulates angiogenesis. Use of an ex vivo angiogenesis inhibition assay and comparison of ΔlaeA, ΔgliP, and wild-type cellular extracts revealed that the pleiotropic effects of A. fumigatus cellular extracts with different mammalian cell lines are not attributable solely to gliotoxin. Gliotoxin is an important inhibitor of angiogenesis, as demonstrated when ΔgliP strains and extracts resulted in about a 50% decrease of inhibition. However, the loss of the secondary metabolites regulated by laeA resulted in angiogenesis similar to the uninfected control [29]. This novel suggestion of the role gliotoxin plays in modulating host angiogenesis adds to our understanding of the pathobiology of A. fumigatus and clearly indicates that other, as-yet-unknown SMs are important in invasive aspergillosis.
Melanins
Melanins are amorphous polymers shown to have a role in protecting both plant and human pathogenic fungi (the latter including Cryptococcus neoformans, A. fumigatus, Wangiella dermatitidis, and Paracoccidioides brasiliensis) against host defenses. Two different types of melanin are found within human pathogenic fungi: dihydroxynaphthalene (DHN) melanin and eumelanin. DHN melanin is biosynthesized using a polyketide synthase (PKS) enzyme to cyclize malonyl-CoA precursors and is ultimately assembled into a polymer using a laccase enzyme. Eumelanin is biosynthesized using diphenolic precursors such as 3,4 dihydroxyphenylalanine (L-DOPA) assembled with a laccase enzyme [42•, 43•, 44]. Eumelanin is the pigment found in C. neoformans, and disruption of a single laccase enzyme results in an albino mutant that is attenuated in virulence in animal models. Though eumelanin production is an important virulence factor, we are focusing on the DHN melanins, as the biosynthesis of this type of melanin follows, in part, a prototypical SM schema. However, the hallmark SM clustering of biosynthetic genes is not strictly conserved for melanins. In many fungi, the DHN melanin structural genes are often scattered throughout the genome (as reviewed elsewhere [45]), but in the pathogenic A. fumigatus, there is a cluster of six genes that are all involved in DHN melanin biosynthesis (Fig. 1b).
Albino mutants of A. fumigatus, Aspergillus nidulans, and W. dermatitidis are not significantly impaired in normal growth and development in laboratory growth medium, as would be predicted for the loss of a secondary metabolite. In DHN melanin biosynthesis, an iterative PKS initiates the pathway by condensing acyl-CoA molecules in a head-to-tail joining and cyclization. Differences in PKS substrate use have been reported between fungi, with Colletotrichum lagenarium using only malonyl-CoA [46]. In A. fumigatus, the initiating enzyme, pksP, condenses one acetyl-CoA with four malonyl-CoA molecules to produce a heptaketide napthopyrone (Fig. 1b). Disruption of pksP, also known as alb1, results in albino mutants with severely attenuated virulence and smooth spores [47–49•].
Curiously, mutants of genes further down the biosynthetic pathway, which result in pink or brown pigmentation of the spores, do not present a clear picture in virulence attributes. Tsai et al. [50] reported preliminary studies of the scytalone reductase deletant, Δarp1, which functions at the midpoint of the pathway. Disruption of arp1 results in reddish-pink spores and is the only other reported virulence determinant from this pathway. Disruption of the final enzymatic assembly into the amorphic polymer by disruption of the abr2 laccase encoding enzyme results in brown spores but no increase in susceptibility to reactive oxygen species or decrease in virulence in an intranasal mouse-infection mode [48]. Although Δabr2 strains clearly show an overall decrease in laccase activity, the gross spore morphologic changes associated with disruption of all melanin pathway intermediates are absent.
The resting spores are the first contact with the host, and DHN melanin is found only in the conidia. As the spore begins to swell during germination, the melanin coating begins to break down, and it is not detectable at the point when a germ tube appears. Several properties of this pigment coating have been described that may contribute to its role in virulence in the establishment of infection. DHN melanins partially counteract the defenses of the host immune system by absorbing toxic reactive oxygen species secreted by macrophages and neutrophils. A recent report suggests that DHN melanins may function by shielding fungal ligands from host receptors [49•]. These include several conserved fungal pathogen-associated molecular patterns (PAMPs) displayed on the conidial surface, such as β-glucan and mannose proteins. Both purified melanins and wild-type conidia were weakly proinflammatory. In contrast, ΔpksP conidia elicited greater cytokine production than wild-type conidia, indicating that PAMPs displayed on the cell surface are masked by the melanin layer. These immunomodulatory and protective properties, combined with the loss of virulence in albino mutants, reveal that this secondary metabolite is more than a rudimentary pigment; it is critical in the pathogenicity of A. fumigatus.
Siderophores
Most fungal siderophores are produced by the condensation of the nonproteinogenic amino acid ornithine using NRPS enzymes. These hydroxamate siderophores form tight associations with Fe+++ ions and are used by the fungi to obtain iron in a low-iron environment. These high-affinity metabolites are not the only mechanism used by the fungi to obtain iron. and in some cases, such as in A. fumigatus, disruption of siderophore biosynthesis is not lethal. The question of whether siderophores should be classified as primary or secondary metabolites is unresolved, but we include them here for their shared biochemical attributes (the use of NRPS genes, gene clustering, nonlethal disruptions) and their clearly demonstrated role as virulence determinants in mammalian models. This section focuses on the role of siderophores in A. fumigatus virulence; for an excellent review on the broader role of siderophores in virulence of fungi see Haas et al. [51••].
Schrettl et al. [52] demonstrated distinct roles for the different siderophores produced by A. fumigatus. From the common precursor L-ornithine, two biosynthetic pathways that use unique NRPS enzymes are formed: the intracellular hydroxyferricrocin (HFC) siderophore and the extracellular triacetylfusarine C (TAFC) siderophore (Fig. 1c). Intracellular iron is stored as an FC-iron chelate, comprising more than 47% of the total conidial iron content. FC provides protection against the oxidative damaging properties of excess iron and has been shown to accumulate during intracellular iron excess [53].
The effect on the pathobiology of A. fumigatus of intracellular and extracellular siderophores, as well as several biosynthetic intermediates, has been examined with an in vivo neutropenic mouse model. Disruption of TAFC and HFC biosynthesis by deletion of the first biosynthetic pathway enzyme, an L-Ornithine N5-Oxygenase, encoded by sidA, yielded avirulent strains. Supplementation of the intracellular HFC precursor to ΔsidA strains partially rescued virulence (40% mouse survival at 12 days), suggesting that siderophores are critical in the initial phase of infection. Blocking the intracellular HFC pathway by disrupting the NRPS gene, ΔsidC, decreased virulence (50% survival at 12 days) and supports the role of the siderophores in establishing disease. Deletion of the genes involved in the extracellular TAFC biosynthetic pathway resulted in severe attenuation of disease (75% to 95% survival at 12 days), demonstrating that the primary role of extracellular siderophores is to promote hyphal growth under iron-limiting conditions, when a secondary iron uptake system present in A. fumigatus, reductive iron assimilation, is insufficient [52].
Iron availability affects host-pathogen interactions. As part of an innate strategy of the human host to combat infection, the availability of free iron in the host is lowered in response to inflammation induced by microbial pathogens. The production of siderophores to access host iron reservoirs is not a strict determinant of virulence: A. nidulans (a nonpathogen) and A. fumigatus both produce the same siderophores. Also, other human pathogens, such as Candida albicans and C. neoformans, lack siderophores. These other pathogens employ one or more of four different uptake mechanisms identified within the fungi, as reviewed by Haas et al. [51••], but no efflux mechanism has yet been identified, suggesting that control of iron uptake is the major homeostatic mechanism. All four systems are rarely found in a single species, but redundancy is a common theme. Within the Aspergilli, the nonpathogenic species A. nidulans employs only the siderophore-mediated uptake mechanism, whereas the iron uptake of the pathogenic A. fumigatus is redundant, employing both siderophores and reductive iron assimilation. The demonstrated role of siderophores in virulence and a lack of homologous biosynthetic machinery in humans make inhibition of these enzymes potential targets for antifungal therapies.
Other Toxic Secondary Metabolites with Unknown Roles in Virulence
The list of confirmed secondary metabolites that function as virulence determinants is currently limited. In A. fumigatus, several immunosuppressive toxins are known to be produced, but their assessment as virulence determinants has been hampered by a lack of knowledge of the encoding biosynthetic pathways. Gene clusters encoding biosynthetic enzymes for fumitremorgin, pseurotin A, helvolic acid, and fumigaclavine have been determined using a variety of methods. The gene clusters encoding fumitremorgin and pseurotin A were elucidated by in vivo disruption and overexpression of the precursor encoding NRPS brevianamide synthetase and hybrid PKS/NRPS PsoA, respectively, in the genome reference strain AF293 [54, 55]. Helvolic acid and fumigaclavine C biosynthetic gene clusters were identified by heterologous expression of pathway genes in nonproducing organisms and biochemical confirmation of precursor formation [56, 57]. Currently there are no known animal tests to assess the role of these metabolites by using mutant strains of A. fumigatus. Other toxins known to be produced include fumiquinazolines, fumagillin, monomethylsulochrin, phthioic acid, pyripyropene A, restrictocin and trypacidin, but the encoding genes have not been elucidated in A. fumigatus [58]. The advances made in connecting the observed mycotoxins of this pathogenic organism with the biosynthetic enzymes combined with in vivo disruption mutants will allow for assessment of the role of each SM in animal models of virulence (Table 2).
Conclusions
As research into the biosynthesis of fungal secondary metabolites is allowing for the creation of SM null or overexpression mutants, we are poised to gain deeper understanding into the role that these bioactive compounds play in human mycoses—not just in A. fumigatus but also in other pathogenic fungi that have hallmark secondary metabolite gene clusters in their genomes. Ultimately, deletions and/or heterologous cluster expression may allow us to understand the role of SM in aiding fungal pathogenicity. Is it the production of fungal metabolites that confer an advantage inside a host? (That is, could the nonpathogenic species A. nidulans evolve into a pathogen by the addition of a helvolic acid biosynthetic pathway, allowing this species to inhibit cilial clearing?) Is it the ability to evade host defenses, such as the masking of PAMPs conferred by DHN melanins? Or is it an ability to suppress the host defenses with a natural product, such as the suppression by gliotoxin of neutrophil phagocytosis?
The ability to modulate a large proportion of SM by manipulating the global regulator laeA indicated that virulence will not be attributable to a single metabolite. The ΔlaeA strain of A. fumigatus abrogated the production of gliotoxin and significantly reduced virulence in neutropenic mouse models. This reduced virulence indicates that there are other secondary metabolites affected by ΔlaeA in addition to gliotoxin, as gliotoxin was not a virulence factor in neutropenic models. We predict that an understanding of the LaeA mechanism will provide useful information in understanding how to simultaneously regulate multiple SM clusters. There is a need to assay each metabolite’s contribution to virulence in isolation, but it will be critical to identify the combinations of metabolites that confer virulence in the different immunocompromised animal models.
Disruption mutants have been reported for the helvolic acid, pseurotin A, and fumitremorgin biosynthetic pathways, and the results of virulence assays in animal models will be informative for directing future research. Metabolites that can be classified as virulence determinants should be put forward as candidates for additional disruption analysis of each pathway enzymatic step, as has been done in the siderophore biosynthetic pathway. This basic knowledge will provide insights as to which steps contribute to the ability of the fungi to colonize a human host and is essential to any therapeutic strategy targeting specific pathogen pathways.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Davies J: Recombinant DNA and the Production of Small Molecules. Washington, DC: ASM Press; 1985.
Rohlfs M, Albert M, Keller NP, Kempken F: Secondary chemicals protect mould from fungivory. Biol Lett 2007, 3:523–525.
Proctor RH, Hohn TM, McCormick SP: Reduced virulence of Gibberella zeae caused by disruption of a trichothecene toxin biosynthetic gene. Mol Plant Microbe Interact 1995, 8:593–601.
Paterson RR, Lima N: Toxicology of mycotoxins. EXS 2010, 100:31–63.
Richard JL: Some major mycotoxins and their mycotoxicoses—an overview. Int J Food Microbiol 2007, 119:3–10.
• Cichewicz RH: Epigenome manipulation as a pathway to new natural product scaffolds and their congeners. Nat Prod Rep 2009, 27:11–22. This article reviews the manipulation of chromatin-modifying enzymes to upregulate cryptic gene clusters and identify the resultant secondary metabolites.
Brase S, Encinas A, Keck J, Nising CF: Chemistry and biology of mycotoxins and related fungal metabolites. Chem Rev 2009, 109:3903–3990.
Hoffmeister D, Keller NP: Natural products of filamentous fungi: enzymes, genes, and their regulation. Nat Prod Rep 2007, 24, 393–416.
Nierman WC, Pain A, Anderson MJ, et al.: Genomic sequence of the pathogenic and allergenic filamentous fungus Aspergillus fumigatus. Nature 2005, 438:1151–1156.
Maicas S, Moreno I, Nieto A, et al.: In silico analysis for transcription factors with Zn(II)2C6 binuclear cluster DNA-binding domains in Candida albicans. Comp Funct Genomics 2005, 6:345–356.
Bok JW, Chung D, Balajee SA, et al.: GliZ, a transcriptional regulator of gliotoxin biosynthesis, contributes to Aspergillus fumigatus virulence. Infect Immun 2006, 74:6761–6768.
Woloshuk CP, Foutz KR, Brewer JF, et al.: Molecular characterization of aflR, a regulatory locus for aflatoxin biosynthesis. Appl Environ Microbiol 1994, 60:2408–2414.
Proctor RH, Hohn TM, McCormick SP, Desjardins AE: Tri6 encodes an unusual zinc finger protein involved in regulation of trichothecene biosynthesis in Fusarium sporotrichioides. Appl Environ Microbiol 1995, 61:1923–1930.
Yu JH, Keller N: Regulation of secondary metabolism in filamentous fungi. Annu Rev Phytopathol 2005, 43:437–458.
Bok JW, Keller NP: LaeA, a regulator of secondary metabolism in Aspergillus spp. Eukaryot Cell 2004, 3:527–535.
Bok JW, Balajee SA, Marr KA, et al.: LaeA, a regulator of morphogenetic fungal virulence factors. Eukaryot Cell 2005, 4:1574–1582.
Kale SP, Milde L, Trapp MK, et al.: Requirement of LaeA for secondary metabolism and sclerotial production in Aspergillus flavus. Fungal Genet Biol 2008, 45:1422–1429.
Fedorova ND, Khaldi N, Joardar VS, et al.: Genomic islands in the pathogenic filamentous fungus Aspergillus fumigatus. PLoS Genet 2008, 4:e1000046.
Kosalkova K, Garcia-Estrada C, Ullan RV, et al.: The global regulator LaeA controls penicillin biosynthesis, pigmentation and sporulation, but not roquefortine C synthesis in Penicillium chrysogenum. Biochimie 2009, 91:214–225.
Bayram O, Krappmann S, Ni M, et al.: VelB/VeA/LaeA complex coordinates light signal with fungal development and secondary metabolism. Science 2008, 320:1504–1506.
Amaike S, Keller NP: Distinct roles for VeA and LaeA in development and pathogenesis of Aspergillus flavus. Eukaryot Cell 2009, 8:1051–1060.
Perrin RM, Fedorova ND, Bok JW, et al.: Transcriptional regulation of chemical diversity in Aspergillus fumigatus by LaeA. PLoS Pathog 2007, 3:e50.
Stinnett SM, Espeso EA, Cobeno L, et al.: Aspergillus nidulans VeA subcellular localization is dependent on the importin alpha carrier and on light. Mol Microbiol 2007, 63:242–255.
Bok JW, Noordermeer D, Kale SP, Keller NP: Secondary metabolic gene cluster silencing in Aspergillus nidulans. Mol Microbiol 2006, 61:1636–1645.
Reyes-Dominguez Y, Bok JW, Berger H, et al.: Heterochromatic marks are associated with the repression of secondary metabolism clusters in Aspergillus nidulans. Mol Microbiol 2010, 76:1376–1386.
Shwab EK, Bok JW, Tribus M, et al.: Histone deacetylase activity regulates chemical diversity in Aspergillus. Eukaryot Cell 2007, 6:1656–1664.
Dagenais TR, Giles SS, Aimanianda V, et al.: Aspergillus fumigatus LaeA-mediated phagocytosis is associated with a decreased hydrophobin layer. Infect Immun 2010, 78:823–829.
Sugui JA, Pardo J, Chang YC, et al.: Role of laeA in the Regulation of alb1, gliP, conidial morphology, and virulence in Aspergillus fumigatus. Eukaryot Cell 2007, 6:1552–1561.
Ben-Ami R, Lewis RE, Leventakos K, Kontoyiannis DP: Aspergillus fumigatus inhibits angiogenesis through the production of gliotoxin and other secondary metabolites. Blood 2009, 114:5393–5399.
• Aimanianda V, Bayry J, Bozza S, et al.: Surface hydrophobin prevents immune recognition of airborne fungal spores. Nature 2009, 460:1117–1121. This paper demonstrates that the RodA protein coating the conidia surface renders the spores immunologically inert.
McDonagh A, Fedorova ND, Crabtree J, et al.: Sub-telomere directed gene expression during initiation of invasive aspergillosis. PLoS Pathog 2008, 4:e1000154.
Menzel AEO, Wintersteiner O, Hoogerheide JC: The isolation of gliotoxin and fumigacin from culture filtrates of Aspergillus fumigatus. J Biol Chem 1944, 152:419–429.
Gardiner DM, Howlett BJ: Bioinformatic and expression analysis of the putative gliotoxin biosynthetic gene cluster of Aspergillus fumigatus. FEMS Microbiol Lett 2005, 248:241–248.
Balibar CJ, Walsh CT: GliP, a multimodular nonribosomal peptide synthetase in Aspergillus fumigatus, makes the diketopiperazine scaffold of gliotoxin. Biochemistry 2006, 45:15029–15038.
Cramer RA Jr, Gamcsik MP, Brooking RM, et al.: Disruption of a nonribosomal peptide synthetase in Aspergillus fumigatus eliminates gliotoxin production. Eukaryot Cell 2006, 5:972–980.
Golden MC, Hahm SJ, Elessar RE, et al.: DNA damage by gliotoxin from Aspergillus fumigatus. An occupational and environmental propagule: adduct detection as measured by 32P DNA radiolabelling and two-dimensional thin-layer chromatography. Mycoses 1998, 41:97–104.
Kupfahl C, Geginat G, Hof H: Gliotoxin-mediated suppression of innate and adaptive immune functions directed against Listeria monocytogenes. Med Mycol 2006, 44(7):591–599.
Spikes S, Xu R, Nguyen CK, et al.: Gliotoxin production in Aspergillus fumigatus contributes to host-specific differences in virulence. J Infect Dis 2008, 197:479–486.
Kupfahl C, Heinekamp T, Geginat G, et al.: Deletion of the gliP gene of Aspergillus fumigatus results in loss of gliotoxin production but has no effect on virulence of the fungus in a low-dose mouse infection model. Mol Microbiol 2006, 62:292–302.
Sugui JA, Pardo J, Chang YC, et al.: Gliotoxin is a virulence factor of Aspergillus fumigatus: gliP deletion attenuates virulence in mice immunosuppressed with hydrocortisone. Eukaryot Cell 2007, 6:1562–1569.
• Kwon-Chung KJ, Sugui JA: What do we know about the role of gliotoxin in the pathobiology of Aspergillus fumigatus? Med Mycol 2009, 47(Suppl 1):S97–S103. This article reviews the equivocal results of five studies concerning gliotoxin as a virulence factor in mouse models and highlights that the immune suppression treatment used was the determining factor in all studies.
• Liu GY, Nizet V: Color me bad: microbial pigments as virulence factors. Trends Microbiol 2009, 17:406–413. This paper reviews a broad range of pigments and their roles in the virulence of bacteria, fungi, and protozoa pathogens.
• Karkowska-Kuleta J, Rapala-Kozik M, Kozik A: Fungi pathogenic to humans: molecular bases of virulence of Candida albicans, Cryptococcus neoformans and Aspergillus fumigatus. Acta Biochim Pol 2009, 56:211–224. This article reviews molecular mechanisms of virulence in addition to secondary metabolism for three important fungal pathogens.
Gomez BL, Nosanchuk JD: Melanin and fungi. Curr Opin Infect Dis 2003, 16:91–96.
Keller NP, Hohn TM: Metabolic pathway gene clusters in filamentous fungi. Fungal Genet Biol 1997, 21:17–29.
Fujii I, Mori Y, Watanabe A, et al.: Heterologous expression and product identification of Colletotrichum lagenarium polyketide synthase encoded by the PKS1 gene involved in melanin biosynthesis. Biosci Biotechnol Biochem 1999, 63:1445–1452.
Tsai HF, Chang YC, Washburn RG, et al: The developmentally regulated alb1 gene of Aspergillus fumigatus: its role in modulation of conidial morphology and virulence. J Bacteriol 1998, 180:3031–3038.
Sugareva V, Hartl A, Brock M, et al.: Characterisation of the laccase-encoding gene abr2 of the dihydroxynaphthalene-like melanin gene cluster of Aspergillus fumigatus. Arch Microbiol 2006, 186:345–355.
• Chai LY, Netea MG, Sugui J, et al.: Aspergillus fumigatus conidial melanin modulates host cytokine response. Immunobiology 2009 Nov 23 (Epub ahead of print). This paper advances our understanding of how melanins protect conidia from host defenses.
Tsai HF, Wheeler MH, Chang YC, et al.: A developmentally regulated gene cluster involved in conidial pigment biosynthesis in Aspergillus fumigatus. J Bacteriol 1999, 181:6469–6477.
•• Haas H, Eisendle M, Turgeon BG: Siderophores in fungal physiology and virulence. Annu Rev Phytopathol 2008, 46:149–187. This is a thorough review of iron acquisition mechanisms, siderophore biosynthesis, iron storage, iron metabolism regulation, and virulence in both plants and animals.
Schrettl M, Bignell E, Kragl C, et al.: Distinct roles for intra- and extracellular siderophores during Aspergillus fumigatus infection. PLoS Pathog 2007, 3:1195–1207.
Wallner A, Blatzer M, Schrettl M, et al.: Ferricrocin, a siderophore involved in intra- and transcellular iron distribution in Aspergillus fumigatus. Appl Environ Microbiol 2009, 75:4194–4196.
Maiya S, Grundmann A, Li X, et al.: Identification of a hybrid PKS/NRPS required for pseurotin A biosynthesis in the human pathogen Aspergillus fumigatus. Chembiochem 2007, 8:1736–1743.
Maiya S, Grundmann A, Li SM, et al.: The fumitremorgin gene cluster of Aspergillus fumigatus: identification of a gene encoding brevianamide F synthetase. Chembiochem 2006, 7:1062–1069.
Unsold IA, Li SM: Reverse prenyltransferase in the biosynthesis of fumigaclavine C in Aspergillus fumigatus: gene expression, purification, and characterization of fumigaclavine C synthase FGAPT1. Chembiochem 2006, 7:158–164.
Mitsuguchi H, Seshime Y, Fujii I, et al.: Biosynthesis of steroidal antibiotic fusidanes: functional analysis of oxidosqualene cyclase and subsequent tailoring enzymes from Aspergillus fumigatus. J Am Chem Soc 2009, 131:6402–6411.
Frisvad JC, Rank C, Nielsen KF, Larsen TO: Metabolomics of Aspergillus fumigatus. Med Mycol 2009, 47(Suppl 1):S53–S71.
Reeves EP, Reiber K, Neville C, et al.: A nonribosomal peptide synthetase (Pes1) confers protection against oxidative stress in Aspergillus fumigatus. FEBS J 2006, 273:3038–3053.
Kremer A, Westrich L, Li SM: A 7-dimethylallyltryptophan synthase from Aspergillus fumigatus: overproduction, purification and biochemical characterization. Microbiology 2007, 153:3409–3416.
Lodeiro S, Xiong Q, Wilson WK, et al.: Protostadienol biosynthesis and metabolism in the pathogenic fungus Aspergillus fumigatus. Org Lett 2009, 11:1241–1244.
Ames BD, Walsh CT: Anthranilate-activating modules from fungal nonribosomal peptide assembly lines. Biochemistry 2010, 49:3351–3365.
Pihet M, Vandeputte P, Tronchin G, et al.: Melanin is an essential component for the integrity of the cell wall of Aspergillus fumigatus conidia. BMC Microbiol 2009, 9:177.
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Garvey, G.S., Keller, N.P. Fungal Secondary Metabolites and Their Fundamental Roles in Human Mycoses. Curr Fungal Infect Rep 4, 256–265 (2010). https://doi.org/10.1007/s12281-010-0032-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12281-010-0032-8