Abstract
Nonalcoholic fatty liver disease (NAFLD) is rapidly becoming the most common cause of fatal liver diseases such as cirrhosis, liver cancer, and indications for orthotopic liver transplantation. Given its high prevalence, the absence of FDA-approved drugs for NAFLD is noticeable. In the pathogenesis of NAFLD, it is well known that mitochondrial dysfunction arises as a result of changes in ETC complexes and the membrane potential (Δψm), as well as decreased ATP synthesis. Due to their fundamental role in energy metabolism and cell death decision, alterations in mitochondria are considered to be critical factors causing NAFLD. Reduced levels of β-oxidation, along with increased lipogenesis, result in lipid accumulation in hepatocytes, and the subsequent production of reactive oxygen species and hepatocyte injury, which contribute to hepatic inflammation and fibrosis through the activations of Kupffer cells and hepatic stellate cells. Here, we review the latest findings describing the involvement of mitochondrial processes in the development of NAFLD and discuss the potential targets against which therapeutics for this disease can be developed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nonalcoholic fatty liver disease (NAFLD) has been identified as a global epidemic and it is mostly likely to occur in association with type 2 diabetes and obesity (Milic and Stimac 2012; Loomba and Sanyal 2013). NAFLD constitutes a spectrum of liver disorders that begins with simple steatosis (SS), which can progress to nonalcoholic steatohepatitis (NASH), nonalcoholic steatofibrosis (NASF), cirrhosis, and hepatocellular carcinoma (HCC) in advanced stages (Younes and Bugianesi 2019).
Insulin resistance, oxidative stress, and inflammation are believed to play central roles in the pathogenesis of NAFLD (Lewis and Mohanty 2010). Despite an understanding of how lipid accumulates in the liver (SS), the mechanisms by which NASH and NASF develop are unclear. Numerous hypotheses have been proposed for NAFLD pathogenesis. According to the classical “two-hit” hypothesis, NAFLD is a sequential disease whereby insulin resistance (“the first hit”) promotes an increased flux of free fatty acids (FFAs) into hepatocytes. If these FFAs are not appropriately metabolized or secreted, simple steatosis can develop (Day and James 1998). Simple steatosis then predisposes the liver to a “second hit” including mitochondrial dysfunction, ER stress, bacterial endotoxins of intestinal origin, and inflammation. This classical hypothesis has been modified to indicate that NAFLD may be a consequence of parallel “multi-hits” (Tilg and Moschen 2010). In this model, insulin resistance results in increased lipogenesis and excessively elevated uptake of FFAs into hepatocytes. This lipotoxicity primes the liver for injury arising from “multiple and parallel hits” (oxidative stress and the activation of proinflammatory and fibrogenic pathways including the activation of Kupffer cells and hepatic stellate cells), which leads to NASH and NASF (Berlanga et al. 2014).
Mitochondria are key organelles that play a vital role in energy generation from glucose, glutamine and lipid metabolism. Alterations in mitochondrial structure and function are considered a hallmark of NAFLD. Early observations in patient with NASH described the presence of dysfunctional mitochondria in hepatocytes (Garcia-Ruiz et al. 2013). Moreover, studies in experimental models have shown a wide range of changes in mitochondrial functions (Sunny et al. 2017). Besides their function in ATP generation by oxidative phosphorylation, mitochondria also plays important roles in many other cellular events, including fatty acid breakdown by β-oxidation, the synthesis of ketone bodies, the oxidative catabolism of amino acids, metabolite production via the tricarboxylic acid cycle (TCA cycle), and the generation of reactive oxygen species (ROS) (Kelly and Scarpulla 2004; Murgia et al. 2009; Murphy 2009). Hence, alterations in mitochondrial function may have a broad impact on cellular integrity and thus stand out as an important mechanism underlying the pathogenesis of NAFLD. The aim of this review is to provide a general overview of mitochondrial perturbations in terms of energy metabolism and their potential implications in NAFLD.
Mitochondria dysfunction in NAFLD: oxidative stress and ROS production
Under conditions of normal mitochondrial homeostasis, a cell can effectively remove physiological ROS through antioxidant mechanisms as well as by enabling metabolic adaptations that inhibit substrate delivery to the TCA cycle, a series of enzyme-catalyzed chemical reactions used by aerobic organisms to release energy. In NAFLD, however, both increased mitochondrial ROS production and decreased activity of ROS scavenging mechanisms (e.g., GSH, SOD2, and catalase) could potentiate the effects of oxidative stress through oxidization of polyunsaturated fatty acids, leading to the production of aldehyde by-products such as 4-hydroxy-2-nonenal (HNE) and malondialdehyde (MDA) (Yin et al. 2015). Also, oxidative stress induces protein oxidation and lipid peroxidation promoting alterations in the mitochondrial genome. These mechanisms may eventually result in a deleterious cycle of mitochondrial damage and mitochondria-originating oxidative stress (Mantena et al. 2009).
Clinically, liver tissues from patients with NASH have high mitochondrial levels of ROS and ROS-mediated mitochondrial DNA (mtDNA) damage (Pessayre 2007). Similarly, hepatic tissues from obese (ob/ob) mice have been shown to have increased levels of lipogenesis from glucose (Kaplan and Leveille 1981, Begriche et al. 2010), an increased formation of mitochondrial ROS, higher levels of oxidative stress, enhanced lipid peroxidation, reduced levels of mitochondrial ETC components (Larosche et al. 2010), and decreased ATP levels (Chavin et al. 1999; Lin et al. 2000; Begriche et al. 2010). Compared with normal liver, fatty livers from ob/ob mice have increased levels of tumor necrosis factor (TNF) (Lin et al. 2000; Begriche et al. 2006) and FFAs, and a higher degree of proton leakage, which results in a decrease in ATP synthesis (Begriche et al. 2010).
Since patients with NAFLD have elevated mitochondrial fatty acid oxidation (FAO) and the TCA cycle, increased supply of reducing equivalents to the electron transport chain is sustained. This prolonged dysfunction in the respiratory complexes promotes the production of superoxide (Aharoni-Simon et al. 2011) (Fig. 1). Additionally, ROS driven mitochondria dysfunction has been reported to activate adenosine monophosphate–activated protein kinase (AMPK) and c-Jun N-terminal kinase (JNK), which are known to be mitogen-activated protein kinases (MAPKs) (Meakin et al. 2014; Herzig and Shaw 2018; Win et al. 2018). Activation of these signaling pathways plays a critical role in the development of liver diseases and injuries, such as steatosis, NASH, fibrosis, and HCC (Tilg and Moschen 2010; Lewis and Mohanty 2010; Quinlan et al. 2013).
Effects of oxidative stress and ROS on mitochondria. In the fatty liver of patients with NAFLD, mitochondria are exposed to prolonged oxidative stress and increased fatty acid oxidation and flux through the TCA cycle. This eventually leads to dysfunction in the ETC and the increased production of ROS. Increased production of ROS has a deleterious effect on mitochondria by causing the conversion of superoxide to hydrogen peroxide, which induces a stress signaling response and mitochondrial damage. Also, long exposure to oxidative stress impairs DNA repair system of mitochondria. As a result, detrimental effects, such as malfunction in nuclear DNA, a depletion of mtDNA, and increased levels of 8-hydroxy-2′-deoxyguanosine. In addition to these deleterious effects in mitochondria, increased ROS production also activate the AMPK, JNK, and MAPK pathways to cause serious injury in hepatocytes
While mitochondria have been known to be the major site of ROS production in the cell, complex I and III are considered to be major sites of superoxide production (Tell et al. 2013; Kotiadis et al. 2014). More recent studies have demonstrated that other mitochondrial enzymes are also associated with a decline in mitochondrial homeostasis. Both glycerol 3-phosphate dehydrogenase and 2-oxoglutarate dehydrogenase have been suggested to be involved in maintaining mitochondrial redox potent (Quinlan et al. 2013). An enzyme called superoxide dismutase converts superoxide into hydrogen peroxide which can then cause mitochondrial damage and/or initiate stress signaling responses.
As mitochondrial enzyme contributes to destruction of mitochondrial homeostasis, cardiolipin, a unique phospholipid found in the inner mitochondrial membrane, is highly sensitive to oxidative stress, resulting in mitochondrial dysfunction that includes the loss of ETC complex activity and the induction of mitochondrial permeability transition (MPT) pore opening (Li et al. 2010). Furthermore, cytochrome c, which is released from cardiolipin into the cytosol can activate the caspase-mediated apoptotic pathway leading to subsequent cell death (Kagan et al. 2005). Aside from inner mitochondria membrane, elevated ROS production have been suggested to be linked with mitochondrial outer membrane permeabilization (MOMP), altered mitochondrial membrane potential (ΔΨm), and a loss of mitochondrial integrity in NAFLD (Rector et al. 2010).
Along with mitochondria membrane’s permeability destruction, accumulating evidence suggests that oxidative stress can cause alterations in mtDNA. MtDNA is particularly susceptible to oxidative damage due to the fact that it is immediately adjacent to the site of ROS production and DNA repair systems. NAFLD is characterized by both mtDNA depletion and increased levels of 8-hydroxy-2′-deoxyguanosine (8-OHdG), an oxidized form deoxyguanosine. In addition, oxidative damage of nuclear DNA may also ultimately lead to mitochondrial dysfunction by compromising the transcription of nuclear-encoded mitochondrial genes. For example, the expression levels of critical factors involved in mitochondrial metabolism and biogenesis such as TFAM, NRF-2, and PGC-1α, have been reported to be decreased in NAFLD (Aharoni-Simon et al. 2011). Thus, the mechanisms for mitochondrial alterations and inadequate adaptation have strong connection with changes in mitochondrial ROS formation and subsequent ROS signaling, which ultimately cause dysfunctions in mitochondrial biogenesis and mitophagy (Table 1). These alterations also regulate changes in mitochondrial levels of GSH, FFAs, and lipid peroxidation products.
Mitochondrial quality control: mitophagy in NAFLD
Changes in morphology of mitochondria is very critical for long-term viability of cell. These cellular events, which are also known as the mitochondrial fission and fusion, is controlled by different molecules (e.g., MFN2, OPA1, and DRP1) (Table 2). Along with mitochondrial morphology control, mitophagy is the selective degradation of damaged mitochondria through autophagy. Mitophagy has recently suggested to play a key role in NAFLD. In the pathogenies of NAFLD, ALCAT1, a lysocardiolipin acyltransferase that catalyzes the pathological remodeling of cardiolipin, can cause mitochondrial dysfunction, including inhibition of mitophagy and OXPHOS (Wang et al. 2015). Recently, p62 has been found to promote mitochondrial ubiquitination and mitophagy through the recruitment of two subunits of a cullin-RING ubiquitin E3 ligase complex, Keap1 and Rbx1, protecting against the development of NAFLD (Yamada et al. 2018). AMPK is important in preventing hepatic lipid accumulation and inflammation (Day et al. 2017). AMPK can induce mitophagy through a dual mechanism involving both the activation of ULK1 and suppression of the mammalian target of rapamycin (mTOR) complex (Egan et al. 2011). Given that mitochondrial fission is required for the induction of mitophagy (Tanaka et al. 2010), it is intriguing that AMPK-mediated fission is induced by the mitochondrial fission factor, a mitochondrial outer-membrane receptor for DRP1 (Toyama et al. 2016).
The role of mitochondria in the development of simple steatosis
High-caloric fat diets can cause the dysregulation of lipid and glucose metabolism, resulting in the accumulation of triglycerides (TGs) and free fatty acids (FFAs) in the liver (Eccleston et al. 2011). Under these circumstances, hepatic metabolism is shifted to allow recovery from the hepatic lipid burden. This shift includes increased FAO, followed by induction of the tricarboxylic acid (TCA) cycle and enhanced oxidative phosphorylation (OXPHOS) (Sunny et al. 2011). AMPK activates catabolic pathway such as fatty acid and glucose oxidation pathways by inducing the activation of PGC-1〈 (Meakin et al. 2014; Herzig and Shaw 2018). PGC-1〈 interacts with peroxisome proliferator–activated receptor 〈 (PPAR〈) to induce the expression of several enzymes involved in fatty acid-metabolism, including carnitine palmitoyltransferase-1 (CPT-1) and acyl-CoA dehydrogenases, ultimately promoting mitochondrial fatty acid ®-oxidation (Fromenty and Pessayre 1995; Pessayre et al. 2012). CPT-1 catalyzes the import of FFAs into the mitochondria. CPT-1 is inhibited by malonyl-CoA (Pessayre et al. 2001; Gusdon et al. 2014) which is formed by acetyl-CoA carboxylase during the initial step in the synthesis of FFAs from acetyl-CoA (Gusdon et al. 2014; Meakin et al. 2014). Excess levels of carbohydrates result in the increased production of acetyl-CoA, subsequently leading to the inhibition of CPT-1 and ultimately and inhibition of ®-oxidation (McGarry and Foster 1980; Fromenty and Pessayre 1995; Pessayre et al. 2002; Gusdon et al. 2014). Activation of CPT-1 can inhibit liver injury in patient with NAFLD as demonstrated by decreased serum levels of AST, ALT, bilirubin, and mtDNA (Lim et al. 2010). This entire process precisely regulates mitochondrial energy metabolism in the liver of healthy individuals, but not in the liver of patient with NAFLD (Larosche et al. 2007; Babbar and Sheikh 2013; Gusdon et al. 2014; Sunny et al. 2017).
Mitochondrial contribution to the transition from simple steatosis to NASH and NASF
Liver biopsies from patient diagnosed with NASH and obesity have shown ultrastructural damage to the mitochondria (Sanyal et al. 2001; Hinke et al. 2007). Even with exposure to oxidative stress and ROS, there is continuous mitochondrial adaptation on morphology (mitochondrial fission and fusion), dynamics of energy expenditure and gene expression, which are also known as mitochondrial ‘remodeling’ (Sunny et al. 2017). In NAFLD, increased FFAs and de novo lipogenesis, and accumulation of TGs induce adaptations of mitochondrial oxidative metabolism, for instance increased hepatic TCA cycle due to following failure of cellular functions: incomplete β-oxidation, impairment of ketogenesis, and decreased mitochondrial respiratory chain and ATP synthesis (Sunny et al. 2017). Despite the efforts of the liver to overcome lipid accumulation, the mitochondrial adaptative response is insufficient to protect against lipotoxicity due to the continuous and chronic deposition of FFAs. This phenomenon has been demonstrated in a choline-deficient NAFLD mouse model, which showed that there were higher levels of mitochondrial biogenesis and mitochondrial mass in fatty livers in the early stage of disease than in liver tissue from healthy animals (Babbar and Sheikh 2013; Mansouri et al. 2018). At later times, mitochondrial dysfunction manifest as alterations in the ETC complexes and membrane potential (Δψm), and decreased ATP synthesis (Teodoro et al. 2008). Consequently, the capacity of mitochondria to overcome the increased import of FFAs is lost in the more advanced stages of NAFLD. Moreover, in later stages, disease progression is accelerated by inhibition of CPT-1, impaired mitochondrial FAO, and chronic ATP depletion caused by the increased hepatic expression of UCP2 (Serviddio et al. 2008). These findings suggest that the ability of mitochondria to adapt to increased FFAs seen in the early stages of NAFLD development (simple steatosis) decreases as NAFLD progresses to NASH.
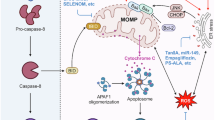
All NASH etiologies are linked with the increased formation of mitochondrial ROS (Tell et al. 2013). Generally, oxidative stress and lipid peroxidation have been reported to activate NF-κB to induce the production of pro-inflammatory cytokines (TNF-α, IL-1β, Il-6 and IL-8), which cause apoptosis and necrosis in hepatocytes (Pessayre et al. 2001, 2002; Carter-Kent et al. 2008; Tell et al. 2013; Rodrigues et al. 2017). This mitochondria-associated vicious cycles may include lipid accumulation, lipid peroxidation, ROS formation, depletion of antioxidants, altered mitochondrial quality control, and mitochondrial damage-induced inflammation (Larosche et al. 2007; Pessayre 2007; Zhang et al. 2010; Begriche et al. 2011; Marques et al. 2012; Tell et al. 2013; Feillet-Coudray et al. 2014; Marques et al. 2015). Damaged mitochondria and the subsequent necrosis of hepatocytes produce mitochondria-derived danger associated molecular patterns (DAMPs) (Fig. 2). Mitochondria possess bacteria-like characteristics, including the presence of hypomethylated CpG motifs in the mitochondrial genome, formylpeptides, and other danger signals. These mitochondrial DAMPs activate NOD like receptor family pyrin domain contain 3 (NLRP3) inflammasome and other innate immune system via pattern recognition receptors such as TLRs (Murgia et al. 2009; Murphy 2009; Elsheikh et al. 2010; Marques et al. 2015). For example, once released from damaged hepatocytes in HFD-fed mice, mtDNA has been shown to interacts with the TLR9 on Kupffer cells and hepatic stellate cells to stimulate the innate immune and fibrogenic responses, as has been suggested to occur in the pathogenesis of NASH (Begriche et al. 2006; Murgia et al. 2009; Murphy 2009; Garcia-Martinez et al. 2016). Finally, ROS-associated lipid peroxidation, mitochondrial DAMPs, and subsequent activation of caspases establish chronic liver injury by the infiltration of inflammatory cells (Pessayre et al. 2002; Murgia et al. 2009; Tell et al. 2013; Handa et al. 2014).
Mitochondria-mediated mechanisms of steatohepatitis and fibrosis in the pathogenesis of NAFLD. The increased accumulation of damaged/dysfunctional mitochondria within hepatocyte results in cell necrosis and induces the leakage of mitochondrial DAMPs, such as mtDNA, N-formyl peptides, and ATP. These signals subsequently trigger the activation of Toll-like receptor 9 (TLR9) and formyl peptide receptor 1 (FPR1), which in turn activates the IRFs and NF-kB, and thereby the production of inflammatory cytokines. mtDNA and ATP also activate the inflammasomes NLRP3 and AIM2, respectively. Multiple inflammatory cytokines and the activation of inflammasomes provide a chronic inflammatory milieu, which contributes the development of steatohepatitis and fibrosis
The levels of microRNA miR-21 have been reported to be increased in the liver of human patients and mice with NASH, and in whom caspase-2 is activated (Rodrigues et al. 2017). The mTOR/NF-κB pathway-mediated miR-21 activation inhibits PPAR-α and promotes mitochondrial dysfunction and hepatocyte injury. In addition, miR-26a, miR-33a and miR-141-3p have been found to regulate mitochondrial function in the pathogenesis of NAFLD. miR-26a overexpression protects against hepatocyte apoptosis and regulates fatty acid and cholesterol homeostasis (Ali et al. 2018). miR-33a specifically inhibits mitochondrial complex I activity and its knockdown protected HFD-induced mitochondrial dysfunction (Nie et al. 2018). miR-141-3p is dramatically up-regulated in HFD-fed mice and could promotes mitochondrial dysfunction by inhibition of phosphatase and tensin homolog (PTEN) (Ji et al. 2015). Cell death caused opening of the MPT pore which seems to be a critical event in hepatocyte cell death (Chavin et al. 1999).
Moreover, ER stress plays critical factors in the pathogenesis of NAFLD as aggregation of unfolded protein response (UPR) causing ER stress is required for hepatic lipid metabolism as protective stress response (Henkel and Green 2013). Comparing levels of ER stress related transcriptions factors such as activating transcription factor 6 (ATF6), X-box–binding protein 1 (XBP1 s) and C/EBP homologous protein (CHOP), liver tissue from patients with NAFLD and NASH have higher expression compared to normal individuals’ liver tissue (Lee et al. 2017a, b). ER chaperons (e.g., GRP78 and GRP94), which takes huge role in mediating protein misfolding were downregulated in liver tissue from patient diagnosed with NAFLD and NASH compared to normal liver tissue (Lee et al. 2017a, b). Mitochondrial dysfunction in NASH also decreases ATP synthesis, which may cause endoplasmic reticulum (ER) stress and activation of the UPR. The UPR is linked to the activation of de novo lipogenesis and further enhances the development of steatosis (Lee et al. 2017a, b). Recent studies have demonstrated that prolonged ER stress, or chronic activation of the UPR, also induces hepatocyte injury and inflammation through a CHOP-dependent signaling pathway (Willy et al. 2015). Moreover, the mis folding of apoB protein, a major component of very-low density lipoprotein (VLDL), impairs lipid export from the liver and exacerbates steatosis in mice (Uchiyama et al. 2006).
Increased mitochondrial cholesterol accumulation is also associated with the transition from steatosis to NASH. In patient with NASH, the cholesterol content has been shown to be negatively correlated with mitochondrial GSH (mtGSH) levels (Gan et al. 2014). Decreased mt GSH levels may arise as a result of damage to the mtGSH transport system, which transports GSH from the cytosol to mitochondria as well as causes alterations in their membrane permeability upon induction by high cholesterol level. High levels of cholesterol have also been found to sensitize hepatocytes to TNF- and Fas-induced apoptosis and to cause mitochondrial GSH depletion in ob/ob mice (Mari et al. 2006).
Clinical research findings involving mitochondria-mediating drugs
Clinical trials have been conducted to evaluate the efficacy of several drug candidates in patient with NAFLD. Metformin and pioglitazone are generally recommended for treatment in select NAFLD patients (Lazaridis and Tsochatzis 2017). However, no other drugs have been approved for the treatment of NAFLD by the Food and Drug Administration (FDA) in the USA. The results of various therapeutic approaches using pioglitazone and metformin have recently been summarized (Le and Loomba 2012). Metformin has been suggested to inhibit mitochondrial complex activity and subsequently activate AMPK (Hinke et al. 2007). Metformin was found to be efficient in decreasing the serum levels of ALT in patient with NAFLD who were not diabetic (Le and Loomba 2012). Similarly, pioglitazone, a peroxisome proliferator-activated receptor gamma agonist, was effective in ameliorating NASH in non-diabetic patients with NAFLD (Promrat et al. 2004). However, in addition to these beneficial effects, pioglitazone has side effects, such as osteopenia, fluid retention, and weight gain (Issa et al. 2017). Thus, it is generally recommended to lose weight through a modification of lifestyle and diet (Mishra and Younossi 2007).
Therapeutic approaches using antioxidants have also been implemented to protect against oxidative stress-induced liver injury. Among them, vitamin E has been widely used to treat patients with NAFLD (Ji et al. 2014). Indeed, vitamin E can effectively alleviate the liver injury and histological features in patients with NASH (Sato et al. 2015). However, the beneficial effect of vitamin E in normalizing serum ALT level was not found in a meta-analysis. Additionally, vitamin E therapy was not found to be effective for the child patients with NAFLD (Lavine et al. 2011). Therefore, further studies aimed at examining long-term tolerance and efficacy of vitamin E in specific subsets of patients are required, for example patient with NASH or diabetes-associated cirrhosis (Musso et al. 2013). Accumulating evidence has also suggested that plant-derived antioxidants including resveratrol, epigallo-catechin gallate, curcumin, coumestrol, silybin, anthocyanidins, and allyl-isothiocyanate can all improve the severity of NAFLD by increasing mitochondrial function. However, the selective permeability of the inner mitochondrial membrane (IMM) remains the major obstacle to mitochondria-targeted treatments. Several drugs have been used to deliver antioxidants to mitochondria. For instance, MitoQ, a lipophilic triphenylphosphonium (TPP) cation, can induce negatively charged hydrophobic IMM. Therefore, MitoQ may remain and act as an effective antioxidants with the IMM effectively (Smith et al. 2003; Asin-Cayuela et al. 2004; Rokitskaya et al. 2008). Indeed, MitoQ treatment has been shown to effectively improve cardiolipin-mediated mitochondrial integrity and metabolic syndrome features, and to induce antiapoptotic effects through the inhibition of caspase-3 activation and cytochrome c release (Dhanasekaran et al. 2004; Feillet-Coudray et al. 2014; Fouret et al. 2015). Similarly, a mitochondria-targeted vitamin E has been shown to be an effective agent in preventing peroxide-mediated transferrin-iron transport to mitochondria and eventually protecting against apoptosis (Dhanasekaran et al. 2005). Unfortunately, very few clinical trials have been conducted using these agents (Table 3).
Encouragingly, several phase 3 clinical trials of other mitochondria-related drugs have been initiated to assess their therapeutic efficacy in patient with NAFLD (Konerman et al. 2018; Cai et al. 2019) (Fig. 3). Elafibranor is a dual peroxisome proliferation-activated receptor (PPAR)-α/δ agonist and has multiple protective effects in the pathogenesis of NAFLD (Staels et al. 2013). As mentioned previously, PPAR-α has been shown in several studies in humans and animals to attenuate triglyceride and FFAs accumulation, and the resulting hepatic inflammation (Tailleux et al. 2012; Staels et al. 2013). PPAR-δ can also decreases fatty acid uptake and regulate energy metabolism, including glucose and hepatic inflammation. Furthermore, PPAR-δ can improve insulin sensitivity by regulating lipid metabolism in adipose tissue (Riserus et al. 2008; Poulsen et al. 2012). Currently, one phase III trial of elafibranor is being conducted to evaluate the efficacy and safety in patients with NASH (Konerman et al. 2018). Apoptosis signal-regulating kinase 1 (ASK1) has been demonstrated to be a major player in the pathogenesis of oxidative stress-mediated hepatic injury, inflammation, and fibrosis. Its inhibitor, selonsertib, is also being explored in patient with NASH. There are currently two phase III trials of selonsertib, investigating its efficacy in patients with compensated cirrhosis and bridging fibrosis (F3) due to NASH (Konerman et al. 2018).
Mechanism of action of therapeutic targets for the treatment of NAFLD. As shown in previous figures, mitochondrial dysfunction has strong correlation with NAFLD. Along with many efforts to develop treatment for NAFLD, several mitochondria-targeting drugs are on the clinical trial. Elafibranor, dual peroxisome proliferation-activated receptor (PPAR)-α/δ agonist, is one of the drugs. As it is well known for PPAR-α agonist, it would attenuate hepatic inflammation associated with lipid metabolism and mitochondrial dysfunction. Next, selonsertib is an apoptosis signal-regulating kinase 1 (ASK1) inhibitor. This drug would inhibit mitochondrial malfunction in regards of apoptotic signaling from hepatocyte, which would activate kupffer cell. Aside from those two drugs, cenicriviroc is an antifibrogenic agent that target dual inflammatory receptor CCR2/CCR5. This drug would ameliorate progression of steatohepatitis to fibrogenesis targeting hepatic stellate cell. ASK Apoptosis signal-regulating kinase, CB1 cannabinoid receptor type, CCR C–C chemokine receptor, ER endoplasmic reticulum, FGF fibroblast growth factor, FFA free fatty acids, FXR farnesoid X receptor, GLP-1 glucagon-like peptide-1, PPAR peroxisome proliferator-activated receptor, ROS reactive oxygen species, SHP small heterodimer partner, SREBP sterol regulatory element binding protein
Conclusion
The pathogenesis of NAFLD involves metabolic dysregulation, inflammation and fibrosis. Numerous clinical trials have been conducted targeting each of these components. Currently, it is well accepted that a combination therapeutic strategy should be adopted with a backbone treatment and a complementary treatment for patients with NAFLD. Backbone treatment generally includes metabolic modulation, while complementary treatment targets the modulation of inflammation or fibrosis. Of note, mitochondrial processes are involved in both modulations in therapeutic targets for NAFLD. Mitochondria regulate ROS formation, oxidative stress, and lipid metabolism in the early stage of simple steatosis. Over time, the increased injury to hepatocytes produce various DAMPs, which in turn promote steatohepatitis and fibrosis. Thus, maintaining mitochondrial integrity by mitophagy may be a key factor for protecting against treating NAFLD. Furthermore, preclinical and clinical research addressing therapeutic approaches that target mitochondrial process will be required in the near future.
References
Aharoni-Simon M, Hann-Obercyger M, Pen S, Madar Z, Tirosh O (2011) Fatty liver is associated with impaired activity of PPARgamma-coactivator 1alpha (PGC1alpha) and mitochondrial biogenesis in mice. Lab Invest 91:1018–1028
Ali O, Darwish HA, Eldeib KM, Abdel Azim SA (2018) miR-26a potentially contributes to the regulation of fatty acid and sterol metabolism in vitro human HepG2 cell model of nonalcoholic fatty liver disease. Oxid Med Cell Longev 2018:8515343
Asin-Cayuela J, Manas AR, James AM, Smith RA, Murphy MP (2004) Fine-tuning the hydrophobicity of a mitochondria-targeted antioxidant. FEBS Lett 571:9–16
Babbar M, Sheikh MS (2013) Metabolic stress and disorders related to alterations in mitochondrial fission or fusion. Mol Cell Pharmacol 5:109–133
Begriche K, Igoudjil A, Pessayre D, Fromenty B (2006) Mitochondrial dysfunction in NASH: causes, consequences and possible means to prevent it. Mitochondrion 6:1–28
Begriche K, Massart J, Fromenty B (2010) Effects of beta-aminoisobutyric acid on leptin production and lipid homeostasis: mechanisms and possible relevance for the prevention of obesity. Fundam Clin Pharmacol 24:269–282
Begriche K, Massart J, Robin MA, Borgne-Sanchez A, Fromenty B (2011) Drug-induced toxicity on mitochondria and lipid metabolism: mechanistic diversity and deleterious consequences for the liver. J Hepatol 54:773–794
Berlanga A, Guiu-Jurado E, Porras JA, Auguet T (2014) Molecular pathways in non-alcoholic fatty liver disease. Clin Exp Gastroenterol 7:221–239
Cai J, Zhang XJ, Li H (2019) Progress and challenges in the prevention and control of nonalcoholic fatty liver disease. Med Res Rev 39:328–348
Carter-Kent C, Zein NN, Feldstein AE (2008) Cytokines in the pathogenesis of fatty liver and disease progression to steatohepatitis: implications for treatment. Am J Gastroenterol 103:1036–1042
Chavin KD, Yang S, Lin HZ, Chatham J, Chacko VP, Hoek JB, Walajtys-Rode E, Rashid A, Chen CH, Huang CC, Wu TC, Lane MD, Diehl AM (1999) Obesity induces expression of uncoupling protein-2 in hepatocytes and promotes liver ATP depletion. J Biol Chem 274:5692–5700
Childress ES, Alexopoulos SJ, Hoehn KL, Santos WL (2018) Small molecule mitochondrial uncouplers and their therapeutic potential. J Med Chem 61:4641–4655
Day CP, James OF (1998) Steatohepatitis: a tale of two “hits”? Gastroenterology 114:842–845
Day EA, Ford RJ, Steinberg GR (2017) AMPK as a therapeutic target for treating metabolic diseases. Trends Endocrinol Metab 28:545–560
Dhanasekaran A, Kotamraju S, Kalivendi SV, Matsunaga T, Shang T, Keszler A, Joseph J, Kalyanaraman B (2004) Supplementation of endothelial cells with mitochondria-targeted antioxidants inhibit peroxide-induced mitochondrial iron uptake, oxidative damage, and apoptosis. J Biol Chem 279:37575–37587
Dhanasekaran A, Kotamraju S, Karunakaran C, Kalivendi SV, Thomas S, Joseph J, Kalyanaraman B (2005) Mitochondria superoxide dismutase mimetic inhibits peroxide-induced oxidative damage and apoptosis: role of mitochondrial superoxide. Free Radic Biol Med 39:567–583
Ding WX, Guo F, Ni HM, Bockus A, Manley S, Stolz DB, Eskelinen EL, Jaeschke H, Yin XM (2012) Parkin and mitofusins reciprocally regulate mitophagy and mitochondrial spheroid formation. J Biol Chem 287:42379–42388
Eccleston HB, Andringa KK, Betancourt AM, King AL, Mantena SK, Swain TM, Tinsley HN, Nolte RN, Nagy TR, Abrams GA, Bailey SM (2011) Chronic exposure to a high-fat diet induces hepatic steatosis, impairs nitric oxide bioavailability, and modifies the mitochondrial proteome in mice. Antioxid Redox Signal 15:447–459
Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, Vasquez DS, Joshi A, Gwinn DM, Taylor R, Asara JM, Fitzpatrick J, Dillin A, Viollet B, Kundu M, Hansen M, Shaw RJ (2011) Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science 331:456–461
Elsheikh A, Lavergne SN, Castrejon JL, Farrell J, Wang H, Sathish J, Pichler WJ, Park BK, Naisbitt DJ (2010) Drug antigenicity, immunogenicity, and costimulatory signaling: evidence for formation of a functional antigen through immune cell metabolism. J Immunol 185:6448–6460
Feillet-Coudray C, Fouret G, Ebabe Elle R, Rieusset J, Bonafos B, Chabi B, Crouzier D, Zarkovic K, Zarkovic N, Ramos J, Badia E, Murphy MP, Cristol JP, Coudray C (2014) The mitochondrial-targeted antioxidant MitoQ ameliorates metabolic syndrome features in obesogenic diet-fed rats better than Apocynin or Allopurinol. Free Radic Res 48:1232–1246
Filadi R, Pendin D, Pizzo P (2018) Mitofusin 2: from functions to disease. Cell Death Dis 9:330
Fouret G, Tolika E, Lecomte J, Bonafos B, Aoun M, Murphy MP, Ferreri C, Chatgilialoglu C, Dubreucq E, Coudray C, Feillet-Coudray C (2015) The mitochondrial-targeted antioxidant, MitoQ, increases liver mitochondrial cardiolipin content in obesogenic diet-fed rats. Biochim Biophys Acta 1847:1025–1035
Fromenty B, Pessayre D (1995) Inhibition of mitochondrial beta-oxidation as a mechanism of hepatotoxicity. Pharmacol Ther 67:101–154
Galloway CA, Lee H, Brookes PS, Yoon Y (2014) Decreasing mitochondrial fission alleviates hepatic steatosis in a murine model of nonalcoholic fatty liver disease. Am J Physiol Gastrointest Liver Physiol 307:G632–641
Gan LT, Van Rooyen DM, Koina ME, McCuskey RS, Teoh NC, Farrell GC (2014) Hepatocyte free cholesterol lipotoxicity results from JNK1-mediated mitochondrial injury and is HMGB1 and TLR4-dependent. J Hepatol 61:1376–1384
Garcia-Martinez I, Santoro N, Chen Y, Hoque R, Ouyang X, Caprio S, Shlomchik MJ, Coffman RL, Candia A, Mehal WZ (2016) Hepatocyte mitochondrial DNA drives nonalcoholic steatohepatitis by activation of TLR9. J Clin Invest 126:859–864
Garcia-Ruiz C, Baulies A, Mari M, Garcia-Roves PM, Fernandez-Checa JC (2013) Mitochondrial dysfunction in non-alcoholic fatty liver disease and insulin resistance: cause or consequence? Free Radic Res 47:854–868
Gusdon AM, Song KX, Qu S (2014) Nonalcoholic fatty liver disease: pathogenesis and therapeutics from a mitochondria-centric perspective. Oxid Med Cell Longev 2014:637027
Handa P, Maliken BD, Nelson JE, Morgan-Stevenson V, Messner DJ, Dhillon BK, Klintworth HM, Beauchamp M, Yeh MM, Elfers CT, Roth CL, Kowdley KV (2014) Reduced adiponectin signaling due to weight gain results in nonalcoholic steatohepatitis through impaired mitochondrial biogenesis. Hepatology 60:133–145
Henkel A, Green RM (2013) The unfolded protein response in fatty liver disease. Semin Liver Dis 33:321–329
Herzig S, Shaw RJ (2018) AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol 19:121–135
Hinke SA, Martens GA, Cai Y, Finsi J, Heimberg H, Pipeleers D, Van de Casteele M (2007) Methyl succinate antagonises biguanide-induced AMPK-activation and death of pancreatic beta-cells through restoration of mitochondrial electron transfer. Br J Pharmacol 150:1031–1043
Issa D, Wattacheril J, Sanyal AJ (2017) Treatment options for nonalcoholic steatohepatitis—a safety evaluation. Expert Opin Drug Saf 16:903–913
Ji HF, Sun Y, Shen L (2014) Effect of vitamin E supplementation on aminotransferase levels in patients with NAFLD, NASH, and CHC: results from a meta-analysis. Nutrition 30:986–991
Ji J, Qin Y, Ren J, Lu C, Wang R, Dai X, Zhou R, Huang Z, Xu M, Chen M, Wu W, Song L, Shen H, Hu Z, Miao D, Xia Y, Wang X (2015) Mitochondria-related miR-141-3p contributes to mitochondrial dysfunction in HFD-induced obesity by inhibiting PTEN. Sci Rep 5:16262
Kagan VE, Tyurin VA, Jiang J, Tyurina YY, Ritov VB, Amoscato AA, Osipov AN, Belikova NA, Kapralov AA, Kini V, Vlasova, II, Zhao Q, Zou M, Di P, Svistunenko DA, Kurnikov IV, Borisenko GG (2005) Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat Chem Biol 1:223–232
Kaplan ML, Leveille GA (1981) Development of lipogenesis and insulin sensitivity in tissues of the ob/ob mouse. Am J Physiol 240:E101–107
Kelly DP, Scarpulla RC (2004) Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev 18:357–368
Konerman MA, Jones JC, Harrison SA (2018) Pharmacotherapy for NASH: current and emerging. J Hepatol 68:362–375
Kotiadis VN, Duchen MR, Osellame LD (2014) Mitochondrial quality control and communications with the nucleus are important in maintaining mitochondrial function and cell health. Biochim Biophys Acta 1840:1254–1265
Land WG (2015) The Role of Damage-Associated Molecular Patterns (DAMPs) in Human Diseases: Part II: DAMPs as diagnostics, prognostics and therapeutics in clinical medicine. Sultan Qaboos Univ Med J 15:e157–170
Larosche I, Letteron P, Fromenty B, Vadrot N, Abbey-Toby A, Feldmann G, Pessayre D, Mansouri A (2007) Tamoxifen inhibits topoisomerases, depletes mitochondrial DNA, and triggers steatosis in mouse liver. J Pharmacol Exp Ther 321:526–535
Larosche I, Letteron P, Berson A, Fromenty B, Huang TT, Moreau R, Pessayre D, Mansouri A (2010) Hepatic mitochondrial DNA depletion after an alcohol binge in mice: probable role of peroxynitrite and modulation by manganese superoxide dismutase. J Pharmacol Exp Ther 332:886–897
Lavine JE, Schwimmer JB, Van Natta ML, Molleston JP, Murray KF, Rosenthal P, Abrams SH, Scheimann AO, Sanyal AJ, Chalasani N, Tonascia J, Unalp A, Clark JM, Brunt EM, Kleiner DE, Hoofnagle JH, Robuck PR (2011) Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA 305:1659–1668
Lazaridis N, Tsochatzis E (2017) Current and future treatment options in non-alcoholic steatohepatitis (NASH). Expert Rev Gastroenterol Hepatol 11:357–369
Le TA, Loomba R (2012) Management of non-alcoholic fatty liver disease and steatohepatitis. J Clin Exp Hepatol 2:156–173
Lee J, Homma T, Fujii J (2017a) Mice in the early stage of liver steatosis caused by a high fat diet are resistant to thioacetamide-induced hepatotoxicity and oxidative stress. Toxicol Lett 277:92–103
Lee S, Kim S, Hwang S, Cherrington NJ, Ryu DY (2017b) Dysregulated expression of proteins associated with ER stress, autophagy and apoptosis in tissues from nonalcoholic fatty liver disease. Oncotarget 8:63370–63381
Lewis JR, Mohanty SR (2010) Nonalcoholic fatty liver disease: a review and update. Dig Dis Sci 55:560–578
Li J, Romestaing C, Han X, Li Y, Hao X, Wu Y, Sun C, Liu X, Jefferson LS, Xiong J, Lanoue KF, Chang Z, Lynch CJ, Wang H, Shi Y (2010) Cardiolipin remodeling by ALCAT1 links oxidative stress and mitochondrial dysfunction to obesity. Cell Metab 12:154–165
Lim CY, Jun DW, Jang SS, Cho WK, Chae JD, Jun JH (2010) Effects of carnitine on peripheral blood mitochondrial DNA copy number and liver function in non-alcoholic fatty liver disease. Korean J Gastroenterol 55:384–389
Lin HZ, Yang SQ, Chuckaree C, Kuhajda F, Ronnet G, Diehl AM (2000) Metformin reverses fatty liver disease in obese, leptin-deficient mice. Nat Med 6:998–1003
Liu H, Dai C, Fan Y, Guo B, Ren K, Sun T, Wang W (2017) From autophagy to mitophagy: the roles of P62 in neurodegenerative diseases. J Bioenerg Biomembr 49:413–422
Loomba R, Sanyal AJ (2013) The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol 10:686–690
Mansouri A, Gattolliat CH, Asselah T (2018) Mitochondrial dysfunction and signaling in chronic liver diseases. Gastroenterology 155:629–647
Mantena SK, Vaughn DP, Andringa KK, Eccleston HB, King AL, Abrams GA, Doeller JE, Kraus DW, Darley-Usmar VM, Bailey SM (2009) High fat diet induces dysregulation of hepatic oxygen gradients and mitochondrial function in vivo. Biochem J 417:183–193
Mari M, Caballero F, Colell A, Morales A, Caballeria J, Fernandez A, Enrich C, Fernandez-Checa JC, Garcia-Ruiz C (2006) Mitochondrial free cholesterol loading sensitizes to TNF- and Fas-mediated steatohepatitis. Cell Metab 4:185–198
Marques PE, Amaral SS, Pires DA, Nogueira LL, Soriani FM, Lima BH, Lopes GA, Russo RC, Avila TV, Melgaco JG, Oliveira AG, Pinto MA, Lima CX, De Paula AM, Cara DC, Leite MF, Teixeira MM, Menezes GB (2012) Chemokines and mitochondrial products activate neutrophils to amplify organ injury during mouse acute liver failure. Hepatology 56:1971–1982
Marques PE, Oliveira AG, Pereira RV, David BA, Gomides LF, Saraiva AM, Pires DA, Novaes JT, Patricio DO, Cisalpino D, Menezes-Garcia Z, Leevy WM, Chapman SE, Mahecha G, Marques RE, Guabiraba R, Martins VP, Souza DG, Mansur DS, Teixeira MM, Leite MF, Menezes GB (2015) Hepatic DNA deposition drives drug-induced liver injury and inflammation in mice. Hepatology 61:348–360
McGarry JD, Foster DW (1980) Regulation of hepatic fatty acid oxidation and ketone body production. Annu Rev Biochem 49:395–420
Meakin PJ, Chowdhry S, Sharma RS, Ashford FB, Walsh SV, McCrimmon RJ, Dinkova-Kostova AT, Dillon JF, Hayes JD, Ashford ML (2014) Susceptibility of Nrf2-null mice to steatohepatitis and cirrhosis upon consumption of a high-fat diet is associated with oxidative stress, perturbation of the unfolded protein response, and disturbance in the expression of metabolic enzymes but not with insulin resistance. Mol Cell Biol 34:3305–3320
Melser S, Lavie J, Benard G (2015) Mitochondrial degradation and energy metabolism. Biochim Biophys Acta 1853:2812–2821
Milic S, Stimac D (2012) Nonalcoholic fatty liver disease/steatohepatitis: epidemiology, pathogenesis, clinical presentation and treatment. Dig Dis 30:158–162
Mishra P, Younossi ZM (2007) Current treatment strategies for non-alcoholic fatty liver disease (NAFLD). Curr Drug Discov Technol 4:133–140
Murgia M, Giorgi C, Pinton P, Rizzuto R (2009) Controlling metabolism and cell death: at the heart of mitochondrial calcium signalling. J Mol Cell Cardiol 46:781–788
Murphy MP (2009) How mitochondria produce reactive oxygen species. Biochem J 417:1–13
Musso G, Anty R, Petta S (2013) Antioxidant therapy and drugs interfering with lipid metabolism: could they be effective in NAFLD patients? Curr Pharm Des 19:5297–5313
Nassir F, Ibdah JA (2014) Role of mitochondria in nonalcoholic fatty liver disease. Int J Mol Sci 15:8713–8742
Nie H, Yu X, He H, Zhou L, Li Q, Song C, Wang D, Ren T, Chen Z, Huang H, Dai X, Zhou Y (2018) Hepatocyte miR-33a mediates mitochondrial dysfunction and hepatosteatosis by suppressing NDUFA5. J Cell Mol Med 22:6285–6293
Perry RJ, Zhang D, Zhang XM, Boyer JL, Shulman GI (2015) Controlled-release mitochondrial protonophore reverses diabetes and steatohepatitis in rats. Science 347:1253–1256
Pessayre D (2007) Role of mitochondria in non-alcoholic fatty liver disease. J Gastroenterol Hepatol 22(Suppl 1):S20–27
Pessayre D, Berson A, Fromenty B, Mansouri A (2001) Mitochondria in steatohepatitis. Semin Liver Dis 21:57–69
Pessayre D, Mansouri A, Fromenty B (2002) Nonalcoholic steatosis and steatohepatitis. V. Mitochondrial dysfunction in steatohepatitis. Am J Physiol Gastrointest Liver Physiol 282:G193–199
Pessayre D, Fromenty B, Berson A, Robin MA, Letteron P, Moreau R, Mansouri A (2012) Central role of mitochondria in drug-induced liver injury. Drug Metab Rev 44:34–87
Poulsen L, Siersbaek M, Mandrup S (2012) PPARs: fatty acid sensors controlling metabolism. Semin Cell Dev Biol 23:631–639
Promrat K, Lutchman G, Uwaifo GI, Freedman RJ, Soza A, Heller T, Doo E, Ghany M, Premkumar A, Park Y, Liang TJ, Yanovski JA, Kleiner DE, Hoofnagle JH (2004) A pilot study of pioglitazone treatment for nonalcoholic steatohepatitis. Hepatology 39:188–196
Quinlan CL, Perevoshchikova IV, Hey-Mogensen M, Orr AL, Brand MD (2013) Sites of reactive oxygen species generation by mitochondria oxidizing different substrates. Redox Biol 1:304–312
Rector RS, Thyfault JP, Uptergrove GM, Morris EM, Naples SP, Borengasser SJ, Mikus CR, Laye MJ, Laughlin MH, Booth FW, Ibdah JA (2010) Mitochondrial dysfunction precedes insulin resistance and hepatic steatosis and contributes to the natural history of non-alcoholic fatty liver disease in an obese rodent model. J Hepatol 52:727–736
Riserus U, Sprecher D, Johnson T, Olson E, Hirschberg S, Liu A, Fang Z, Hegde P, Richards D, Sarov-Blat L, Strum JC, Basu S, Cheeseman J, Fielding BA, Humphreys SM, Danoff T, Moore NR, Murgatroyd P, O’Rahilly S, Sutton P, Willson T, Hassall D, Frayn KN, Karpe F (2008) Activation of peroxisome proliferator-activated receptor (PPAR)delta promotes reversal of multiple metabolic abnormalities, reduces oxidative stress, and increases fatty acid oxidation in moderately obese men. Diabetes 57:332–339
Rodrigues PM, Afonso MB, Simao AL, Carvalho CC, Trindade A, Duarte A, Borralho PM, Machado MV, Cortez-Pinto H, Rodrigues CM, Castro RE (2017) miR-21 ablation and obeticholic acid ameliorate nonalcoholic steatohepatitis in mice. Cell Death Dis 8:e2825
Rokitskaya TI, Klishin SS, Severina II, Skulachev VP, Antonenko YN (2008) Kinetic analysis of permeation of mitochondria-targeted antioxidants across bilayer lipid membranes. J Membr Biol 224:9–19
Rousset S, Alves-Guerra MC, Mozo J, Miroux B, Cassard-Doulcier AM, Bouillaud F, Ricquier D (2004) The biology of mitochondrial uncoupling proteins. Diabetes 53(Suppl 1):S130–S135
Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, Luketic VA, Shiffman ML, Clore JN (2001) Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology 120:1183–1192
Sato K, Gosho M, Yamamoto T, Kobayashi Y, Ishii N, Ohashi T, Nakade Y, Ito K, Fukuzawa Y, Yoneda M (2015) Vitamin E has a beneficial effect on nonalcoholic fatty liver disease: a meta-analysis of randomized controlled trials. Nutrition 31:923–930
Serviddio G, Bellanti F, Tamborra R, Rollo T, Capitanio N, Romano AD, Sastre J, Vendemiale G, Altomare E (2008) Uncoupling protein-2 (UCP2) induces mitochondrial proton leak and increases susceptibility of non-alcoholic steatohepatitis (NASH) liver to ischaemia-reperfusion injury. Gut 57:957–965
Smith RA, Porteous CM, Gane AM, Murphy MP (2003) Delivery of bioactive molecules to mitochondria in vivo. Proc Natl Acad Sci USA 100:5407–5412
Staels B, Rubenstrunk A, Noel B, Rigou G, Delataille P, Millatt LJ, Baron M, Lucas A, Tailleux A, Hum DW, Ratziu V, Cariou B, Hanf R (2013) Hepatoprotective effects of the dual peroxisome proliferator-activated receptor alpha/delta agonist, GFT505, in rodent models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology 58:1941–1952
Sunny NE, Parks EJ, Browning JD, Burgess SC (2011) Excessive hepatic mitochondrial TCA cycle and gluconeogenesis in humans with nonalcoholic fatty liver disease. Cell Metab 14:804–810
Sunny NE, Bril F, Cusi K (2017) Mitochondrial adaptation in nonalcoholic fatty liver disease: novel mechanisms and treatment strategies. Trends Endocrinol Metab 28:250–260
Tailleux A, Wouters K, Staels B (2012) Roles of PPARs in NAFLD: potential therapeutic targets. Biochim Biophys Acta 1821:809–818
Tanaka A, Cleland MM, Xu S, Narendra DP, Suen DF, Karbowski M, Youle RJ (2010) Proteasome and p97 mediate mitophagy and degradation of mitofusins induced by Parkin. J Cell Biol 191:1367–1380
Tell G, Vascotto C, Tiribelli C (2013) Alterations in the redox state and liver damage: hints from the EASL basic school of hepatology. J Hepatol 58:365–374
Teodoro JS, Rolo AP, Duarte FV, Simoes AM, Palmeira CM (2008) Differential alterations in mitochondrial function induced by a choline-deficient diet: understanding fatty liver disease progression. Mitochondrion 8:367–376
Tilg H, Moschen AR (2010) Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology 52:1836–1846
Toyama EQ, Herzig S, Courchet J, Lewis TL Jr, Loson OC, Hellberg K, Young NP, Chen H, Polleux F, Chan DC, Shaw RJ (2016) Metabolism. AMP-activated protein kinase mediates mitochondrial fission in response to energy stress. Science 351:275–281
Uchiyama S, Shimizu T, Shirasawa T (2006) CuZn-SOD deficiency causes ApoB degradation and induces hepatic lipid accumulation by impaired lipoprotein secretion in mice. J Biol Chem 281:31713–31719
Wang L, Liu X, Nie J, Zhang J, Kimball SR, Zhang H, Zhang WJ, Jefferson LS, Cheng Z, Ji Q, Shi Y (2015) ALCAT1 controls mitochondrial etiology of fatty liver diseases, linking defective mitophagy to steatosis. Hepatology 61:486–496
Willy JA, Young SK, Stevens JL, Masuoka HC, Wek RC (2015) CHOP links endoplasmic reticulum stress to NF-kappaB activation in the pathogenesis of nonalcoholic steatohepatitis. Mol Biol Cell 26:2190–2204
Win S, Than TA, Zhang J, Oo C, Min RWM, Kaplowitz N (2018) New insights into the role and mechanism of c-Jun-N-terminal kinase signaling in the pathobiology of liver diseases. Hepatology 67:2013–2024
Xiong W, Ma Z, An D, Liu Z, Cai W, Bai Y, Zhan Q, Lai W, Zeng Q, Ren H, Xu D (2019) Mitofusin 2 participates in mitophagy and mitochondrial fusion against angiotensin II-induced cardiomyocyte injury. Front Physiol 10:411
Yamada T, Murata D, Adachi Y, Itoh K, Kameoka S, Igarashi A, Kato T, Araki Y, Huganir RL, Dawson TM, Yanagawa T, Okamoto K, Iijima M, Sesaki H (2018) Mitochondrial stasis reveals p62-mediated ubiquitination in Parkin-independent mitophagy and mitigates nonalcoholic fatty liver disease. Cell Metab 28(588–604):e585
Yi HS (2019) Implications of mitochondrial unfolded protein response and mitokines: a perspective on fatty liver diseases. Endocrinol Metab (Seoul) 34:39–46
Yin X, Zheng F, Pan Q, Zhang S, Yu D, Xu Z, Li H (2015) Glucose fluctuation increased hepatocyte apoptosis under lipotoxicity and the involvement of mitochondrial permeability transition opening. J Mol Endocrinol 55:169–181
Younes R, Bugianesi E (2019) A spotlight on pathogenesis, interactions and novel therapeutic options in NAFLD. Nat Rev Gastroenterol Hepatol 16:80–82
Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ (2010) Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464:104–107
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) Grant by the Korea government (MEST; MRC, 2017R1A5A2015541, NRF-2017R1C1B2004423 and 2019R1A2C1090178).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lee, J., Park, JS. & Roh, Y.S. Molecular insights into the role of mitochondria in non-alcoholic fatty liver disease. Arch. Pharm. Res. 42, 935–946 (2019). https://doi.org/10.1007/s12272-019-01178-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-019-01178-1