Abstract
Ginseng’s major active components, ginsenosides, have been known to show anti-cancer, neuroprotective, and anti-inflammatory activities. Ultrasonication processed Panax ginseng berry extract (UGB) contains various ginsenosides. The components are different from Panax ginseng berry extract (GBE). This study was aimed to investigate the cytotoxic mechanism of UGB in HepG2 cells, human hepatocellular carcinoma cell line. HepG2 cells were treated with UGB (0, 10, 20 μg/ml). Cell growth and cellular apoptosis were evaluated by MTT assay and Annexin V/Pi staining, respectively. Intracellular Reactive oxygen species (ROS) levels were also determined by 2′, 7′-dichlorofluorescin diacetate (DCFDA) staining. The expressions of Bax, Bcl-2 and caspase-3, the apoptotic markers, were evaluated by Western Blot. UGB dose-dependently inhibited cell growth and induced apoptotic cell death. Intracellular ROS levels were increased. UGB increased the expression of the cleaved form of caspase-3. Furthermore, UGB induced apoptosis of HepG2 cells through Bax activation and Bcl-2 inhibition. In conclusion, UGB induced apoptosis through an intrinsic pathway in HepG2 cells suggesting that UGB might play a role as a novel substance for anti-cancer effect.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Apoptosis, the process of programmed cell death is known to play a vital role in various processes such as immune system functioning or cell death. Due to the ability to modulate the cell’s life cycle, apoptosis is believed to have immense therapeutic potential (Elmore 2007).
Signals including viral infection, radiation and chemical induce apoptosis (Steller 1995). Apoptosis can be induced through 2 pathways, the extrinsic pathway which is associated with activation of cell surface death receptors and the intrinsic pathway which is associated with mitochondria (Debatin and Krammer 2004; Elrod and Sun 2008). Apoptosis starts when various stimuli activate apoptosis pathways including the intrinsic pathway. Activation of intrinsic apoptosis pathway leads to release of apoptotic mediators from mitochondria. The Bcl-2 family, including anti- and pro-apoptotic proteins, is key regulator in intrinsic apoptosis pathway (Park et al. 2014). Little change in the balance of Bcl-2 family proteins may cause promotion or inhibition of cell death (Shroff et al. 2007; Ola et al. 2011).
In this study, Bax was evaluated as pro-apoptotic protein and Bcl-2 was studied as anti-apoptotic protein among Bcl-2 family proteins. Initiation of apoptotic signaling makes Bax undergo conformational shift and become mitochondria membrane-associated organelle. Activated Bax induces the activation of caspase-3 (Hsu et al. 1997; Wolter et al. 1997; Gross et al. 1998; Nechushtan et al. 1999; Kim and Jin 2004). Bcl-2 inhibits cell death which is induced by diverse stimuli such as chemotherapeutic agents and this indicate Bcl-2 as cell death negative regulator. Generally, the ratio of pro-apoptotic proteins to anti-apoptotic proteins is known to be important in regulation of the resistance to apoptotic stimuli or cell sensitivity (Ola et al. 2011). Released apoptotic mediators from mitochondria can induce the activation of caspase-3, which is known to play a central role in the execution phase of cell apoptosis (Hengartner 2000; Mao et al. 2013). Reactive oxygen species (ROS) generation is a well-known factor that induces apoptosis. Studies have indicated that ROS control the expression of Bcl-2 family proteins. It has been revealed that Bcl-2 expression is suppressed by ROS while Bax expression is increased by ROS (Li et al. 2004).
Ginsenosides are known to show anti-cancer, neuroprotective, and anti-inflammatory activities (Tode et al. 1993; Kim et al. 1999; Oh et al. 2004; Leung et al. 2007; Kim et al. 2008; Zhang et al. 2012; Chen et al. 2013). Ultrasonication processed Panax ginseng berry extract (UGB) contains various ginsenosides. Ginsenoside Rh1, Rg3, Rg2 and F4 are main components of UGB. There are less Ginsenoside Rh1, Rg3, Rg2 and F4 in Panax ginseng berry extract (GBE). Ginsenoside Rh1 is known to prevent various inflammations containing allergy (Park et al. 2004). Rg3 is known to show antitumor, anti-diabetic and anti-oxidant activities (Xu et al. 2008; Sun and Zhou 2010; Wei et al. 2012; Zhang et al. 2012). Rg2 is a ginsenoside which have memory-protective and neuroprotective activities (Li et al. 2007; Zhang et al. 2008; Shuangyan et al. 2012). F4 is known to induce apoptosis (Chen et al. 2013). Ratios of ginsenoside Rg3, F4, and Rk1 which have anti-cancer activities are higher in UGB than those in GBE.
In this study, it was hypothesized that UGB may induce HepG2 cell apoptosis. If the hypothesis is possible, cell apoptosis induced by the UGB may be associated with the intrinsic apoptosis pathway. Levels of intracellular ROS production and expressions of Bcl-2, Bax, and cleaved caspase-3 proteins were evaluated.
Materials and methods
Reagents
UGB was kindly obtained from Professor Sung Kwon Ko, Semyung University. Fetal bovine serum (FBS), Dulbecco’s modified Eagle’s medium (DMEM) and phenol red free DMEM were purchased from Gibco (Carlsbad, CA, USA). Antibiotic–antimycotic (streptomycin, amphotericin B, penicillin) and trypsin-ethylenediamine tetra acetic acid (EDTA) were purchased from Invitrogen (Grand Island, NY, USA). Bovine serum albumin (BSA) was purchased from Bio-Technology (St Louis, MO, USA). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT), Hank’s balanced salt solution-modified (HBSS), 2′,7′-dichlorofluorescin diacetate (DCFDA), 4-(2-hydroxyethyle)-1-piperazine-N′-2-ethane sulfonic acid (HEPES), leupeptin, aprotini, phenylmethyl-sulfonylfluoride (PMSF), ethylene glycol-bis-(β-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA), EDTA, β-mercaptoethanol, were purchased from Sigma Chemical Co (St. Louis, MO, USA). Phosphate-buffered saline (PBS) is purchased from Welgene Inc. (Daegu, South Korea). Annexin V-FITC Apoptosis Detection Kit was purchased from eBioscience (San Diego, CA, USA). Bax, Bcl-2, Actin antibodies were purchased from Santa Cruz Biotechnology (Delaware, CA, USA). Caspase-3 antibody was purchased from Cell signaling (Beverly, MA, USA). Goat anti-rabbit IgG-HRP, rabbit anti-goat IgG-HRP were purchased from Bethyl (Montgomery, TX, USA). Enhanced chemiluminescence (ECL) agents were purchased from PerkinElmer Life Sciences (Boston, MA, USA). Ammonium persulfate, N,N,N′,N′-tetramethylethylene diamine (TEMED), nitrocellulose (NC) membrane, Tris/Glycine/SDS buffer, Tris/Glycine buffer, and 30 % acrylamide/bis solution were purchased from BioRad (Richmond, CA, USA).
Preparation and analysis of ultrasonication-processed P. ginseng berry extract (UGB)
UGB and GBE water solvent extracts were kindly provided by professor Sung Kwon Ko of Semyung University. We analyzed the constituents of UGB and GBE using a Waters 1525 binary high-performance liquid chromatography (HPLC) system (Waters, US). The separation of UGB was performed on an analytical column (Eurospher, 100-5 C18, 250 mm × 3.0 mm, 5 μm, Knauer, Germany) by gradient elution at room temperature. The process of elution was carried out according to the following conditions: 0 min, 17 % of A; 25 min, 25 % of A; 50 min, 40 % of A; 105 min, 60 % of A; 110 min, 100 % of A. The flow rate was 0.8 ml/min, the injection volume was 20 μl and the chromatograms were obtained using a uv/vis Waters 2478 Dual λ Absorbance Detector (Water, USA) at 203 nm.
Cell culture
The human liver carcinoma cell line, HepG2 (KCLB 88065), was purchased from Korean Cell Line Bank (Seoul, Korea). Cells were grown in DMEM supplemented with 10 % FBS, 1 % Antibiotic–antimycotic. The cells were cultured in a humidified CO2 incubator at 37 °C. HepG2 cells were seeded in 6-well dishes and when the adherent cells became confluent, the cells were treated with UGB for 24 h.
Cell viability assay
The cell viability was assessed using MTT reduction assay. HepG2 cells were plated at a density of 2 × 105/well in 6-well plates and maintained in DMEM containing 10 % FBS and 1 % Antibiotic–antimycotic at 37 °C under microaerophilic conditions for 24 h. The media was changed with serum-free DMEM and incubated for 24 h to silence gene activity and arrest cell growth, followed by treatment with UGB (5, 10, 15, 20, 25, 50, 75 µg/ml) for 24 h. After the treatment HepG2 cells were incubated with MTT solution (final, 5 mg/ml) for 4 h in a humidified CO2 incubator at 37 °C. After the incubation, the supernatant was removed and DMSO was treated. Absorbance was assessed at 590 nm with a microplate reader (Molecular Devices, Sunnyvale, CA, USA).
Annexin-V staining assay
After the treatment with UGB (10, 20 µg/ml), HepG2 cells were collected and suspended in 1 ml Annexin V binding buffer containing 5 µL of propidium iodide (PI) and Annexin V-FITC. Using a fluorescence-activated cell sorting (FACS) Calibur™ flow cytometer equipped with Cell Quest Pro software (Becton–Dickinson Immunocytometry System; Franklin Lakes, NJ, USA), the fluorescence was measured. The fluorescence data was collected from 10,000 cells.
Measurement of ROS generation
Levels of intracellular ROS were estimated by DCFDA staining assay. After the treatment with UGB (0, 10, 20 µg/ml), HepG2 cells were incubated with 20 µM carboxy-H2DCFDA in a humidified CO2 incubator at 37 °C. The fluorescence was measured using FACS. The fluorescence intensity is proportional to the level of ROS produced by the cells. The fluorescence data was collected from 10,000 cells.
Western blot analysis
After the treatment with UGB (0, 10, 20 µg/ml), protein samples of each well were collected. Same amounts of proteins from samples were separated using SDS–polyacrylamide. Then the gels (Laemmli’s SDS-PAGE 10 %) were transferred to NC membranes by Power Pac 1000 (Bio-Rad, Melville, NY, USA). For blocking nonspecific bindings, the membranes were incubated in nonfat dry milk. After the incubation, the membranes were incubated 24 h at 4 °C with primary antibodies raised against cleaved caspase-3, Bax, Bcl-2. After the incubation with the primary antibodies, the membranes were incubated for 2 h with the secondary antibodies. By ECL agent, the immune-reactive proteins were detected. The expression of Actin was also detected to confirm the uniformity of protein loading. The results were analyzed using Quantity One analysis software (Bio-Rad, Richmond, CA, USA). The percentages of the expressions against control bands were calculated.
Analysis of data
Data represent the mean values from triplicate measurements. The data was expressed as the mean ± S.E.M. and analyzed with a Student’s t test. A P < 0.05 was considered statistically significant.
Results
The phytochemistry of UGB
Table 1 shows the composition ratio of the GBE and UGB analyzed by the HPLC systems. The ginsenosides Rg2, Rg3, Rh1, Rh4, Rk1, Rk3 and F4 were significantly increased after ultrasonication. Among these ginsenosides, the composition ratio of ginsenosides Rg2, Rh1 and F4 was largely increased from that produced during red ginseng’s process of manufacture. Furthermore, ginsenosides Rg3 and Rk1 were newly produced in UGB, which were not identified in GBE.
The growth of HepG2 cell was suppressed by ultrasonication processed Panax ginseng berry extract
To estimate the effects of UGB on HepG2 cell viability, HepG2 cells were treated with UGB (5, 10, 15, 20, 25, 50, 75 µg/ml) for 24 h. After the treatment, cell viability was evaluated by using MTT assay (Fig. 1). UGB dose dependently decreased the cell viability of HepG2 cell. Cell viability was approximately 50 % when HepG2 cells were treated with 20 µg/ml UGB.
Effect of ultrasonication processed Panax ginseng berry extract on cell viability in HepG2 cells. Cell viability was evaluated using the MTT assay. HepG2 cells were treated with various concentrations of UGB for 24 h. After the treatment, cells were incubated with MTT solution for 4 h. After the incubation, the supernatant was removed and DMSO was treated. Absorbance was assessed at 590 nm with a microplate reader. **P < 0.01 indicates significant differences compared to the control group
The apoptosis level of HepG2 cell was raised by ultrasonication processed Panax ginseng berry extract
By fluorometric analysis after annexin V/PI staining, apoptosis induced by UGB was quantified. Apoptosis ratio of control HepG2 cells was relatively low (3.85 %) (Fig. 2). The ratio of apoptosis dose dependently increased when treated with UGB (10 µg/ml: 15.3 %, 20 µg/ml: 41.4 %).
Effect of ultrasonication processed Panax ginseng berry extract on cell apoptosis in HepG2 cells. HepG2 cell apoptosis was evaluated using the Annexin V-FITC/PI double staining assay. HepG2 cells were treated with a 0, b 10, c 20 µg/ml of UGB for 24 h. After the treatment, cells were treated with suspended in 1 ml Annexin V binding buffer containing 5µL of propidium iodide and Annexin V-FITC. The degree of cell apoptosis was quantified by measuring fluorescence with a FACS Calibur™ flow cytometer
The level of intracellular ROS was raised by ultrasonication processed Panax ginseng berry extract
To estimate the level of intracellular ROS by flow cytometry, carboxy-H2DCFDA, a ROS sensitive fluorometric probe, was used (Fig. 3). The levels of intracellular ROS of HepG2 cells were raised dose dependently when treated with UGB (10 µg/ml: 148.9 % 20 µg/ml: 151.1 % compared to control).
Effect of ultrasonication processed Panax ginseng berry extract on intracellular ROS level in HepG2 cell. Intracellular ROS level in HepG2 cell was evaluated by DCFDA staining assay. HepG2 cells were treated with a 0, b 10, c 20 µg/ml of UGB for 24 h. After the treatment, HepG2 cells were incubated with 20 µM carboxy-H2DCFDA. The degree of intracellular ROS level was evaluated by measuring fluorescence with FACS. The fluorescence was measured using FACS. The fluorescence intensity is proportional to the level of ROS produced by the cells. a ROS production in the differently treated group of UGB. b ROS production shown using bar diagrams. **P < 0.01 indicates a significant difference compared to the control group
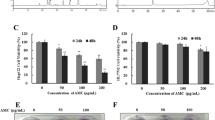
The expression level of cleaved caspase-3 was raised by ultrasonication processed Panax ginseng berry extract
In the intrinsic apoptosis pathway caspase-3 plays vital role downstream of the Bcl-2 family during apoptosis by initiating cellular breakdown. To investigate whether UGB treatment activates caspase-3, western blot assay was used (Fig. 4). The expression level of cleaved caspase-3 protein was raised dose dependently after treatment with UGB (10 µg/ml: 155.5 % 20 µg/ml: 220.3 % compared to control).
Effects of ultrasonication processed Panax ginseng berry extract on expression of cleaved caspase-3 protein in HepG2 cells. Expression of cleaved caspase-3 protein in HepG2 cell was evaluated by Western Blot assay. HepG2 cells were treated with 0, 10, and 20 µg/ml of UGB for 24 h. After the treatment, cell proteins were harvested for Western Blot to examine the cleaved caspase-3 expression level. Quantitative caspase-3 expression level was normalized to Actin. *P < 0.05, **P < 0.01 indicate significant differences compared to the control group
The Bax/Bcl-2 ratio was decrease by ultrasonication processed Panax ginseng berry extract
The Bcl-2 family, which includes Bcl-2 and Bax, regulates various steps of the intrinsic apoptosis pathway. Bcl-2 inhibits cell apoptosis but Bax promotes programmed cell death. To investigate whether UGB regulate the expression of apoptotic genes, expression levels of Bcl-2 and Bax in HepG2 cells were measured after the treatment with UGB (Figs. 5, 6) The expression level of Bcl-2 (normalized to Actin) was decreased dose dependently after treatment with UGB (10 µg/ml: 93.5 %, 20 µg/ml: 72.0 % compared to control), whereas the expression levels of Bax (normalized to Actin) were increased dose dependently after treatment with UGB (10 µg/ml: 181.0 %, 20 µg/ml: 216.9 % compared to control). Therefore the Bax/Bcl-2 ratio was dose-dependently increased after the treatment with UGB.
Effect of ultrasonication processed Panax ginseng berry extract on expressions of Bcl-2 protein and Bax protein in HepG2 cell. Expressions of Bcl-2 protein and Bax protein were evaluated by Western Blot assay. HepG2 cells were treated with 0, 10, and 20 µg/ml of UGB for 24 h. Cell proteins were harvested for Western Blot to examine the Bcl-2 expression level. Quantitative Bcl-2 expression levels were normalized to Actin. **P < 0.01 indicates significant differences compared to the control group
Discussion
It has been researched that various ginsenosides show anti-cancer, neuroprotective, and anti-inflammatory activities (Tode et al. 1993; Oh et al. 2004; Leung et al. 2007; Zhang et al. 2012; Chen et al. 2013; Choi et al. 2014; Lim et al. 2014). Main ginsenosides in UGB are ginsenoside Rh1, Rg3, Rg2 and F4. There are less ginsenoside Rh1, Rg3, Rg2 and F4 in GBE. Ginsenoside Rh1, main component of UGB, is known to show anti-allergic activity and anti-inflammatory activity (Park et al. 2004). Ginsenoside Rg3, which is also main component of UGB, is known to show anti-tumor, anti-diabetic, and anti-oxidant activities (Xu et al. 2008; Sun and Zhou 2010; Wei et al. 2012; Zhang et al. 2012). Ginsenoside Rg2 is known to show memory protective and neuroprotective activities (Li et al. 2007; Zhang et al. 2008; Shuangyan et al. 2012). Ginsenoside F4 is known to induce apoptosis (Chen et al. 2013). Ratios of ginsenoside Rg3, F4, and Rk1 which have anti-cancer activities are higher in UGB than those in GBE. In this study, in vitro evidence showing apoptosis effect of UGB through the intrinsic apoptosis pathway is provided. HepG2 cell, human hepatocellular carcinoma cell line, is used to study the relationship between UGB and cell apoptosis. This study reveals that UGB induces HepG2 cell apoptosis through the intrinsic apoptosis pathway.
Apoptosis is a process of programmed cell death. It has a vital role in cell number modulation (Fadeel and Orrenius 2005; Elmore 2007). The result from the MTT assay shows that UGB dose dependently decreases the cell growth of HepG2 cell (Fig. 2). In MTT assay in our study, the 20 µg/ml of UGB caused approximately 50 % decrease in cell viability compared to that of the control group. We evaluated the induction of HepG2 cell apoptosis by UGB through flow cytometry after Annexin V/PI staining in this study. The result shows that UGB induces HepG2 cell apoptosis dose dependently (Fig. 3). In annexin V/PI staining assay in our study, 20 µg/ml of UGB increased the ratio of apoptosis cells from 3.9 % (control) to 41.4 % (20 µg/ml) (Figs. 6, 7).
Apoptosis pathway in ultrasonication processed Panax ginseng berry extract-induced HepG2 cell apoptosis. When the HepG2 cells were treated with UGB, intracellular ROS levels were increased. Also, the expressions of anti-apoptotic Bcl-2 protein were decreased and the expressions of pro-apoptotic Bax protein were increased. The expressions of cleaved caspase-3 were increased. HepG2 cell growth was decreased. Finally, HepG2 cell apoptosis level was increased
Caspases and Bcl-2 family, apoptosis factors, are associated in modulating apoptosis process (Hengartner 2000; Fuchs and Steller 2011; Martinou and Youle 2011). By Western Blot assay, the expressions of cleaved caspase-3, Bax, Bcl-2 proteins were evaluated. Cleaved caspase-3 is known to play a central role in the execution phase of cell apoptosis (Porter and Janicke 1999; Hengartner 2000; Mao et al. 2013). The result shows that UGB dose dependently increased the expression of caspase-3 (Fig. 4) leading to apoptosis. In Western Blot assay in our study, 20 µg/ml of UGB caused approximately 120 % increase of the expression of cleaved caspase-3 protein compared to that of the control group.
The result of Western Blot assay used to evaluate the expression of Bcl-2 protein shows UGB dose dependently decreased the expression of Bcl-2 protein (Figs. 5, 6). In Western Blot assay in our study, 20 µg/ml of UGB induced approximately 30 % decrease of the expression of Bcl-2 protein compared to that of the control group.
The result of Western Blot assay used to evaluate the expression of Bax protein shows UGB dose dependently increased the expression of Bax protein (Fig. 6). In Western Blot assay in our study, 20 µg/ml of UGB caused approximately 120 % increase of the expression of Bax protein compared to that of the control group.
ROS is used in immune cells including phagocytic cells to support cell functions, so low level of ROS is important for survival (Boxer et al. 1979; Victor et al. 2004). However, high level of ROS can cause cell damage. It is known that ROS regulate the expression of Bcl-2 family proteins (Li et al. 2004). That is why we evaluated the levels of ROS in HepG2 cells. Levels of ROS were evaluated through DCFDA staining assay. The result of the assay shows UGB dose dependently increases the level of ROS production in HepG2 cell (Fig. 3). In DCFDA assay in our study, 20 µg/ml of UGB caused approximately 50 % increase of the ROS production level.
In summary, it was investigated whether UGB, which includes various ginsenosides, induces HepG2 cell apoptosis. Additionally, we investigated the signaling pathway associated with HepG2 cell apoptosis induced by UGB. The results show us that HepG2 cell apoptosis is induced by UGB and this apoptosis is associated with expression of caspase-3 protein, expression of Bcl-2 protein, expression of Bax, and levels of intracellular ROS production which are involved in the intrinsic apoptosis pathway (Fig. 7).
By modulating the cell’s life cycle, apoptosis is believed to have immense therapeutic potential (Elmore 2007). In various cancers, the failure to induce apoptotic cell death has been implicated. Many anticancer drugs have ability to induce apoptosis (Kim et al. 2003). That’s why the ability of UGB to induce apoptosis in HepG2 cell, the human liver carcinoma cell line, makes UGB as a novel substance for anti-cancer effect.
In conclusion, UGB induces apoptosis through the intrinsic pathway in HepG2 cells suggesting that UGB might play a role as a novel substance for anti-cancer effect.
References
Boxer LA, Harris RE, Baehner RL (1979) Regulation of membrane peroxidation in health and disease. Pediatrics 64:713–718
Chen B, Shen YP, Zhang DF, Cheng J, Jia XB (2013) The apoptosis-inducing effect of ginsenoside F4 from steamed notoginseng on human lymphocytoma JK cells. Nat Prod Res 27:2351–2354
Choi K, Yoon J, Lim HK, Ryoo S (2014) Korean red ginseng water extract restores impaired endothelial function by inhibiting arginase activity in aged mice. Korean J Physiol Pharmacol 18:95–101
Debatin KM, Krammer PH (2004) Death receptors in chemotherapy and cancer. Oncogene 23:2950–2966
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35:495–516
Elrod HA, Sun SY (2008) PPARgamma and apoptosis in cancer. PPAR Res 2008:704165
Fadeel B, Orrenius S (2005) Apoptosis: a basic biological phenomenon with wide-ranging implications in human disease. J Intern Med 258:479–517
Fuchs Y, Steller H (2011) Programmed cell death in animal development and disease. Cell 147:742–758
Gross A, Jockel J, Wei MC, Korsmeyer SJ (1998) Enforced dimerization of BAX results in its translocation, mitochondrial dysfunction and apoptosis. EMBO J 17:3878–3885
Hengartner MO (2000) The biochemistry of apoptosis. Nature 407:770–776
Hsu YT, Wolter KG, Youle RJ (1997) Cytosol-to-membrane redistribution of Bax and Bcl-X(L) during apoptosis. Proc Natl Acad Sci USA 94:3668–3672
Kim YS, Jin SH (2004) Ginsenoside Rh2 induces apoptosis via activation of caspase-1 and -3 and up-regulation of Bax in human neuroblastoma. Arch Pharm Res 27:834–839
Kim YS, Jin SH, Lee YH, Kim SI, Park JD (1999) Ginsenoside Rh2 induces apoptosis independently of Bcl-2, Bcl-xL, or Bax in C6Bu-1 cells. Arch Pharm Res 22:448–453
Kim HJ, Mun JY, Chun YJ, Choi KH, Ham SW, Kim MY (2003) Effects of a naphthoquinone analog on tumor growth and apoptosis induction. Arch Pharm Res 26:405–410
Kim YJ, Kwon HC, Ko H, Park JH, Kim HY, Yoo JH, Yang HO (2008) Anti-tumor activity of the ginsenoside Rk1 in human hepatocellular carcinoma cells through inhibition of telomerase activity and induction of apoptosis. Biol Pharm Bull 31:826–830
Leung KW, Yung KK, Mak NK, Chan YS, Fan TP, Wong RN (2007) Neuroprotective effects of ginsenoside-Rg1 in primary nigral neurons against rotenone toxicity. Neuropharmacology 52:827–835
Li D, Ueta E, Kimura T, Yamamoto T, Osaki T (2004) Reactive oxygen species (ROS) control the expression of Bcl-2 family proteins by regulating their phosphorylation and ubiquitination. Cancer Sci 95:644–650
Li N, Liu B, Dluzen DE, Jin Y (2007) Protective effects of ginsenoside Rg2 against glutamate-induced neurotoxicity in PC12 cells. J Ethnopharmacol 111:458–463
Lim HJ, Lee HY, Lim DY (2014) Inhibitory effects of ginsenoside-rb2 on nicotinic stimulation-evoked catecholamine secretion. Korean J Physiol Pharmacol 18:431–439
Mao Z, Xia W, Wang J, Chen T, Zeng Q, Xu B, Li W, Chen X, Xu S (2013) Perfluorooctane sulfonate induces apoptosis in lung cancer A549 cells through reactive oxygen species-mediated mitochondrion-dependent pathway. J Appl Toxicol 33:1268–1276
Martinou JC, Youle RJ (2011) Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev Cell 21:92–101
Nechushtan A, Smith CL, Hsu YT, Youle RJ (1999) Conformation of the Bax C-terminus regulates subcellular location and cell death. EMBO J 18:2330–2341
Oh GS, Pae HO, Choi BM, Seo EA, Kim DH, Shin MK, Kim JD, Kim JB, Chung HT (2004) 20(S)-Protopanaxatriol, one of ginsenoside metabolites, inhibits inducible nitric oxide synthase and cyclooxygenase-2 expressions through inactivation of nuclear factor-kappaB in RAW 264.7 macrophages stimulated with lipopolysaccharide. Cancer Lett 205:23–29
Ola MS, Nawaz M, Ahsan H (2011) Role of Bcl-2 family proteins and caspases in the regulation of apoptosis. Mol Cell Biochem 351:41–58
Park EK, Choo MK, Han MJ, Kim DH (2004) Ginsenoside Rh1 possesses antiallergic and anti-inflammatory activities. Int Arch Allergy Immunol 133:113–120
Park MY, Jeong YJ, Kang GC, Kim MH, Kim SH, Chung HJ, Jung JY, Kim WJ (2014) Nitric oxide-induced apoptosis of human dental pulp cells is mediated by the mitochondria-dependent pathway. Korean J Physiol Pharmacol 18:25–32
Porter AG, Janicke RU (1999) Emerging roles of caspase-3 in apoptosis. Cell Death Differ 6:99–104
Shroff EH, Snyder C, Chandel NS (2007) Role of Bcl-2 family members in anoxia induced cell death. Cell Cycle 6:807–809
Shuangyan W, Ruowu S, Hongli N, Bei Z, Yong S (2012) Protective effects of Rg2 on hypoxia-induced neuronal damage in hippocampal neurons. Artif Cells Blood Substit Immobil Biotechnol 40:142–145
Steller H (1995) Mechanisms and genes of cellular suicide. Science 267:1445–1449
Sun HQ, Zhou ZY (2010) Effect of ginsenoside-Rg3 on the expression of VEGF and TNF-alpha in retina with diabetic rats. Int J Ophthalmol 3:220–223
Tode T, Kikuchi Y, Hirata J, Kita T, Imaizumi E, Nagata I (1993) Inhibitory effects of oral administration of ginsenoside Rh2 on tumor growth in nude mice bearing serous cyst adenocarcinoma of the human ovary. Nihon Sanka Fujinka Gakkai Zasshi 45:1275–1282
Victor VM, Rocha M, De la Fuente M (2004) Immune cells: free radicals and antioxidants in sepsis. Int Immunopharmacol 4:327–347
Wei X, Su F, Su X, Hu T, Hu S (2012) Stereospecific antioxidant effects of ginsenoside Rg3 on oxidative stress induced by cyclophosphamide in mice. Fitoterapia 83:636–642
Wolter KG, Hsu YT, Smith CL, Nechushtan A, Xi XG, Youle RJ (1997) Movement of Bax from the cytosol to mitochondria during apoptosis. J Cell Biol 139:1281–1292
Xu TM, Cui MH, Xin Y, Gu LP, Jiang X, Su MM, Wang DD, Wang WJ (2008) Inhibitory effect of ginsenoside Rg3 on ovarian cancer metastasis. Chin Med J (Engl) 121:1394–1397
Zhang G, Liu A, Zhou Y, San X, Jin T, Jin Y (2008) Panax ginseng ginsenoside-Rg2 protects memory impairment via anti-apoptosis in a rat model with vascular dementia. J Ethnopharmacol 115:441–448
Zhang C, Liu L, Yu Y, Chen B, Tang C, Li X (2012) Antitumor effects of ginsenoside Rg3 on human hepatocellular carcinoma cells. Mol Med Rep 5:1295–1298
Acknowledgments
This research was supported by High Value-added Food Technology Development program, Ministry of Agriculture, Food and Rural Affairs (113021-03).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that there are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Jung, H., Bae, J., Ko, S.K. et al. Ultrasonication processed Panax ginseng berry extract induces apoptosis through an intrinsic apoptosis pathway in HepG2 cells. Arch. Pharm. Res. 39, 855–862 (2016). https://doi.org/10.1007/s12272-016-0760-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-016-0760-6