Abstract
Reactive oxygen species (ROS) is associated with cancer progression in different cancers, including melanoma. It also affects specificity protein (Sp1), a transcription factor. Flavonoid morin is known to inhibit growth of cancer cells, including lung cancer and breast cancer. Herein, we hypothesized that morin can inhibit cancer activities in melanoma by altering ROS generation. The aim of this study is to determine the effects of morin and its underlying mechanisms in melanoma cells. Effects of morin on cell proliferation and apoptosis were determined using standardized assays. Changes in pro-apoptotic and anti-apoptotic proteins were analyzed by western blot analysis. Cellular ROS levels and mitochondrial function were evaluated by measuring DCF-DA fluorescence and rhodamine-123 fluorescence intensities, respectively. Morin induced ROS production and apoptosis, as presented by increased proportion of cells with Annexin V-PE(+) staining and sub-G0/G1 peak in cell cycle analysis. It also downregulated Sp1, Mcl-1, Bcl-2, and caspase-3 but upregulated cleaved caspase-3, Bax, and PUMA. In immunohistochemical staining, Sp1 was overexpressed in melanoma tissues compared to normal skin tissues. Collectively, our data suggest that morin can induce apoptosis of melanoma cells by regulating pro-apoptotic and anti-apoptotic proteins through ROS, and may be a potential substance for treatment of melanoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer is a major cause of death with a very high mortality rate in both developed and developing countries (Jemal et al. 2011). Among various types of cancers, cutaneous melanoma is one of the most common types of skin cancers with high rate of metastasis and mortality (Hofmann et al. 2000). Cutaneous melanoma arises from melanocytes thus it is deeply pigmented. It is usually primarily diagnosed by presentation of the skin lesion. Cutaneous melanoma progression is associated with oxidative stress and various cell signaling pathways (Hambright et al. 2015).
Oxidative stress interferes with normal oxidation–reduction balance. It generates reactive oxygen species (ROS) that can damage cells and cause changes in the signaling process (Choi et al. 2014). ROS are byproducts of cellular metabolism and act as important signaling molecules. They are removed through antioxidizing defense mechanisms including the Keap1/Nrf2 pathway (Nguyen et al. 2009). Increase of ROS in cancer cells under oxidative stress can activate growth signaling process and cause more cell growth to result in proliferation of cancer cells. However, due to high dependency on ROS, cancer cells react more sensitive to the increase of oxidative stress, especially in melanoma cells (Cabello et al. 2012; Lee et al. 2012; Guterres et al. 2013). Accordingly, targeting cancer cells with substances that can increase ROS beyond certain threshold can selectively kill cancer cells while having little impact on normal cells (Lau et al. 2008).
Specificity protein 1 (Sp1) is a transactivation molecule in Sp/Krüppel-like factor (KLF) group (Briggs et al. 1986). Sp1 can lead to activation of various genes involved in cell proliferation, cell cycles, and cell apoptosis (Karlseder et al. 1996; Black et al. 2001; Deniaud et al. 2006). Furthermore, Sp1 has been shown to be expressed at high levels in various cancers including thyroid cancer, glioma, colorectal cancer, lung cancer, pancreatic cancer, gastric cancer, and breast cancer (Chiefari et al. 2002; Wang et al. 2003; Yao et al. 2004; Wang et al. 2007; Jiang et al. 2008; Guo et al. 2010; Guan et al. 2012; Hsu et al. 2012). ROS is related to adjustment of Sp1 and its downstream proteins (Wang et al. 2008; Gandhy et al. 2012; Jin et al. 2013). Mcl-1, a protein related to Sp1 (Choi et al. 2013), is essential for the survival of various cell lineage. It is frequently amplified in human cancers, making it special among Bcl-2 family members that contribute to cell survival (Opferman et al. 2003, 2005; Dzhagalov et al. 2007; Arbour et al. 2008; Dzhagalov et al. 2008; Beroukhim et al. 2010). Bcl-2 is also one of Sp1-related proteins associated with cell survival (Duan et al. 2005).
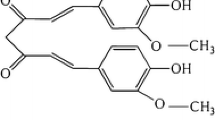
Morin (3,5,7,2′,4′-pentahydroxyflavone) is a flavonoid derived from the Moraceae family for the first time. Morin acts as anti-oxidant and neuroprotectant (Noor et al. 2012; Ola et al. 2014; Du et al. 2016). Furthermore, it has been reported that morin can control inflammatory reactions, thus inhibiting carcinogenesis and cancer progression (Kawabata et al. 1999; Brown et al. 2003). Morin exhibits anti-cancer activities by regulating cell proliferation and apoptosis in lung cancer, colorectal cancer, breast cancer, leukemia, and other human cancers (Kuo et al. 2007; Jin et al. 2014; Lee et al. 2016; Sithara et al. 2017; Yao et al. 2017). However, whether morin has an impact on the progression of melanoma and underlying mechanism is not well known. In the present study, we investigated cellular responses, including cellular ROS levels, mitochondrial function, apoptosis, and Sp1 expression, in melanoma cells treated with morin.
Materials and methods
Tissue sample collection and preparation
Melanoma specimens were obtained from patients who had undergone surgery in the Department of Plastic and Reconstructive Surgery between December 2015 and November 2018. The institutional review board of Soonchunhyang University Seoul Hospital reviewed and approved this research protocol involving the use of tissue samples. Six melanoma tissues were obtained from three male and three female patients and were diagnosed by pathologist. Normal skin tissues were collected from the backs of six women who had breast reconstruction with a latissimus dorsi flap. For immunohistochemical analysis, archival formalin-fixed, paraffin-embedded tissues were used.
Cell culture
The human melanoma cell lines, G361 and SK-MEL-2, and human fibroblast cell line, KEL FIB, were purchased from the American Type Culture Collection (Manassas, VA, USA). These cells were cultured at 37 °C in a submerged monolayer in DMEM supplemented with 5% fetal calf serum, 100 U of penicillin/mL, and 100 μg of streptomycin/mL in a humidified air with 5% CO2.
Cell viability assay
The cells were seeded into a 96-well microtiter plate at density of 5 × 103 cells/well and then treated with 0, 50, 100, 200, 300, or 400 μM morin (BP0959; Biopurify Phytochemicals Ltd., Chengdu, China) for 24, 48, and 72 h, of which chemical structure is shown in Fig. 1a. After incubation, cells were reacted with tetrazolium dye (MTT, Sigma, St. Louis, MO, USA, final concentration: 0.1 mg/mL) for 4 h and mixed with 200 μL of dimethyl sulfoxide. Absorbance value at wavelength of 540 nm was measured with a GloMax-Multi Microplate Multimode Reader (Promega, Madison, WI, USA). The percentage of viable cells was determined by comparing absorbance values with those of vehicle-treated control cells (100%).
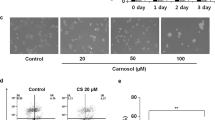
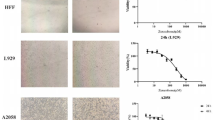
Effects of flavonoid morin in KEL FIB, G-361, and SK-MEL-2 cells a Chemical structure of morin [2-(2,4-dihydroxyphenyl)- 3,5,7 trihydroxy-4H-1-benzopyran-4-one]. b The percentage of cell viability was measured by MTT assay. Cells were treated with various concentration (0, 50, 100, 200, 300, 400 μM) of morin for 48 h. c Phase contrast images of KEL FIB, G361 and SK-MEL-2 treated with morin (0, 100, 200, 300 μM). The quantitative data were shown as mean ± SD for three independent experiments. *P < 0.05 compared to untreated controls
Annexin V-PE binding assay
Apoptotic-cell distribution was determined with a MuseTM Annexin V & Dead Cell kit (MCH100105; Merck Millipore Co., Darmstadt, Germany) according to the manufacturer’s protocol. The kit includes a fluorescent-dye phycoerythrin (PE) conjugated to Annexin V to detect phosphatidylserine on the external membrane of apoptotic cells and 7-AAD (7- amino-actinomycin D) as a dead cell marker. Briefly, trypsinized cells were harvested by centrifugation at 500×g for 7 min at 4 °C, mixed with MuseTM Annexin V and Dead Cell reagent, and analyzed using a Muse Cell Analyzer (Merck KgaA, Darmstadt, Germany).
Measurement of intracellular ROS levels
Intracellular ROS levels were evaluated by measuring DCF-DA (Sigma) fluorescence intensity. Briefly, cells were stained with 10 μM DCF-DA for 30 min at 37 °C in the dark. Following incubation, cells were washed twice with 1x PBS, trypsinized, resuspended in 1x PBS, and immediately analyzed with a MACSQuant Analyzer flow cytometer and MACSQuantify™ software (Version 2.5; MiltenyiBiotec GmbH, BergischGladbach, Germany). DCF fluorescence was detected using a 530 nm bandpass filter. Each measurement was based on the mean fluorescence intensity of 1 × 104 cells.
Mitochondrial membrane potential (MMP) disruption assay
Cells were seeded into six-well culture plates at density of 5 × 104 cells/well and cultured for 24 h. Cells were then incubated with morin alone or in combination with N-acetylcysteine (NAC, Sigma) for another 48 h. These cells were then trypsinized, harvested by centrifugation at 500×g for 7 min at 4 °C, washed twice with 1 × PBS, and stained with serum-free DMEM medium containing Rhodamine 123 (Sigma, 30 nM) at 37 °C for 30 min. Fluorescence intensity was measured and analyzed using a MACSQuant analyzer flow cytometer and MACSQuantify™ software.
Cell cycle assay analysis
Percentages of cells in G1, S, and G2/M phases were measured by quantifying DNA content in PI-stained cells. Briefly, trypsinized cells (~ 106 cell/mL) were pelleted by centrifugation at 500×g for 7 min at 4 °C, fixed in 70% ice-cold ethanol overnight at − 20 °C, and incubated with Muse™ Cell Cycle reagent (Merck Millipore, Billerica, MA, USA). Data from 1 x 104 single-cell events were collected with a MACSQuant Analyzer flow cytometer and analyzed using MACSQuantify™ software (Version 2.5; MiltenyiBiotec GmbH, BergischGladbach, Germany).
Western blot analysis
Total cell lysates were extracted with a 1 × RIPA buffer. Cell lysates containing 30 μg of proteins were resolved on NuPAGE 4–12% bis–tris polyacrylamide gel and then electro-transferred onto a PVDF membrane. The membrane was probed with 1:500 diluted primary antibodies in casein blocking buffer at 4 °C for 24 h followed by incubation with 1:5000 dilution of secondary antibody coupled to horseradish peroxidase in casein blocking buffer at room temperature for 2 h. Signals were detected using an ECL detection kit. Membranes were then stripped and re-blotted with anti-β-actin antibody (A2228; Sigma, St. Louis, MO, USA) as a loading control. The following primary antibodies were used: specific antibodies against Sp1 or Caspase-3 were purchased from Santa Cruz Biotechnology (Dallas, TX, USA), and specific antibodies against Mcl-1, Bcl-2, Cleaved Caspase-3, Bax, and PUMA were purchased from Cell Signaling Technology (Danvers, MA, USA). Secondary antibodies used were HRP-conjugated anti-rabbit IgG, anti-goat IgG, and anti-mouse IgG, and these were purchased from Santa Cruz Biotechnology (Dallas, TX, USA).
Small interfering RNA (siRNA) transfection
RNA interference assay was performed using an Sp1-targeting siRNA (HSS110105, HSS110106, HSS186048; Invitrogen, Carlsbad, CA, USA). Briefly, cells were seeded onto six-well and 96-well plates, and then transfected at 40% confluency with the Sp1-targeting siRNA duplex or the Stealth RNAi negative control duplex (12935-200; Invitrogen, Carlsbad, CA, USA) using lipofectamine RNAi MAX (Invitrogen, Carlsbad, CA, USA). They were processed for western blotting.
Immunohistochemical analysis
For immunohistochemical (IHC) analysis, four micron paraffin sections were prepared and stained according to the avidin–biotin staining technique. Briefly, slides were deparaffinized and cleared in xylene, rehydrated with ethanol, and placed in citrate buffer (0.01 M, pH 6.0) solution, with subsequent heating using ultrashort wave for 15 min. The sections were incubated overnight at 4 °C with anti-Sp1 antibody (1:500), followed by incubation in biotin-labelled secondary antibody for 30 min and then streptavidin-peroxidase for another 30 min. The staining was visualized using 3,3′-diaminobenzidine tetrahydrochloride. Negative controls were stained at the same time and in the same way, substituting the primary antibody with PBS. Assessment of IHC was scored as 0, 1 + (mild), 2 + (moderate), and 3 + (strong)
Results
Effects of morin treatment on fibroblast and melanoma cells
To determine whether morin shows cancer cell-specific cytotoxicity in melanoma cells, both cell viability and morphology was analyzed by MTT assay and phase contrast images, respectively, in KEL FIB cells and melanoma cells, including G361 and SK-MEL-2 cells. As shown in Fig. 1b, the viability of G361 and SK-MEL-2 cells decreased dose-dependently in response to different concentrations (50, 100, 200, 300 and 400 μM) of morin, while the viability of KEL FIB cells didn’t change significantly. Furthermore, at post 48 h treatment with 0, 100, 200, and 300 μM of morin, the morphology of melanoma cells changed dramatically and some cells appeared to be detached compared to KEL FIB cells (Fig. 1c). These results suggest that morin may have preferential cytotoxicity on melanoma cells.
Apoptotic effects of morin treatment on G361 cells
We investigated whether effects of morin treatment on cell viability were associated with apoptosis. As shown in Fig. 2a, cell viability of G361 cells was decreased by treatment of morin in time and dose-dependent manner. Then, we experimented to figure out changes of apoptosis-associated proteins by western blotting. The results showed that levels of cleaved caspase-3, Bax, and PUMA were increased while level of caspase-3 was decreased, as the concentration of morin was increased (Fig. 2b). The proportion of cells showing early and late phases of apoptosis was increased as concentration of morin increased (Fig. 2c). Cell cycle analysis demonstrated that the amount of sub-g0/g1 phase cells meaning apoptosis was increased with rising concentrations (100, 200, and 300 μm) of morin (fig. 2d). With the increasing morin concentrations, the percentage of cells in G2/M phase was increased, meaning a delay in G2 to M phase transition, and those in G1 and S phases were decreased compared to untreated controls. These findings suggest that morin may inhibit cell growth and promote apoptosis of G361 cells.
Apoptotic effects of morin on G361 cells. Cells were treated with various concentration (0, 100, 200, 300, 400 μM) of morin for 48 h. a The percentage of cell viability was measured by MTT assay. b The levels of apoptosis-associated proteins were measured after morin treatment by western blotting. ß-actin was used as a loading control. c The percentage of apoptotic cells after Annexin V-PE binding was analyzed using a Muse cell analyzer. d Cell distribution in G0/G1, S, and G2/M phases was analyzed using flow cytometry after staining with propidium iodide (20 μg/mL). The quantitative data were shown as mean ± SD for three independent experiments. *P < 0.05 compared to untreated controls
Effects of morin treatment on oxidative stress of G361 cells
To determine whether apoptotic effects of morin treatment was correlated with oxidative stress, cells were treated with morin and intracellular ROS levels were analyzed by flow cytometry using DCF-DA. In the representative histogram, treatment with 0, 100, 200, and 300 μM of morin for 48 h increased the production of ROS to 2.20, 15.57, 25.54, and 31.14%, respectively (Fig. 3a). Mitochondrial transmembrane potential (ΔΨm) was also measured by flow cytometry using fluorescent dye rhodamine-123. As shown in Fig. 3b, morin caused a dose-dependent decrease in the number of cells with intact ΔΨm. The percentage of cells with ΔΨm loss increased 2.08, 16.67, 27.95, and 31.33% when morin concentration used for treatment was 0, 100, 200, and 300 μM, respectively. These findings suggest that the stability of ΔΨm is significantly impaired by morin due to increased ROS levels.
Quantification of ROS formation and loss of mitochondrial membrane potential by Rhodamine 123 staining. G361 cells were treated with various concentrations of morin (0, 100, 200, 300 μM) for 48 h. a After incubation, cells were stained with DCF-DA and analyzed using flow cytometer. Data analyzing fluorescence intensity from triplicate measurements. b After incubation, cells were stained with Rhodamine 123 analyzed using flow cytometer. Data analyzing fluorescence intensity from triplicate measurements. *P < 0.05 compared to untreated controls
Apoptotic effects of reactive oxygen species following morin treatment on G361 cells
To determine whether apoptosis was caused by ROS in G361 cells, cells were treated with morin and ROS scavenger N-acetylcysteine (NAC), alone or in combination. As shown in Fig. 4a, western blot analysis demonstrated that morin treatment decreased expression levels of Sp1, Mcl-1, Bcl-2, and caspase-3 but increased expression levels of cleaved caspase-3, Bax, and PUMA in G361 cells. However, morin-induced changes of protein expression were effectively reversed in the presence of NAC. In addition, pretreatment of cells with NAC resulted in significant improvement of cell viability (Fig. 4b). Changes of cell morphology were also reduced based on phase contrast images (Fig. 4c). NAC pretreatment also decreased the proportion of cells undergoing apoptosis based on Annexin V-PE staining results (Fig. 4d) and the sub-G0/G1 peak (Fig. 4e) compared to morin treatment alone. These results suggest that morin would lead to apoptosis of G361 cells through ROS by regulating Sp1, Sp1-related proteins, and pro-apoptotic proteins.
Apoptotic effects of reactive oxygen species following morin treatment on G361 cells. Cells were pretreated with 5 mM NAC for 1 h prior to exposure to 0 or 300 μM concentrations of morin for another 48 h. a The levels of Sp1, anti-apoptotic and pro-apoptotic proteins in total cell lysates were measured by western blotting. b The percentage of viable cells was measured by MTT assay. c Phase contrast images of cells treated with morin. d The percentage of apoptotic cells after Annexin V-PE binding was analyzed using a Muse cell analyzer. e Cell distribution in G0/G1, S, and G2/M phases was analyzed using flow cytometry after staining with propidiumiodide(20 μg/mL). The quantitative data were shown as mean ± SD for three independent experiments. *P < 0.05 compared to untreated controls
Pro-oxidant effects of morin on G361 cells
To confirm that morin could act as a pro-oxidant in G361 cells, we used NAC to reduce intracellular ROS levels. After cells were treated with morin and NAC, intracellular ROS levels were analyzed by flow cytometry with DCF-DA. As shown in the representative histogram (Fig. 5a), treatment with morin at final concentration of 300 μM for 48 h produced ROS to approximately 30.93%. However, the accumulation of ROS was significantly attenuated by NAC during morin treatment to approximately 17.44%. The ΔΨm was also measured by flow cytometry with fluorescent dye rhodamine-123. As shown in Fig. 5b, the percentage of cells with ΔΨm loss increased from 1.90 to 34.37% when morin concentration was increased from 0 to 300 μM. However, pretreatment with NAC reduced it to approximately 1.76% and 14.34%, respectively. These findings suggest that ROS scavenging could prevent mitochondrial damage by protecting substantial loss of ΔΨm in morin-treated G361 cells, while the stability of ΔΨm was highly damaged by morin.
Pro-oxidant effects of morin on G361 cells. Cells were pretreated with 5 mM NAC for 1 h prior to exposure to 0 or 300 μM concentrations of morin for another 48 h. a The levels of cellular ROS were measured using flow cytometry after staining with DCF-DA (10 μM). A shift of DCF fluorescence to the right indicates an increase in ROS. b The levels of ΔΨm were measured using a flow cytometry after staining with rhodamine 123 (30 nM). *P < 0.05 compared to untreated controls
Effects of morin as a Sp1 inhibitor on melanoma
Suppression of Sp1 is known to activate apoptotic signals for cell death. Here, we examined the effect of morin as a Sp1 inhibitor on melanoma cells. Western blot analysis showed that levels of Sp1, Mcl-1, and Bcl-2 proteins were decreased in G361 and SK-MEL-2 cells while levels of those proteins were almost unchanged in KEL FIB cells, as the concentration of morin was increased (Fig. 6a). Next, the effects of morin on Sp1, Mcl-1, and Bcl-2 expression have been determined by western blotting following Sp1 silencing in G361 and SK-MEL-2 cells. Expressions of Sp1, Mcl-1 and Bcl-2 proteins were downregulated by treating Sp1-targeting siRNA in both cell types; however, these findings were recovered by NAC pretreatment (Fig. 6b). To investigate the association between Sp1 and cell viability, we examined whether Sp1 knockdown affects sensitivity to morin treatment. When cells transfected with Sp1-targeting siRNA were treated with morin, the viability of G361 and SK-MEL-2 cells was reduced to approximately 18.7% and 16.1%, respectively, in morin-untreated cells, and approximately 18% and 14.9%, respectively, in morin-treated cells, compared with that of respective control siRNA-treated cells (Fig. 6c). Next, we examined whether a decrease in the cell viability following Sp1 knockdown is associated with ROS. As shown in Fig. 6d, the increased ROS levels by morin were further elevated by Sp1 knockdown in both G361 and SK-MEL-2. To confirm overexpression of Sp1 in melanoma tissues, Sp1 proteins was examined by immunohistochemistry in the paraffin sections of both six human melanoma tissues and six normal skin tissues. As shown in Fig. 6e, Sp1 was overexpressed significantly in melanoma tissues compared to normal skin tissues. Four melanoma tissues showed 3 + (strong) positive staining of Sp1 while two melanoma tissues demonstrated 2 + (moderate) positive staining of Sp1. However, all of normal skin tissues showed 1 + (mild) positive staining of Sp1. Taken together, these findings demonstrate that morin may induce apoptosis through downregulation of antiapoptotic Mcl-1 and Bcl-2, which is regulated, at least in part, by the ROS-linked suppression of Sp1 expression in melanoma cells.
Expression of Sp1 in melanoma cells and tissues. a The levels of Sp1, Mcl-1, and Bcl-2 proteins were measured following morin treatment by western blotting in KEL FIB, G361, and SK-MEL-2 cells. Strongly positive staining in melanoma tissue (IHC stain, × 400). b G361 and SK-MEL-2 were transfected with 10 nM of Sp1-targeting siRNA (siSp1) or Stealth RNAi control (siC) for 24 h, after which cells were with either morin (300 μM) or NAC (5 mM) for 48 h. The levels of Sp1, Mcl-1, and Bcl-2 proteins were measured by western blotting. c The percentage of viable cells following treatment of either Sp1-targeting siRNA or morin was measured by MTT assay. d The levels of cellular ROS were measured in melanoma cells treated with either Sp1-targeting siRNA or morin by using flow cytometry after staining with DCF-DA (10 μM). e Representative immunohistochemical staining for Sp1 protein expression in paraffin-embedded normal skin and melanoma tissue. Weakly positive staining in normal skin (IHC stain, × 400). The quantitative data were shown as mean ± SD for three independent experiments. *P < 0.05 compared to untreated controls
Discussion
Oxidative stress is induced by imbalance between oxidative substances and antioxidizing defense mechanisms. Oxidative stress is shown to be related to various skin diseases including skin cancer (Klaunig et al. 1995; Marnett et al. 2003). Melanoma, one of the most serious types of skin cancers, is related to oxidative stress (Hambright et al. 2015). It has been reported that oxidative stress was increased in melanoma cells through various mechanisms (Sander et al. 2003). For example, an increase in superoxide anion and a decrease in hydrogen peroxide have been found in melanoma cells. This environment makes the cell easier for oxidization. In addition, the signaling pathway that strengthens the aggressiveness of melanoma cells characterized by high proliferation speeds and drug resistance is activated (Pervaiz and Clement 2007). However, there is an intriguing report that while increased ROS can contribute to carcinogenesis by causing DNA damage, ROS levels beyond a threshold can cause cell aging and cell death, therefore functioning as an anti-tumor substance (Reuter et al. 2010). Hence, substances which induce ROS can also increase oxidative stress in cancer cells, leading to activation of apoptotic signals.
Morin is originally produced from the Moraceae family such as mulberry figs and other Chinese herbs. It has also been discovered as a yellow pigment from almond hulls and old fustic (Lee et al. 2008). Morin is a flavonoid substance having structure of [2-(2, 4-dihydroxyphenyl)-3,5,7 trihydroxy-4H-1-benzopyran-4-one]. It is widely used in herbal medicines for its biological activities such as anti-inflammation, antioxidizing, and anti-cancer effects (Kim et al. 1993; Lee et al. 1993; Sivaramakrishnan and Niranjali Devaraj 2009; Jin et al. 2014). It has been revealed that morin induced apoptosis in various human cancer cells including lung cancer, colorectal cancer, breast cancer, and leukemia (Kuo et al. 2007; Jin et al. 2014; Lee et al. 2016; Sithara et al. 2017; Yao et al. 2017).
Due to limited information, it was very difficult to determine quantitative levels of the cellular morin that can be achieved in vitro conditions versus in vivo situation. However, recent studies have shown that cancer-specific apoptosis occurred after treating high concentration of morin in breast cancer cells (Jin et al. 2014), whereas any significant changes in cell viability did not be observed in normal lung fibroblasts, even though morin was treated up to 500 μM (Lee et al. 2017). Consistently, our data in melanoma cells and fibroblasts support the previous results, indicating that morin has a selective cytotoxic effect on malignant cells. However, large well-designed clinical studies are required to further elucidate the effect of morin.
Our results suggest that apoptosis signaling activated by morin treatment may be associated with increased ROS levels and mitochondrial damage in human melanoma G361 cells. Although there has been no report to confirm relationship between ROS generation and anti-cancer effects of morin in melanoma cells, some papers reported that morin causes cell death by increasing cellular ROS levels in human leukemia cells and colorectal cancer cells (Kuo et al. 2007; Hyun et al. 2015; Sithara et al. 2017). Here, our data underline an importance of Sp1 as an ROS-regulating molecule. Sp1 related to cell proliferation and cell survival contributes to cancer formation (Prathyusha et al. 2017). This rationale is supported by our findings that Sp1 was more expressed in melanoma tissues than in normal skin tissues, and that Sp1 knockdown decreased the viability of G361 and SK-MEL-2 cells, suggesting that overexpression of Sp1 may contribute to melanoma formation. So far, no in vivo studies have been performed to confirm expression of Sp1 protein in melanoma tissues. Furthermore, our findings that morin-induced cytotoxicity was accompanied by the down-regulation of antiapoptotic proteins, including Sp1, Mcl-1 and Bcl-2, and the up-regulation of pro-apoptotic proteins, including cleaved caspase-3, Bax and PUMA, in G361 cells might be at least in part explained by ROS-induced pro-oxidant effects. This possibility is supported by pretreatment with antioxidant NAC, which significantly recovered a series of morin-induced changes such as cell viability, the levels of apoptosis promoting and apoptosis-inhibiting proteins, cellular ROS levels, and ΔΨm. Mc1-1, a protein that inhibits apoptosis, has promoter rich in GC that can directly bind to Sp1 (Choi et al. 2013). Bcl-2, a well-known protein that can inhibit apoptosis, also has promoter that can bind to Sp1 (Duan et al. 2005). In this study, the reduced viability of melanoma cells and the downregulation of Mcl-1 and Bcl-2 expression following Sp1 knockdown suggest that Sp1 may contribute to cell apoptosis and act as a transcriptional regulator of Mcl-1 and Bcl-2. Considering that ROS is a trigger factor for intrinsic apoptosis through interaction with mitochondrial permeability transition complex proteins (Tsujimoto and Shimizu 2007; Bajpai and Nagaraju 2017), dysregulation of ROS by morin treatment in G361 cells can promote mitochondrial apoptosis by downregulating Sp1 and Sp1-related proteins such as Mcl-1 and Bcl-2. Conversely, Sp1-linked molecular pathway may be essential for cell survival in melanoma.
Interestingly, our data demonstrated increased ROS production following downregulation of Sp1 in both G361 and SK-MEL-2 cells. These results suggest that Sp1 may protect cells from oxidative stress. In consistent with our results, there are some reports that Sp1 could attenuate ROS generation to increase cell survival, while downregulation of Sp1 could increase intracellular ROS levels. The upregulation of Sp1 increased Zinc finger protein 179 (Znf179) through the transcriptional activation, which protected cells from ROS-induced damage via upregulation of antioxidant proteins, including peroxireoxin III (Prx3) and superoxide dismutase 2 (SOD2) (Chuang et al. 2017). Moreover, Sp1 is also known as a transcription activator of SOD1 representing a protection against NO toxicity via scavenging the radical superoxide (Baldelli et al. 2008).
Taken together, we observed that morin-induced pro-oxidant environment led to a series of cancer cell-specific apoptotic process in melanoma cells, as evidenced by increased proportion of cells with Annexin V-PE(+) staining and sub-G0/G1 peak in cell cycle analysis, the downregulation of Sp1, Mcl-1, and Bcl-2, and upregulation of cleaved caspase-3, Bax, and PUMA. Despite the fact that future research, including large well-designed clinical studies, is warranted, morin may be a potential candidate substance in treatment of melanoma by activating apoptosis pathway through ROS-mediated damage and Sp1 suppression in vitro.
References
Arbour N, Vanderluit JL, Le Grand JN, Jahani-Asl A, Ruzhynsky VA, Cheung EC, Kelly MA, Mackenzie AE, Park DS, Opferman JT, Slack RS (2008) Mcl-1 is a key regulator of apoptosis during CNS development and after DNA damage. J Neurosci 28:6068–6078
Bajpai R, Nagaraju GP (2017) Specificity protein 1: its role in colorectal cancer progression and metastasis. Crit Rev Oncol Hematol 113:1–7
Baldelli S, Aquilano K, Rotilio G, Ciriolo MR (2008) Glutathione and copper, zinc superoxide dismutase are modulated by overexpression of neuronal nitric oxide synthase. Int J Biochem Cell Biol 40:2660–2670
Beroukhim R, Mermel CH, Porter D, Wei G, Raychaudhuri S, Donovan J, Barretina J, Boehm JS, Dobson J, Urashima M, Mc Henry KT, Pinchback RM, Ligon AH, Cho YJ, Haery L, Greulich H, Reich M, Winckler W, Lawrence MS, Weir BA, Tanaka KE, Chiang DY, Bass AJ, Loo A, Hoffman C, Prensner J, Liefeld T, Gao Q, Yecies D, Signoretti S, Maher E, Kaye FJ, Sasaki H, Tepper JE, Fletcher JA, Tabernero J, Baselga J, Tsao MS, Demichelis F, Rubin MA, Janne PA, Daly MJ, Nucera C, Levine RL, Ebert BL, Gabriel S, Rustgi AK, Antonescu CR, Ladanyi M, Letai A, Garraway LA, Loda M, Beer DG, True LD, Okamoto A, Pomeroy SL, Singer S, Golub TR, Lander ES, Getz G, Sellers WR, Meyerson M (2010) The landscape of somatic copy-number alteration across human cancers. Nature 463:899–905
Black AR, Black JD, Azizkhan-Clifford J (2001) Sp1 and kruppel-like factor family of transcription factors in cell growth regulation and cancer. J Cell Physiol 188:143–160
Briggs MR, Kadonaga JT, Bell SP, Tjian R (1986) Purification and biochemical characterization of the promoter-specific transcription factor, Sp1. Science 234:47–52
Brown J, O’prey J, Harrison PR (2003) Enhanced sensitivity of human oral tumours to the flavonol, morin, during cancer progression: involvement of the Akt and stress kinase pathways. Carcinogenesis 24:171–177
Cabello CM, Lamore SD, Bair WB 3rd, Qiao S, Azimian S, Lesson JL, Wondrak GT (2012) The redox antimalarial dihydroartemisinin targets human metastatic melanoma cells but not primary melanocytes with induction of NOXA-dependent apoptosis. Invest New Drugs 30:1289–1301
Chiefari E, Brunetti A, Arturi F, Bidart JM, Russo D, Schlumberger M, Filetti S (2002) Increased expression of AP2 and Sp1 transcription factors in human thyroid tumors: a role in NIS expression regulation? BMC Cancer 2:35
Choi ES, Han G, Park SK, Lee K, Kim HJ, Cho SD, Kim HM (2013) A248, a novel synthetic HDAC inhibitor, induces apoptosis through the inhibition of specificity protein 1 and its downstream proteins in human prostate cancer cells. Mol Med Rep 8:195–200
Choi CY, Kim JY, Wee SY, Lee JH, Nam DH, Kim CH, Cho MK, Lee YJ, Nam HS, Lee SH, Cho SW (2014) Expression of nuclear factor erythroid 2 protein in malignant cutaneous tumors. Arch Plast Surg 41:654–660
Chuang JY, Kao TJ, Lin SH, Wu AC, Lee PT, Su TP, Yeh SH, Lee YC, Wu CC, Chang WC (2017) Specificity protein 1-zinc finger protein 179 pathway is involved in the attenuation of oxidative stress following brain injury. Redox Biol 11:135–143
Deniaud E, Baguet J, Mathieu AL, Pages G, Marvel J, Leverrier Y (2006) Overexpression of Sp1 transcription factor induces apoptosis. Oncogene 25:7096–7105
Du Y, Qu J, Zhang W, Bai M, Zhou Q, Zhang Z, Li Z, Miao J (2016) Morin reverses neuropathological and cognitive impairments in APPswe/PS1dE9 mice by targeting multiple pathogenic mechanisms. Neuropharmacology 108:1–13
Duan H, Heckman CA, Boxer LM (2005) Histone deacetylase inhibitors down-regulate bcl-2 expression and induce apoptosis in t(14;18) lymphomas. Mol Cell Biol 25:1608–1619
Dzhagalov I, St John A, He YW (2007) The antiapoptotic protein Mcl-1 is essential for the survival of neutrophils but not macrophages. Blood 109:1620–1626
Dzhagalov I, Dunkle A, He YW (2008) The anti-apoptotic Bcl-2 family member Mcl-1 promotes T lymphocyte survival at multiple stages. J Immunol 181:521–528
Gandhy SU, Kim K, Larsen L, Rosengren RJ, Safe S (2012) Curcumin and synthetic analogs induce reactive oxygen species and decreases specificity protein (Sp) transcription factors by targeting microRNAs. BMC Cancer 12:564
Guan H, Cai J, Zhang N, Wu J, Yuan J, Li J, Li M (2012) Sp1 is upregulated in human glioma, promotes MMP-2-mediated cell invasion and predicts poor clinical outcome. Int J Cancer 130:593–601
Guo Z, Zhang W, Xia G, Niu L, Zhang Y, Wang X, Zhang Y, Jiang B, Wang J (2010) Sp1 upregulates the four and half lim 2 (FHL2) expression in gastrointestinal cancers through transcription regulation. Mol Carcinog 49:826–836
Guterres FA, Martinez GR, Rocha ME, Winnischofer SM (2013) Simvastatin rises reactive oxygen species levels and induces senescence in human melanoma cells by activation of p53/p21 pathway. Exp Cell Res 319:2977–2988
Hambright HG, Meng P, Kumar AP, Ghosh R (2015) Inhibition of PI3 K/AKT/mTOR axis disrupts oxidative stress-mediated survival of melanoma cells. Oncotarget 6:7195–7208
Hofmann UB, Westphal JR, Van Muijen GN, Ruiter DJ (2000) Matrix metalloproteinases in human melanoma. J Invest Dermatol 115:337–344
Hsu TI, Wang MC, Chen SY, Yeh YM, Su WC, Chang WC, Hung JJ (2012) Sp1 expression regulates lung tumor progression. Oncogene 31:3973–3988
Hyun HB, Lee WS, Go SI, Nagappan A, Park C, Han MH, Hong SH, Kim G, Kim GY, Cheong J, Ryu CH, Shin SC, Choi YH (2015) The flavonoid morin from Moraceae induces apoptosis by modulation of Bcl-2 family members and Fas receptor in HCT 116 cells. Int J Oncol 46:2670–2678
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61:69–90
Jiang NY, Woda BA, Banner BF, Whalen GF, Dresser KA, Lu D (2008) Sp1, a new biomarker that identifies a subset of aggressive pancreatic ductal adenocarcinoma. Cancer Epidemiol Biomark Prev 17:1648–1652
Jin M, Ande A, Kumar A, Kumar S (2013) Regulation of cytochrome P450 2e1 expression by ethanol: role of oxidative stress-mediated pkc/jnk/sp1 pathway. Cell Death Dis 4:e554
Jin H, Lee WS, Eun SY, Jung JH, Park HS, Kim G, Choi YH, Ryu CH, Jung JM, Hong SC, Shin SC, Kim HJ (2014) Morin, a flavonoid from Moraceae, suppresses growth and invasion of the highly metastatic breast cancer cell line MDA-MB231 partly through suppression of the Akt pathway. Int J Oncol 45:1629–1637
Karlseder J, Rotheneder H, Wintersberger E (1996) Interaction of Sp1 with the growth- and cell cycle-regulated transcription factor E2F. Mol Cell Biol 16:1659–1667
Kawabata K, Tanaka T, Honjo S, Kakumoto M, Hara A, Makita H, Tatematsu N, Ushida J, Tsuda H, Mori H (1999) Chemopreventive effect of dietary flavonoid morin on chemically induced rat tongue carcinogenesis. Int J Cancer 83:381–386
Kim HK, Namgoong SY, Kim HP (1993) Antiinflammatory activity of flavonoids: mouse ear edema inhibition. Arch Pharm Res 16:18
Klaunig JE, Xu Y, Bachowski S, Ketcham CA, Isenberg JS, Kolaja KL, Baker TK, Walborg EF Jr, Stevenson DE (1995) Oxidative stress in nongenotoxic carcinogenesis. Toxicol Lett 82–83:683–691
Kuo HM, Chang LS, Lin YL, Lu HF, Yang JS, Lee JH, Chung JG (2007) Morin inhibits the growth of human leukemia HL-60 cells via cell cycle arrest and induction of apoptosis through mitochondria dependent pathway. Anticancer Res 27:395–405
Lau AT, Wang Y, Chiu JF (2008) Reactive oxygen species: current knowledge and applications in cancer research and therapeutic. J Cell Biochem 104:657–667
Lee SJ, Son KH, Chang HW, Do JC, Jung KY, Kang SS, Kim HP (1993) Antiinflammatory activity of naturally occurring flavone and flavonol glycosides. Arch Pharmacal Res 16:25
Lee HS, Jung K-H, Hong S-W, Park I-S, Lee C, Han H-K, Lee D-H, Hong S-S (2008) Morin protects acute liver damage by carbon tetrachloride (CCl4) in rat. Arch Pharm Res 31:1160–1165
Lee JH, Won YS, Park KH, Lee MK, Tachibana H, Yamada K, Seo KI (2012) Celastrol inhibits growth and induces apoptotic cell death in melanoma cells via the activation ROS-dependent mitochondrial pathway and the suppression of PI3 K/AKT signaling. Apoptosis 17:1275–1286
Lee J, Jin H, Lee WS, Nagappan A, Choi YH, Kim GS, Jung J, Ryu CH, Shin SC, Hong SC, Kim HJ (2016) Morin, a flavonoid from Moraceae, inhibits cancer cell adhesion to endothelial cells and EMT by downregulating VCAM1 and Ncadherin. Asian Pac J Cancer Prev 17:3071–3075
Lee MH, Cha HJ, Choi EO, Han MH, Kim SO, Kim GY, Hong SH, Park C, Moon SK, Jeong SJ, Jeong MJ, Kim WJ, Choi YH (2017) Antioxidant and cytoprotective effects of morin against hydrogen peroxide-induced oxidative stress are associated with the induction of Nrf-2mediated HO-1 expression in V79-4 Chinese hamster lung fibroblasts. Int J Mol Med 39:672–680
Marnett LJ, Riggins JN, West JD (2003) Endogenous generation of reactive oxidants and electrophiles and their reactions with DNA and protein. J Clin Invest 111:583–593
Nguyen T, Nioi P, Pickett CB (2009) The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem 284:13291–13295
Noor H, Cao P, Raleigh DP (2012) Morin hydrate inhibits amyloid formation by islet amyloid polypeptide and disaggregates amyloid fibers. Protein Sci 21:373–382
Ola MS, Aleisa AM, Al-Rejaie SS, Abuohashish HM, Parmar MY, Alhomida AS, Ahmed MM (2014) Flavonoid, morin inhibits oxidative stress, inflammation and enhances neurotrophic support in the brain of streptozotocin-induced diabetic rats. Neurol Sci 35:1003–1008
Opferman JT, Letai A, Beard C, Sorcinelli MD, Ong CC, Korsmeyer SJ (2003) Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature 426:671–676
Opferman JT, Iwasaki H, Ong CC, Suh H, Mizuno S, Akashi K, Korsmeyer SJ (2005) Obligate role of anti-apoptotic MCL-1 in the survival of hematopoietic stem cells. Science 307:1101–1104
Pervaiz S, Clement MV (2007) Superoxide anion: oncogenic reactive oxygen species? Int J Biochem Cell Biol 39:1297–1304
Prathyusha AMVN, Nawadkar R, Chari B (2017) Role of Sp1 Transcriptional Factor in Gastrointestinal Carcinogenesis. In: Nagaraju GP (ed) Role of transcription factors in gastrointestinal malignancies, 1st edn. Springer, Singapore, pp 191–201
Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB (2010) Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med 49:1603–1616
Sander CS, Hamm F, Elsner P, Thiele JJ (2003) Oxidative stress in malignant melanoma and non-melanoma skin cancer. Br J Dermatol 148:913–922
Sithara T, Arun KB, Syama HP, Reshmitha TR, Nisha P (2017) Morin inhibits proliferation of SW480 colorectal cancer cells by inducing apoptosis mediated by reactive oxygen species formation and uncoupling of warburg effect. Front Pharmacol 8:640
Sivaramakrishnan V, Niranjali Devaraj S (2009) Morin regulates the expression of NF-kappaB-p65, COX-2 and matrix metalloproteinases in diethylnitrosamine induced rat hepatocellular carcinoma. Chem Biol Interact 180:353–359
Tsujimoto Y, Shimizu S (2007) Role of the mitochondrial membrane permeability transition in cell death. Apoptosis 12:835–840
Wang L, Wei D, Huang S, Peng Z, Le X, Wu TT, Yao J, Ajani J, Xie K (2003) Transcription factor Sp1 expression is a significant predictor of survival in human gastric cancer. Clin Cancer Res 9:6371–6380
Wang XB, Peng WQ, Yi ZJ, Zhu SL, Gan QH (2007) Expression and prognostic value of transcriptional factor sp1 in breast cancer. Ai Zheng 26:996–1000
Wang X, Wang J, Lin S, Geng Y, Wang J, Jiang B (2008) Sp1 is involved in H2O2-induced PUMA gene expression and apoptosis in colorectal cancer cells. J Exp Clin Cancer Res 27:44
Yao JC, Wang L, Wei D, Gong W, Hassan M, Wu TT, Mansfield P, Ajani J, Xie K (2004) Association between expression of transcription factor Sp1 and increased vascular endothelial growth factor expression, advanced stage, and poor survival in patients with resected gastric cancer. Clin Cancer Res 10:4109–4117
Yao D, Cui H, Zhou S, Guo L (2017) Morin inhibited lung cancer cells viability, growth, and migration by suppressing miR-135b and inducing its target CCNG2. Tumour Biol 39:1010428317712443
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lee, Y.J., Kim, W.I., Kim, S.Y. et al. Flavonoid morin inhibits proliferation and induces apoptosis of melanoma cells by regulating reactive oxygen species, Sp1 and Mcl-1. Arch. Pharm. Res. 42, 531–542 (2019). https://doi.org/10.1007/s12272-019-01158-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-019-01158-5