Abstract
Toxicity and target organ distribution of cerium oxide nanoparticles (CeNPs) were investigated via single intravenous injection and single oral administration, respectively. Rats were sacrificed at 24 h after treatment with doses of 30 and 300 mg/kg, and cerium concentrations were measured in liver, kidney, spleen, lung, blood, urine and feces. Results revealed cerium levels in blood and tissues were considerably low in oral treated groups and most cerium was detected in feces, meaning CeNPs would not be absorbed in the gastro-intestinal system. Conversely, high concentrations of cerium were detected in all tissues of rats after intravenous injection. Liver and spleen were main target organs. Cerium levels in liver were 594.9 ± 95.3 μg/g tissue in 30 mg/kg treat group and 3741.7 ± 932.7 μg/g tissue in 300 mg/kg treat group. Cerium levels in spleen reached almost levels of liver. Cerium was also detected, that is relatively low compared to oral administration, in feces of rats treated via intravenous injection, that supports biliary excretion of CeNPs. Urine excretion of CeNPs was not detected in oral treatment and intravenous injection. In accordance with level of cerium distribution, toxicities based on hematology, serum biochemistry and histopathology were observed in rats treated by intravenous injection while no significance was revealed in orally treated groups.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Ceria nanoparticles (CeNPs) have many applications for industry, drug delivery and cosmetic formulations. Especially, many efforts on development of safe therapeutics using CeNPs have been initiated. Reports on anti-oxidative effects of CeNPs have been published (Kolli et al. 2014; Rocca et al. 2015; Pešic et al. 2015). However, studies on adverse effects on the environment and human health are being reported (Nalabotu et al. 2011; Kumari et al. 2014; Ali et al. 2015). It is why more toxicity tests on the CeNPs should be conducted to clarify safety issues. Regarding adverse effects, evidence of inflammation evaluated by hematological-chemical-clinical assays and histological examination was observed when acute and subacute in vivo toxicity were assessed using CD-1 mouse systems (Poma et al. 2014). Peng, et al. also reported that CeNPs have higher reactivity to catalyzing the generation of reactive oxygen species (ROS). ROS generated by CeNPs caused lung injury in rats: broncho-alveolar lavage (BAL) inflammation, cytotoxicity, lipid peroxidation, and pro-inflammation (Peng et al. 2014). When tomato plants were treated with relatively low concentrations of CeNPs (10 mg/L) through their lifecycle, CeNPs revealed adverse effects including seed quality and development of second generation seedlings. Second generation seedlings grown from seeds collected from treated parent plants with CeNPs (treated second generation seedlings) were generally smaller and weaker, as indicated by smaller biomass, lower water transpiration, and slightly higher reactive oxygen species content (Wang et al. 2013). More on these, and many others in vitro tests revealed CeNPs have cytotoxicity and pro-inflammatory activity in cultured cells.
Recently, a few in vivo tests suggested bioavailability of nanoparticles is too low to exert intrinsic toxicity especially when nanoparticles are treated by oral route (Choi et al. 2015). Toxic doses of orally administered nanoparticles were relatively higher compared to generally known toxic chemicals, that must result from low bioavailability and low concentration of nanoparticles in target organs (Hong et al. 2014). In a study, mice were treated with 500, 1000, or 2000 mg/kg zinc oxide nanoparticles for 14 days by oral administration, and serum biochemistry, hematology, and histopathological analysis was conducted (Patra et al. 2012). Then, no significant changes were found in lactate dehydrogenase (LDH), creatinine (CRE), alkaline phosphatase (ALP), total protein cholesterol, triglyceride, uric acid, and phosphorus in treated mice. Slight swelling in the renal glomerulus was observed only in 2000 mg/kg treated mice, also uniquely displaying an irregular array of veins, loss of sinusoid, and hydrophobic degeneration with fatty liver. The no-observed-adverse-effect level (NOAEL) was 1000 mg/kg. Oral administration of nanoparticles, it seems to be absurd if bioavailability was not considered. NOAEL must be significantly down when nanoparticles were intravenously injected in test animals.
In case of CeNP, a few toxicity tests were conducted after intravenous injection. By results, toxicity was revealed in relatively low dose-treated groups compared to oral groups (Minarchick et al. 2015). Tseng et al. revealed evidence of acute and subacute adverse hepatic responses after an infusion of nanoceria (an aqueous dispersion of 85 mg/kg) into Sprague–Dawley rats. Uptake of nanoceria by Kupffer cells was detected as early as 1 h after infusion. Although frank hepatic necrosis was not observed, retention of nanoceria increased hepatic apoptosis acutely, and this persisted until day 90 (Tseng et al. 2014). They also revealed that a statistically significant elevation of serum aspartate aminotransferase (AST) level was observed at 1 and 20 h, but subsided 30 days after ceria infusion. Elevated apoptosis was observed on day 30 (Tseng et al. 2012). However, direct comparison to different routes of administration, i.e., oral administration and intravenous injection was not conducted.
The purpose of this study was to investigate differences in toxicity and kinetics including tissue distribution and excretion of CeNPs in rats after treatment by two different routes, an intravenous injection and single oral administration. Toxicities as evidenced by serum biochemistry, hematology, and histopathology of target organs were evaluated and compared between the two groups, based on cerium level in target organs.
Materials and methods
CeNPs
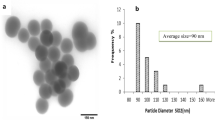
CeNPs were purchased from Sigma-Aldrich (St. Louis, MO, USA). Nanoparticles provided by the manufacturer were in nano-powder form. According to information provided by the manufacturer, average particle size determined by transmission electronic microscopy (TEM) is less than 20 nm.
Animals and treatment
Seven-week-old Sprague–Dawley female rats were purchased from Orient Bio (Gyeonggi-do, Korea). Rats were acclimatized to the laboratory for 1 week before initiation of the experiment. Diet and water were provided ad libitum. The animal facility was maintained at 23 ± 3 °C with relative humidity of at 55 ± 15%, air circulation 12 times each hour, and a 12-h light/dark cycle (light from 8:00 to 20:00) with light intensity of 150–300 Lux. For dosing, CeNPs powder form was diluted with deionized and sterilized water for sonication. Animal study was approved by the Institutional Animal Care and Use Committee, Dongduk Women’s University.
Dosage was 300 mg/kg in high-dose group and 30 mg/kg in low-dose group. Total treated volume of the CeNPs suspension was 10 ml/kg in orally treated groups and 5 ml/kg in intravenous treated group, respectively. For treatment, CeNPs were sonicated by sonifier Vibra-Cell® (Model VC 505, Sonics & Materials, CT, USA) with 13 mm Probe (Model CV334) at 25% amplitude for 8 min. Size-distributions of CeNPs in deionized sterilized water and in fetal bovine serum (1 ml CeNPs solution was mixture with 9 ml FBS) were measured by dynamic light scattering. Five rats were used in each group. At 24 h after treatment, all rats were sacrificed for toxicological examination and CeNPs analysis. All rats were cared for in accordance with principles outlined in the “Guide for the Care and Use of Laboratory Animals” issued by the Animal Care and Committee of NVRQS (National Veterinary Research and Quarantine Service) and guidelines of the Korea Institute of Toxicology.
Sampling and Ce analysis
For biochemistry and hematological assays, rats were sacrificed at 24 h after dosing. Blood and tissues (liver, kidney, lung, and spleen) were obtained from control group and treated groups. Feces and urine were collected daily for 24 h. For Ce analysis, samples were stored at − 80 °C and digested in a solution of 7 ml 70% HNO3 and 1 ml 30% H2O2 using a microwave digestion system (Milestone, Sorisole, Italy) with high temperature and pressure. Rat samples including tissues, blood, urine, and feces were completely solubilized in this condition. After diluting the acidic digested preparation with deionized water, Ce concentration was analyzed using inductively coupled plasma atomic emission spectroscopy (ICP-AES) using an Ultima 2 apparatus (Horiba Jobin, Yvon, France) at the Korean Basic Science Institute (Seoul, Korea) (Lee et al. 2013).
Toxicity tests
All the rsts were observed thoroughly for onset of immediate signs of toxicity. Hematology was analyzed using an Advia 2120 hematological autoanalyzer (Bayer, Whippany, NJ, USA) including total red blood cell (RBC) count, hematocrit (HCT), hemoglobin (HGB) concentration, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), platelet count (PLT), total white blood cell (WBC) count, and differential leukocyte percentage. Serum was used for examination of the following parameters using a model AV400 biochemistry auto-analyzer (Olympus, Tokyo, Japan): total protein (TP), albumin (ALB), A/G ratio, total bilirubin (TBIL), alkaline phosphatase (ALP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), creatinine (CRE), creatinine phosphokinase (CPK), blood urea nitrogen (BUN), total cholesterol (TCHO), triglyceride (TG), glucose (GLU), calcium (Ca), and phosphorus (IP). Serum electrolytes chloride (Cl−), sodium (Na+), and potassium (K+) were measured using a Rapidchem 744 Na/K/Cl ion auto-analyzer (Rapidchem, Erlangen, Germany).
Complete gross postmortem examinations were conducted on all sacrificed rats and three rats were examined for histopathology in high-dose treated groups. Tissues of liver, kidney, and lung were taken from the same three parts of organs and prefixed in 10% (v/v) neutral buffered formaldehyde for histopathological examination. Fixed tissues were trimmed, dehydrated, embedded in paraffin, sectioned, mounted on glass slides, stained with hematoxylin and eosin, and examined by light microscopy.
Statistical analyses
Results are presented as mean ± standard deviation (SD). Differences in parameters between treated groups and control group were assessed by standard two-way analysis of variance (ANOVA). If statistical significance was observed, Duncan’s or Dunnett’s multiple range test was used for comparative analyses among the groups using SPSS version 10.1 K (SPSS, Chicago, IL, USA). A p value < 0.05 was considered statistically significant.
Results
Size distribution of CeNPs in preparation for dosing
Size distributions of CeNPs in deionized water and in 10% FBS are shown in Table 1. Average diameter of CeNPs ranged from 69.2 ± 11.5 to 98.1 ± 7.2 (nm) in deionized water. Nanoparticles in water were stable. When nanoparticles in deionized water were mixed with fetal bovine serum (1:9) to simulate distribution of CeNPs in rat blood after intravenous injection, nanoparticles were also well dispersed without aggregation, and average size ranged from 56.4 ± 48.7 to 201.7 ± 44.0 nm.
Tissue distribution and excretion of Ce after single treatment of CeNPs
Ce concentrations of rat samples measured by ICP-AES at 24 h after oral administration or intravenous injection of CeNPs are shown in Table 2. Blood concentrations of Ce were significantly elevated in intravenously injected rats. Levels were 0.8 ± 0.3 μg/g in low dose and 5.9 ± 2.9 μg/g in high dose, while Ce was not detected in rats treated by oral administration. Even in rats orally treated with high dose of CeNPs (300 mg/kg), no elevation was observed. Like blood level, Ce in tissues including liver, kidneys, lung and spleen were not detected at 24 h after oral administration of CeNPs. However, increased distribution was observed in the high-dose group and the low-dose group after intravenous injection of CeNPs. In all tested tissues including liver, kidney, lung, and spleen, dose-dependent elevations of Ce level were observed. Ce level in the liver after injection was 3741.7 ± 932.7 μg/g in the high-dose group and 594.9 ± 95.3 μg/g in the low-dose group. As shown in Table 2, Ce was also highly distributed in spleen as well as liver. Ce was detected at a level of 3474.1 ± 1230.7 μg/g in high-dose group and 442.0 ± 441.9 μg/g in spleen. Liver and spleen were major targets of CeNPs. Ce was also detected in lungs after intravenous injection but it was about one-tenth level compared to the liver and spleen. Among organs, kidney was a minor target after intravenous injection, compared to the other organs. Ce levels in feces and urine are also shown in Table 2. Absorption of orally administered CeNPs through the gastrointestinal tract was very poor, but was excreted in feces. Ce was detected at a level of 4925.2 ± 3312.9 μg/g in feces of high-dose group and 929.0 ± 515.0 μg/g in low-dose group. The amount of Ce excreted in feces was very high compared to intravenous injection, that means CeNPs would not be absorbed in the gastro-intestinal tract. The rest of CeNPs was still in the gastro-intestinal tract at 24 h after treatment. Small amount of Ce was also detected in feces of rats treated by intravenous injection, considerably low compared to those of oral administration. Excretion via feces after injection suggested biliary excretion of CeNPs. Compared to feces excretion, urine excretion after oral administration and intravenous injection was almost negligible and did not reveal meaningful difference from the vehicle treated control level.
Hematology and serum biochemistry
Hematological and serum biochemical findings in rats sacrificed at 24 h after treatment are shown in Tables 3 and 4, respectively. Although Ce was not detected in blood after oral administration, statistical reduction in the number of white blood cells and lymphocytes were observed. However, other hematological changes were not observed in orally treated rats. In intravenously treated rats, significant reduction of platelet number was observed in the high-dose group. Neutrophil % was increased and lymphocytes % was reduced in the low-dose group and high-dose group, respectively. White blood cells and lymphocyte were also reduced as shown in the orally treated group (Table 3). Results of serum biochemical analysis in rats sacrificed 24 h after CeNPs treatment are summarized in Table 4. Significant changes were not observed in serum biochemical parameters in the orally treated group. Serum parameters in the intravenous treated group as well as orally treated group, were not changed except for A/G ratio. In the high dose-group of intravenous injection, slight reduction of A/G ratio from 1.8 ± 0.1 in control group to 1.6 ± 0.1 in treated group was shown. However, the reduced level does not have toxicologically meaning. Coagulation parameters were also checked and shown in Table 5. As shown in the table, prothrombin time (PT) was increased in high-dose of orally treated group (15.5 ± 0.8 s) compared to the control group (14.1 ± 0.4 s). In case of intravenous injected group, it was increased in low-dose group (16.2 ± 0.7 s) and in high-dose group (17.4 ± 0.9 s). However, activated partial thromboplastin time (APTT) was not changed in the two different treated groups.
Histopathological findings
A histopathological examination was conducted in the control group and the high-dose group. A summary of histological findings is presented in Table 6. In liver, mild infiltration of mononuclear cells was observed in the control group (3/3), orally treated group (2/3), and intravenously treated group (1/3). It is not the effect of CeNPs because cell infiltration was also shown in the non-treated control group. Compared to the control group (0/3) and orally treated group (1/3), hepatocytic vacuolization significantly increased in the intravenously treated group (3/3). Greenish materials were observed in hepatocyte, Kupffer cells and sinusoids of the intravenous treated group, suggesting presence of CeNPs. Focal necrosis was also observed in the one rat of intravenous treated group, while no evidence of necrosis was found in the control group and orally treated group. Representative histopathological image of liver in intravenous group was shown in Fig. 1. In the kidney, greenish material deposit (which seems to be the accumulated CeNPs) were observed only in the intravenous treated group (3/3) as in liver, while no evidence was revealed in the control group and orally treated group (Fig. 2). Greenish deposits were found in blood monocytes and in glomerular capillaries. Multifocal mineralization was revealed only in the intravenous treated group (2/3). No specific lesion in kidney was found in the orally treated group. In the lung as well as liver and kidney, greenish material deposits were observed in alveolar walls and monocytes. Eosinophil crystals and alveolar injury were found with necrotic cell debris (Fig. 3). No specific lesion was found in the control group and orally treated group.
Discussion
Recent studies on bioavailability, biodistribution and kinetics of nanoparticles have rapidly increased because many types of nanoparticles have potential development of biomedicine (Khlebtsov and Dykman 2011; Ruiz et al. 2013; Arami et al. 2015). To exert therapeutic effect or toxicological effect, nanoparticles should be absorbed and distributed to target organs. However, many studies using oral administration of nanoparticles have not demonstrated appropriate pharmacological or toxicological effects and it is the poor bioavailability. Recently, studies of nanoparticles using intravenous routes have been increasingly conducted for target organ distribution and toxico-pharmacological effects (Fraga et al. 2014; Disdier et al. 2015; Elgrabli et al. 2015). When dextran-coated silver nanoparticles were intravenously injected into a rabbit, they were mainly accumulated in liver/spleen region (Ashraf et al. 2015). Organ distribution of gold nanoparticles are influenced by surface charge when treated by intravenous injection (Elci et al. 2016). When rats were treated with nanoparticles by oral administration, levels of target distribution were very low compared to intravenous injection (Park et al. 2011; Choi et al. 2015). As mentioned above, toxicity of nanoparticles appeared in the groups treated with high-dosage of nanoparticles after oral administration (Kim et al. 2013, 2014; Kumari et al. 2014). It was because appropriate administration routes for bioavailability was not considered that systemic circulation and target organ distribution would not have occurred.
Although biomedical applications of ceria nanoparticles were reported and information on in vivo target organ distribution is essential, only a few reports on distribution of ceria nanoparticles in target organs have been produced. Reports are insufficient to ensure bioavailability of orally administered CeNPs. It was reported that radiolabeled CeNPs accumulated mainly in lungs, spleen, and liver.
The physico-chemical properties of CeNPs in gastric juices after oral administration may be rapidly changed. When CeNPs entered stomach, they will be in acidic condition and may be aggregated to form big micro-sized particles. Then, CeNPs would not be absorbed through gastrointestinal tract. However, the size-change in 10% fetal bovine serum is not serious and they are still in nono-size (Table 1). It means that the physico-chemical properties of CeNPs in blood after intravenous administration would be rather stable. As shown in Table 2, CeNPs highly accumulated in liver and spleen. Total amount of accumulated in liver (average weight; 7.59 g) can be calculated as 28.4 mg in high-dose group and 4.5 mg in low-dose group. In case of spleen (average weight; 0.49 g), total amount of ceria can be calculated as 1.7 mg in high-dose group and 0. 2 mg in low-dose group. Although concentration of Ce was almost same in liver and spleen, total accumulated Ce was significantly higher in liver than in spleen. In liver, approximately half (47.3%) of total injected CeNPs in high-dose group (60 mg CeNPs in 200 g body weight) was accumulated and more than half (75.0%) of total injected CeNPs in low-dose group (6 mg CeNPs in 200 g body weight) was accumulated. In case of oral administration, CeNPs were not distributed in all tested targets including liver, kidneys, spleen, lung and liver. But most treated CeNPs were found in feces; 4.93 mg/g in high-dose group and 0.93 mg/g in the low dose-group. Total amount of CeNPs was calculated as 15.2 mg in feces of high-dose group at 24 h after treatment, approximately 25% of orally administered CeNPs. In the low-dose group, total amount of CeNPs in feces of high-dose group at 24 h after treatment was calculated at 1.30 mg, approximately 21.7% of orally administered CeNPs. Feces were collected for 1 day and total amount was calculated (average weight: 3.1 g in high-dose group, 3.5 g in low-dose group). Only 22–25% of total CeNPs treated by oral administration was excreted through feces. More than 70% of total administered CeNPs still remained at 1 day in the gastro-intestinal tract. CeNPs remaining in the body shall be excreted during the next few days. Urine excretion was negligible in our test. As shown in Table 2, relative small amount of CeNPs was excreted through feces after intravenous injection. Although levels were very minor compared to feces, it suggests the possibility of bile excretion of CeNPs.
Regarding toxicity data in Table 3, total white blood cells, platelet and lymph were reduced but neutrophils were increased in the intravenously treated group. Compared to orally treated groups, more changes in hematological parameters were found in the intravenously treated group. Changes in immune/inflammatory cells such as lymphocytes, neutrophils may suggest certain adverse effects. Changes of white blood cells (WBC) count was also in oral treated groups as well as intravenous treated groups. As described before, bioavailability of CeNPs was almost negligible after oral administration. At this moment, we do not understand the meaning of WBC changes in oral treated groups. More information on blood coagulation system needs to be obtained because increased prothrombin time (Table 5) and platelet reduction were revealed in the intravenous treated group (Table 3). Unexpectedly, no meaningful changes in serum biochemical parameters were observed. Although A/G ration was reduced statistically in the injected high-dose group, it does not have physiological meaning (Table 4). AST and ALT levels were not significantly increased although the Ce level in liver was elevated and confirmed in histopathological analysis. It suggested that CeNPs did not strong toxicity in vivo, compared to other nanoparticles such as silver nanoparticles. As shown in Table 2, blood concentration of CeNPs are relatively considerably low compared to target organs including liver, spleen, lung and kidney at 24 h after treatment. CeNPs are easily distributed to target organs that have high volume of distribution. Low blood concentration of CeNPs in this study may have minor effects on blood/serum parameters.
Histopathological analysis of CeNPs-treated groups was summarized in Table 6. Compared to other organs, livers from intravenous treated rats revealed the adverse effects including vacuolization. However, the histopathological changes in the major target organ were not meaningful in orally treated group and intravenous treated group, even-though the CeNPs levels were significantly higher in intravenously treated group.
In summary, bioavailability of CeNPs was negligible in orally treated rats and no toxicologically meaningful effects were observed, while systemic circulation and distribution to target organs were validated to reveal toxicological effects in the intravenously treated group.
References
Ali D, Alarifi S, Alkahtani S, AlKahtane AA, Almalik A (2015) Cerium oxide nanoparticles induce oxidative stress and genotoxicity in human skin melanoma cells. Cell Biochem Biophys 71:1643–1651
Arami H, Khandhar A, Liggitt D, Krishnan KM (2015) In vivo delivery, pharmacokinetics, biodistribution and toxicity of iron oxide nanoparticles. Chem Soc Rev 44:8576–8607
Ashraf A, Sharif R, Ahmad M, Masood M, Shahid A, Anjum DH, Rafique MS, Ghani S (2015) In vivo evaluation of the biodistribution of intravenously administered naked and functionalised silver nanoparticles in rabbit. IET Nanobiotechnol 9:368–374
Choi J, Kim H, Kim P, Jo E, Kim HM, Lee MY, Jin SM, Park K (2015) Toxicity of zinc oxide nanoparticles in rats treated by two different routes: single intravenous injection and single oral administration. J Toxicol Environ Health A 78:226–2243
Disdier C, Devoy J, Cosnefroy A, Chalansonnet M, Herlin-Boime N, Brun E, Lund A, Mabondzo A (2015) Tissue biodistribution of intravenously administrated titanium dioxide nanoparticles revealed blood-brain barrier clearance and brain inflammation in rat. Part Fibre Toxicol 12:27
Elci SG, Jiang Y, Yan B, Kim ST, Saha K, Moyano DF, Yesilbag Tonga G, Jackson LC, Rotello VM, Vachet RW (2016) Surface charge controls the suborgan biodistributions of gold nanoparticles. ACS Nano 10:5536–5542
Elgrabli D, Beaudouin R, Jbilou N, Floriani M, Pery A, Rogerieux F, Lacroix G (2015) Biodistribution and clearance of TiO2 nanoparticles in rats after intravenous injection. PLoS ONE 10:e0124490
Fraga S, Brandão A, Soares ME, Morais T, Duarte JA, Pereira L, Soares L, Neves C, Pereira E, Bastos Mde L, Carmo H (2014) Short- and long-term distribution and toxicity of gold nanoparticles in the rat after a single-dose intravenous administration. Nanomedicine 10:1757–1766
Hong JS, Kim S, Lee SH, Jo E, Lee B, Yoon J, Eom IC, Kim HM, Kim P, Choi K, Lee MY, Seo YR, Kim Y, Lee Y, Choi J, Park K (2014) Combined repeated-dose toxicity study of silver nanoparticles with the reproduction/developmental toxicity screening test. Nanotoxicology 8:349–362
Khlebtsov N, Dykman L (2011) Biodistribution and toxicity of engineered gold nanoparticles: a review of in vitro and in vivo studies. Chem Soc Rev 40:1647–1671
Kim JS, Song KS, Sung JH, Ryu HR, Choi BG, Cho HS, Lee JK, Yu IJ (2013) Genotoxicity, acute oral and dermal toxicity, eye and dermal irritation and corrosion and skin sensitisation evaluation of silver nanoparticles. Nanotoxicology 7:953–960
Kim YR, Park JI, Lee EJ, Park SH, Seong NW, Kim JH, Kim GY, Meang EH, Hong JS, Kim SH, Koh SB, Kim MS, Kim CS, Kim SK, Son SW, Seo YR, Kang BH, Han BS, An SS, Yun HI, Kim MK (2014) Toxicity of 100 nm zinc oxide nanoparticles: a report of 90-day repeated oral administration in Sprague Dawley rats. Int J Nanomedicine 9(Suppl 2):109–126
Kolli MB, Manne NDPK, Para R, Nalabotu SK, Nandyala G, Shokuhfar T, He K, Hamlekhan A, Ma JY, Wehner PS, Dornon L, Arvapalli R, Rice KM, Blough ER (2014) Cerium oxide nanoparticles attenuate monocrotaline induced right ventricular hypertrophy following pulmonary arterial hypertension. Biomaterials 35:9951–9962
Kumari M, Kumari SI, Grover P (2014) Genotoxicity analysis of cerium oxide micro and nanoparticles in Wistar rats after 28 days of repeated oral administration. Mutagenesis 29:467–479
Lee Y, Kim P, Yoon J, Lee B, Choi K, Kil KH, Park K (2013) Serum kinetics, distribution and excretion of silver in rabbits following 28 days after a single intravenous injection of silver nanoparticles. Nanotoxicology 7:1120–1130
Minarchick VC, Stapleton PA, Fix NR, Leonard SS, Sabolsky EM, Nurkiewicz TR (2015) Intravenous and gastric cerium dioxide nanoparticle exposure disrupts microvascular smooth muscle signaling. Toxicol Sci 144:77–89
Nalabotu SK, Kolli MB, Triest WE, Ma JY, Manne ND, Katta A, Addagarla HS, Rice KM, Blough ER (2011) Intratracheal instillation of cerium oxide nanoparticles induces hepatic toxicity in male Sprague-Dawley rats. Int J Nanomedicine 6:2327–2335
Park K, Park EJ, Chun IK, Choi K, Lee SH, Yoon J, Lee BC (2011) Bioavailability and toxicokinetics of citrate-coated silver nanoparticles in rats. Arch Pharm Res 34:153–158
Patra P, Mitra S, Debnath N, Goswami A (2012) Biochemical-, biophysical-, and microarray-based antifungal evaluation of the buffer-mediated synthesized nano zinc oxide: An in vivo and in vitro toxicity study. Langmuir 28:16966–16978
Peng L, He X, Zhang P, Zhang J, Li Y, Zhang J, Ma Y, Ding Y, Wu Z, Chai Z, Zhang Z (2014) Comparative pulmonary toxicity of two ceria nanoparticles with the same primary size. Int J Mol Sci 15:6072–6085
Pešić M, Podolski-Renić A, Stojković S, Matović B, Zmejkoski D, Kojić V, Bogdanović G, Pavićević A, Mojović M, Savić A, Milenković I, Kalauzi A, Radotić K (2015) Anti-cancer effects of cerium oxide nanoparticles and its intracellular redox activity. Chem Biol Interact 232:85–93
Poma A, Ragnelli AM, de Lapuente J, Ramos D, Borras M, Aimola P, Di Gioacchino M, Santucci S, De Marzi L (2014) In vivo inflammatory effects of ceria nanoparticles on CD-1 mouse: evaluation by hematological, histological, and TEM analysis. J Immunol Res 2014:361419
Rocca A, Moscato S, Ronca F, Nitti S, Mattoli V, Giorgi M, Ciofani G (2015) Pilot in vivo investigation of cerium oxide nanoparticles as a novel anti-obesity pharmaceutical formulation. Nanomedicine 11:1725–1734
Ruiz A, Hernández Y, Cabal C, González E, Veintemillas-Verdaguer S, Martínez E, Morales MP (2013) Biodistribution and pharmacokinetics of uniform magnetite nanoparticles chemically modified with polyethylene glycol. Nanoscale 5:11400–11408
Tseng MT, Lu X, Duan X, Hardas SS, Sultana R, Wu P, Unrine JM, Graham U, Butterfield DA, Grulke EA, Yokel RA (2012) Alteration of hepatic structure and oxidative stress induced by intravenous nanoceria. Toxicol Appl Pharmacol 260:173–182
Tseng MT, Fu Q, Lor K, Fernandez-Botran GR, Deng ZB, Graham U, Butterfield DA, Grulke EA, Yokel RA (2014) Persistent hepatic structural alterations following nanoceria vascular infusion in the rat. Toxicol Pathol 42:984–996
Wang Q, Ebbs SD, Chen Y, Ma X (2013) Trans-generational impact of cerium oxide nanoparticles on tomato plants. Metallomics 5:753–759
Acknowledgements
This study was financially supported by National Institute of Environmental Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Kwangsik Park, Juyoung Park, Handule Lee, Jonghye Choi, Wook-Joon Yu and Jinsoo Lee declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Park, K., Park, J., Lee, H. et al. Toxicity and tissue distribution of cerium oxide nanoparticles in rats by two different routes: single intravenous injection and single oral administration. Arch. Pharm. Res. 41, 1108–1116 (2018). https://doi.org/10.1007/s12272-018-1074-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-018-1074-7