Abstract
Sappanchalcone, a bioactive flavonoid isolated from the heartwood of Caesalpinia sappan L. possesses anti-inflammatory effects. We studied the efficacy of sappanchalcone in attenuating collagen-induced arthritis (CIA) in a mouse model of rheumatoid arthritis. Sappanchalcone was purified to homogeneity from the chloroform fraction of the methanolic extract of C. sappan, and identified using mass spectrometry and 1H-nuclear magnetic resonance spectroscopy. CIA-induced male DBA/1J mice were divided into control, sappanchalcone-treated, and methotrexate-treated groups (n = 10 per group). Paw swelling, arthritis severity, radiographic and histomorphometric changes were assessed to measure the protective role of sappanchalcone against chronic disease progression. Sappanchalcone administration significantly reduced clinical arthritis and inflammatory edema in paws. Bone mineral density and trabecular structure were maintained in CIA mice administered sappanchalcone. The levels of pro-inflammatory cytokines (TNF-α, IL-6, and 1L-1β) were significantly lower in the serum of sappanchalcone-treated mice as compared with the control group. Our results suggest that sappanchalcone could be used as an anti-inflammatory and bone-protective agent during the treatment of rheumatoid arthritis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is an autoimmune disease that principally affects the smaller synovial joints, resulting in painful swelling that can lead to bone erosion and joint deformity. The incidence of this disorder is high, and is associated with pain, deformity, disability, and reduced capacity to work. RA affects between 1 and 2 % of the world’s population and increases in prevalence commensurate with aging; it is a systemic illness affecting several organs of the body. Manifestations of RA include subcutaneous nodules, eye inflammation, reduced white blood count, and lung disease. The secretion of pro-inflammatory cytokines into the serum, including interleukin-1 (IL-1) and tumor necrosis factor-alpha (TNF-α), is enhanced during RA, resulting in damage to articular cartilage and bone. Novel cytokines, such as IL-17, IL-18, and RANKL ligand (in the synovium), are also available and secrete enzymes that degrade proteoglycans and collagen leading to bone loss (van der Berg et al. 1999; Lubberts et al. 2001). Soluble mediators of inflammation include prostaglandins, leukotrienes, and matrix metalloproteinases, which either diffuse from the blood or are locally produced within the joint cavity, and act as potent algesic agents during RA disease progression.
Pharmacological disease intervention strategies include non-steroidal anti-inflammatory agents and corticosteroids to reduce inflammation, biologic response modifiers to reduce both inflammation and structural damage to the joints, and disease modifying anti-rheumatic drugs (DMARDs) to slow disease progression (Smolen and Steiner 2003). DMARDs exhibit considerable promise, and are the recommended drug agents during the first 2 years of RA. Although these drugs, which are now considered first-line therapeutic agents for the majority of RA patients, reduce acute inflammation and pain, they do not alter the disease course or prevent joint destruction. In addition, side-effects such as gastro-intestinal disturbances, increased risk of malignancies, and higher costs constraint their prolonged use (Ahmed et al. 2005).
Parallel to conventional drug treatment strategies, alternative herbal medicines are also widely used to treat RA. The efficacy of many plants and plant products is currently being assessed in RA disease models, with several proving to be both safe and effective (Ahmed et al. 2005; Rathore et al. 2007). The most significant of these herbal preparations include gingerol from Zingiber officianalis (Ueki et al. 1964), bromelain from Ananas camosus (Cohen and Goldman 1996), curcumin from Curcuma longa (Deodhar et al. 1980), ethanolic extracts of Nyctanthes arbour tristis (Paul and Saxena 1997), active components of Swertia chirayita (Kumar et al. 2003), aqueous and ethanolic extracts of Crocus sativus (Hosseinzadeh and Younesi 2002), lupeol and 19 α-H lupeol from Strobilanthus callosus (Agarwal and Rangari 2003), alcoholic extract of root of Trewia polycarpa Benth (Chamundeswari et al. 2003), Madimadi-a Korean folk medicine (Kim et al. 2004), and various other traditional Chinese medicines (Sylvester et al. 2001).
Caesalpinia sappan L. (Leguminosae) is a traditional medicinal plant distributed in Asian peninsula countries including India, Burma, Vietnam, Sri Lanka, and China. The heartwood of this plant has long been used as blood tonic, emmenagogue, and expectorant (Zhao et al. 2008). Many active components have been derived from C. sappan, including phenolic compounds such as brazilin, chalcones, protosappanin, and homoisoflavonoids (Fu et al. 2008). Sappanchalcone (a chalcone) exhibits neuroprotective (Moon et al. 2010), anti-inflammatory (Washiyama et al. 2009), and inhibitory effects on antigen-induced beta-hexosaminidase release (Yodsaoue et al. 2009), in addition to anti-influenza virus activity (Liu et al. 2009). These immunomodulatory, anti-inflammatory, and antioxidant activities suggest a potential anti-arthritic effect of the C. sappan extract. The present study assessed the anti-RA effect of sappanchalcone in a collagen-induced mouse model, indexed using pro-inflammatory cytokine levels and physiological, microstructural, and biochemical parameters.
Materials and methods
Plant material and extract preparation
Dried heartwood of C. sappan L. was purchased from the Kyungdong Local Market, Seoul, Korea in March 2012. A voucher specimen (SCHB 12-015) was deposited at the Herbarium of College of Natural Science, Soonchunhyang University, and botanical identification performed by Dr. B.Y. Lee from National Institute of Biological Resources, South Korea. This plant was selected based on its ethnopharmacological use as a treatment for anti-inflammatory diseases.
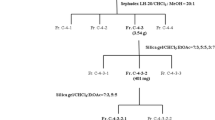
The plant was air-dried and chipped with 6 kg dry powder, extracted three times under reflux with 95 % methanol for 3 days. The extract was filtered through a Buchner funnel using Whatman filter paper No. 1, concentrated using a rotary evaporator under reduced pressure, and subsequently diluted with water. The residue was extracted successively with hexane, chloroform, ethyl acetate, and water-saturated butanol (each in 3,000 mL). Each extract, in addition to the aqueous phase, was dried under reduced pressure to yield a hexane fraction of 1.13 g, a chloroform fraction of 7 g, an ethyl acetate fraction of 176.94 g, a butanol fraction of 8.26 g, and a water-saturated fraction of 4.35 g.
The chloroform fraction was subjected to a silica-gel column (200–300 mesh), and eluted with methanol gradient solvents. Fraction 4 (513 mg) was separated on a Sephadex LH-20 (GE Healthcare) column, and eluted with methanol to yield two fractions. The effective ingredient of fraction 2 (124 mg) was subsequently identified as sappanchalcone.
High performance liquid chromatography (HPLC)
Sappanchalcone was purified using a preparative LC-20 series HPLC system (Shimadzu Corp. Tokyo, Japan), and a reversed phase C18 column (UG120; 250 × 4.6 mm, 5 µm, monitored at 280 nm). The eluent system consisted of a gradient program from 10 to 100 % methanol containing 1.25 % acetic acid at a flow rate of 0.5 mL/min, with a column temperature of 25 °C. At a retention time of 11.147 min, the fraction was collected in repeated HPLC preparations.
Compound characterization
A liquid chromatography–mass spectrometry-ion trap- time-of-flight (LC–MS–IT–TOF) mass spectrometer (Shimadzu Corp., Tokyo, Japan) was used to detect positive and negative ion mode masses, with MS/MS spectra recorded within a scan range of m/z 100–500. The detection voltage and interface temperature were set to 1.60 V and 400 °C. 1H-nuclear magnetic resonance (1H-NMR) spectra was obtained using a JNM-LA 400 NMR (Jeol Ltd., Tokyo, Japan) instrument operated at 400 MHz. Chemical shifts were reported in parts per million (ppm) downfield from an internal tetramethylsilane standard.
Animal preparation and ethics statement
Male DBA/1J mice, aged 7 weeks (20–23 g), were purchased from the Central Lab, Animal Inc., (Seoul, Korea). The animals were allowed an acclimatization period of 7 days at room temperature (22 ± 2 °C), with a 12 h light-dark cycle and relative humidity of 40–70 % before being used for the study. The animals were provided with tap water, and fed on a laboratory diet (crude protein NLT, 20.5 %; crude fat NLT, 3.5 %; crude fiber NMT, 8.0 %; crude ash NMT, 8.0 %; Ca NLT, 0.5 %; and phosphorous NLT, 0.5 %) ad libitum. Mice were given saline or indicated amounts of sappanchalcone and MTX (Sigma-Aldrich, St Louis, MO, USA) by intraperitoneal injection (total injection volume 200 µL). The study was approved by the Bioethics Committee of Soonchunhyang University. The procedures strictly adhered to generally accepted international rules and regulations.
Collagen-induced arthritis (CIA) model and disease scoring
CIA was induced as described previously (Stuart et al. 1979), with only minor modifications. Bovine type II collagen (Central Lab, Seoul, Korea) dissolved in 0.05 M acetic acid (2 mg/mL) were injected intradermally twice (each of 100 µg) at the base of the tail with 0.2 mL of the emulsion in DBA/1J mice; the first injection emulsified with complete Freund’s adjuvant (CFA, Sigma-Aldrich, St Louis, MO, USA) at day 0, and the second booster injection emulsified in incomplete Freund’s adjuvant (ICFA, Sigma-Aldrich, St Louis, MO, USA) at day 21 (Zimmerman et al. 2010). DBA/1J mice were randomly divided into four representative groups (n = 10 per group): normal group without immunization or CIA (collagen-induced arthritis), control group (for saline injection after CIA), and experimental groups for intraperitoneal administration of MTX (a reference anti-rheumatoid drug, 3 mg/kg of mouse body weight; 0.2 mL) or sappanchalcone (10 mg/kg of mouse body weight; 0.2 mL) after CIA. Sappanchalcone and MTX were administered daily and every third day, respectively from day 22 to day 42 after the 2nd booster injection. The thickness of the paw was measured using a Vernier calliper (Ozaki, Tokyo, Japan) at least three times a week for footpad swelling. Each mouse was weighed two times a week.
Arthritis disease scoring
CIA mice were monitored by two independent observers 3–4 times per week to assess degree of inflammation, and extent of erythema, edema of the periarticular tissues, and enlargement, distortion, or ankylosis of the joints. The severity of arthritis was measured using an arthritis index (AI) as follows (Hughes et al. 1994): 0 (no inflammation), 1 (edema or erythema of one joint), 2 (edema or erythema of two joints either on one or two digits), 3 (edema or erythema of more than two joints either on one, two or three digits), and 4 (severe arthritis with deformation or ankylosis of the paws). The AI used the cumulative score of all paws, thereby rendering a maximum possible score of 16.
Microstructural bone examination
Bones of the distal end of the left femur and left calcaneus, the left second metatarsal bone, and the proximal end of the left tibia were imaged using high-resolution microfocal computed tomography (micro-CT; SkyScan 1172, Bruker, Antwerp, Belgium). Bone mineral density (BMD) was assessed with a metaphyseal scan of 3 % of the length of each bone. The following measurement parameters were used: bone volume (in mm3) relative to tissue volume (in mm3 and expressed as percentage); bone surface (in mm3) relative to bone volume (in mm3 and expressed per mm); trabecular thickness (in mm), number, and fractal dimension.
Cytokine and enzyme levels in the serum
Proinflammatory cytokines such as TNF-α, IL-1β, and IL-6 were measured using a mouse immunoassay kit (R&D systems, Minneapolis, MN, USA) according to the manufacturer’s instructions. Enzymes such as aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), and creatinine levels were measured using the Synchron LX-20 analyzer (Beckman-Coulter Inc., Brea, CA).
Statistical analysis
All analyses were completed using the SPSS for Windows software package (ver. 9 SPSS Inc., Chicago, IL, USA). Data are expressed as mean ± SE. Group differences were determined using one-way analysis of variance (ANOVA) followed by Tukey’s post-hoc analysis; a value of p < 0.05 was taken to indicate statistical significance.
Results
Purification and characterization of sappanchalcone
The methanol extract of C. sappan heartwood was sequentially extracted with CHCl3: MeOH, and subjected to column chromatography using a silica gel and Sephadex matrix to obtain two active fractions later identified as sappanchalcone. The schematic representation of the sequential purification process of sappanchalcone is depicted in Fig. 1. Sappanchalcone compound extracted from the heartwood of C. sappan exhibited anti-RA effects in our CIA-induced mouse model.
Preparative HPLC confirmed that sappanchalcone was single and homogenous, with a retention time of 11.147 min (Fig. 2A). Identification with mass spectrometry, of the composition and molecular weight of sappanchalcone revealed positive and negative ion masses of 287.0768 and 285.0569 m/z, respectively (Fig. 2B). 1H-NMR (400 MHz) spectrum indicated the presence of δ 3.90 (3H, s) corresponding to 2′-OMe: 6.44 (1H, dd, J = 2.0, 8.6 Hz); to H-5′: 6.51 (1H, d, J = 2.0 Hz); to H-3′; 6.78 (1H, d, J = 8.0 Hz); to H-5; 6.97 (1H, d, J = 8.4 Hz); to H-6: 7.10 (1H, d, J = 2.0 Hz); and to H-2: 7.35 (1H, d, J = 15.6 Hz) for H-α; 7.48 (1H, d, J = 15.6 Hz) for H-b, and 7.67 (1H, d, J = 15.6 Hz) for H-6′ (Fig. 3).
Purification and characterization of sappanchalcone from Caesalpinia sappan extracts. A Preparative high-performance liquid chromatogram of purified sappanchalcone from C. sappan extract, monitored for 20 min. The Sephadex LH-20 collected fractions were run on a reverse-phase C18 column (4.6 × 250 mm) at 25 °C with a sample injection volume of 20 µL (20 mg/mL) in methanol. The near-homogenous peak fraction was eluted with 100 % methanol in isocratic mode with a flow rate of 0.05 mL/min and detection wavelength of 280 nm. B A liquid chromatography–mass spectrometry-time-of-flight (LC–MS–TOF) spectral map of purified sappanchalcone monitored for 5 min. The positive (+) and negative ion masses (−) were at 287.0768 and 285.0569 respectively, with a scan range of m/z 100–500. The detection voltage and interface temperature were set to 1.60 V and 400 °C. Methanol was used in the mobile phase
Effects of sappanchalcone in the CIA mouse model
A type-II CIA mouse model was developed, with significant arthritis during the 14 days following primary immunization of mice with type-II collagen emulsified with CFA on day 0, and with ICFA on day 21 The CIA mice were administered pure sappanchalcone (10 mg/kg) daily. The anti-arthritis drug MTX, at a concentration of 3 mg/kg, was administered once every 3 days. CIA-induced and non-induced mice were used as control and normal groups, respectively.
As depicted in Fig. 4A, mean body weight significantly decreased by day 25 in mice that developed arthritis, regardless of MTX or sappanchalcone administration. There were no significant differences between the three collagen-sensitized groups compared to normal group. A significant increase in foot pad thickness was observed beyond day 20 in the control CIA mice (Fig. 4B); however, it declined appreciably in the MTX-administered group compared with the control group. Sappanchalcone treatment was associated with a marked decline in the foot pad thickness, compared to MTX treatment. The gross hind paw lesions of CIA-induced mice are depicted in Fig. 4C. Lesions were significantly reduced in both MTX and sappanchalcone-administered mice, suggesting that sappanchalcone can prevent inflammatory arthritis. AI scores reached their maximum at day 42 in all CIA-induced mice groups (Fig. 4D), and were similar in the MTX and sappanchalcone-administered mice during each sensitization stage. The MTX and sappanchalcone treated groups showed a significantly reduced AI score compared with only CIA-induced mice.
Effect of sappanchalcone (SC) on CIA severity in DBA/1J mice, represented in terms of changes in A group mean body weight (g), B foot pad thickness (mm), C gross hind paw lesions, and D clinical arthritis index score. C i, normal group without CIA (collagen-induced arthritis), C ii control group with saline injection after CIA, C iii CIA mice with intraperitoneal (i.p) injection of drug methotrexate (MTX: 3 mg/kg of body weight; injected every third day from day 22 to day 42 after 2nd booster injection), and C-iv, CIA mice with i.p injection of sappanchalcone (10 mg/kg of body weight; injected daily from day 22 to day 42 after the 2nd booster injection). Data are expressed as mean ± SE (n = 10 per group). *p < 0.05 versus control group and # p < 0.05 versus normal group. Fisher’s protected least difference post-hoc test was used for analysis
The areal BMD (aBMD) of the proximal part of the left tibial metaphysis, and the distal part of the left femoral metaphysis, calcaneous, and left second metatarsal bone, are displayed in Fig. 5. The aBMD of the proximal part of the left tibial metaphysis was significantly lower in the control group, and appeared to increase commensurate with MTX and sappanchalcone administration (Fig. 5i). The aBMD of the distal part of the left femoral metaphysis (Fig. 5ii), calcaneous (Fig. 5iii), and the left second metatarsal bone (Fig. 5iv) significantly differed among the three CIA mouse groups (p < 0.05). Prophylactic administration of sappanchalcone and MTX was sufficient to increase the aBMD in CIA mice.
Bone mineral density profiles of i the proximal part of the left tibial metaphysis, ii the distal part of the left femoral metaphysis, iii the distal part of the left calcaneus, and iv the distal part of the left second metatarsal bone. Data are expressed as mean ± SE (n = 10 per group). *p < 0.05 versus control group and # p < 0.05 versus normal group. Fisher’s protected post hoc test was used for analysis
To further investigate changes at the microstructural level, we used micro-CT to analyze the proximal part of the left tibial metaphysis, the distal part of the left femoral metaphysis, and the left leg (Fig. 6). Compared to the non-CIA induced normal controls, CIA-induced animals were characterized by gross distortion of the trabecular structure. In the proximal part of the left tibial metaphysis (Fig. 6a), the distal part of the left femoral metaphysis (Fig. 6b), and the left leg (Fig. 6c), disjointed trabecular structures were observed in CIA-induced control group (Fig. 6ii). In the MTX and sappanchalcone-administered groups, the trabecular structure was maintained (Fig. 6iii, iv). Therapeutic administration of MTX was associated with marginally improved trabecular architecture maintenance compared to sappanchalcone. Prophylactic administration of sappanchalcone was sufficient to preserve the trabecular structure of CIA-induced mice.
Microfocal computed tomography (micro-CT) images of mice (DBA/1J) with collagen-induced arthritis (CIA). A Appearance of a the proximal part of the left tibial metaphysis, b the distal part of the left femoral metaphysis, c the left leg, and d the bone of the knee joints in microfocal computer tomography images. i Normal group, ii control group, iii CIA mice with therapeutic administration of methotrexate, iv CIA mice with prophylactic administration of sappanchalcone. B Radiographic changes in adjuvant-induced arthritis
The microstructure of cancellous bone at the distal part of the left femur measured by micro-CT in the four study groups is described in Table 1. Bone and tissue volume were significantly lower in the collagen-sensitized groups versus the control group (p < 0.05 for all comparisons). Bone surface and volume were significantly higher in CIA groups, but trabecular thickness was significantly lower in the three collagen-sensitized groups compared to the normal group (p < 0.05 for all comparisons). There were no significant differences in the trabecular number or separation parameters of the CIA-induced and non-induced mice. The fractal dimension was lower in the collagen administered, sensitized groups.
Effects of sappanchalcone on pro-inflammatory mediators of arthritis
Because TNF-α, IL-1β, and IL-6 are major inflammatory cytokines in RA, we investigated the effect of sappanchalcone on these mediators in blood serum (Fig. 7). A significant increase in inflammatory cytokines in the CIA group indicated acute arthritis (p < 0.05). Sappanchalcone administration led to a significant decline in the blood serum levels of TNF-α (Fig. 7i), IL-1β (Fig. 7ii), and IL-6 (Fig. 7iii).
Effect of sappanchalcone (SC) on the serum levels of inflammatory mediators in CIA mice. The levels of inflammatory cytokines, including TNF-α (A), IL-1β (B), and IL-6 (C), were determined by ELISA on day 43. Data are expressed as mean ± SE (n = 10 mice per group). **p < 0.001, *p < 0.05 versus control group, and ## p < 0.001, # p < 0.05 versus normal group
Effects of sappanchalcone on organ-to-body weight and enzyme levels
We observed changes in organ-to-body weight and enzyme levels in non-CIA and CIA-induced mice (Table 2). There were significant differences in the liver weights of the normal and collagen-sensitized groups. Sappanchalcone administration led to a significant increase in liver weight compared to normal group. A significant decline in the kidney weight of MTX- treated mice, and an increase in spleen weight of sappanchalcone-treated mice was noticed compared to the normal group (p < 0.05). AST and ALT levels in serum were markedly higher in the control group, although these levels declined following sappanchalcone administration. The increase in the creatinine levels were non-significant in the CIA-groups compared to the normal group (p < 0.05).
Discussion
This report is the first to demonstrate that sappanchalcone from the dried heartwood of C. sappan regulates the level of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6 in a CIA mouse model. It was previously demonstrated that these proinflammatory cytokines play a role in the inflammation and destruction of joints during RA (Myers et al. 1997; Smolen et al. 2005). TNF-α is an early-stage inflammation mediator, which induces IL-6 synthesis, resulting in inflammatory T cell activation. Sappanchalcone controlled serum levels of TNF-α and IL-6 in our collagen-sensitized model, a result that establishes the anti-inflammatory properties of sappanchalcone during RA onset in CIA mice. The immunosuppressive properties of sappanchalcone have been demonstrated using brazilein, another bioactive flavonoid derived from the heartwood of C. sappan, which decreases the mRNA levels of TNF-α and IL-6 in ischemic animals and lipopolysaccharide-induced microglial cell lines (Shen et al. 2007). C. sappan extracts, and their isolated compounds, are potent inhibitors of proinflammatory mediators of joint inflammation; this effect involves the inhibition of nitric oxide (NO) and cyclooxygenase-2 (COX-2) by interfering with their signalling pathways (Wu et al. 2011). In preliminary study brazilin, an active compound of C. sappan was reported to show inhibition of NF-κB factor that disrupts the formation of upstream IL-1R signalling complex (Jeon et al. 2014). The anti-inflammatory effects of sappanchalcone in mouse macrophage cell lines (Washiyama et al. 2009), and human periodontal ligament cells (Jeong et al. 2011), have previously been documented. This study provides a supporting evidence for the analgesic and anti-inflammatory effects of sappanchalcone, which is traditionally used in Oriental and Chinese medicine (Baek et al. 2000). Sappanchalcone may also directly inhibit growth and apoptosis in primary and metastatic oral squamous cell carcinoma (OSCCs) by stimulating caspase-9 and -3 activities (Lee et al. 2011).
The protective role of sappanchalcone observed in the present study, against inflammation in CIA mice, accords with the immunosuppressive effects of Dipsacus asper root extract (Jung et al. 2011). In collagen-sensitized mice (particularly DBA/1 mice, which respond to chick, bovine, porcine, and human type-II collagen), in which inflammation is elicited by increased serum levels of pro-inflammatory cytokine, inhibition of such cytokines could be beneficial for RA. It is suggested that the main inhibitory mechanism of chalcone compounds may be the inactivation of the nuclear factor kB (NF-kB) (Go et al. 2005; Nowakowska 2007). In an earlier report, brazilin (show similar molecular structure as sappanchalcone), an active compound isolated from C. sappan L. is reported as a potent NF-kB inhibitor that selectively disrupts the formation of the upstream IL-1R signalling complex especially the IL-1β-induced polyubiquitination of IRAK1 and its interaction with IKK-γ counterpart (Jeon et al. 2014). We assessed the effects of both sappanchalcone and MTX on RA, because MTX has previously been used to treat RA, in addition to other forms of inflammatory arthritis, and certain forms of childhood arthritis. Although principally used to treat RA, MTX also possesses carcinogenic, mutagenic, and teratogenic effects (Choudhury et al. 2000). We suggest that sappanchalcone represents a cost-effective and efficacious treatment for inflammatory conditions including RA.
To assess the anti-inflammatory properties of an isolated sappanchalcone compound, we monitored localized mouse paw edema in collagen-sensitized groups. Paw swelling and clinical arthritis scores were used to evaluate the degree of inflammation. For most of the CIA-susceptible strains of mice, the first signs of arthritis development are visible between days 18 and 25 after immunization. The incidence of arthritis in CIA-susceptible strains of mice is generally very high, reaching 80–100 % in most strains (David et al. 2007). Our results were in conformity with the data of previous papers (Sakaguchi et al. 2004; Lee et al. 2009). The data reported here provide direct evidence for a regulatory effect of sappanchalcone and MTX on cytokine production and two clinical parameters (footpad thickness and AI scores) in CIA mice in vivo. In particular, when treatment with sappanchlacone was administrated a clear improvement both in the clinical score and in the cytokine levels was observed at a dose of 10 mg/kg per mouse body weight. The inhibitory effects of arthritis was observed after the clinical signs by CIA such as an increase in footpad thickness and AI scores. These results indicate that sappanchalcone specially modulates, in particular a reduction in the levels of proinflammatory cytokines (TNF-α, IL-1β, and IL-6) after CIA condition. The observed effect on clinical parameters of CIA by sappanchalcone could be at least partially due to its effect on TNF production. This reduction of TNF levels would then lead to prevention of CIA. This may affect macrophage activation and, therefore, may represent an immunosuppressive regulatory mechanism (Neurath et al. 1999). Another compound, brazilin, also inhibited carrageenan-induced rat paw edema, although C. sappan showed a greater effect compared to the isolated compound (Washiyama et al. 2009). This suggests that active constituent of C. sappan, including sappanchalcone and certain protosappanins, are better able to attenuate paw edema in mammals (Tanno et al. 2006).
We also obtained micro-CT images of induced arthritis in the left tibial metaphysis, femoral metaphysis, and leg. Microstructural analysis of cancellous bone at the distal left femur, similar to the histomorphometric analysis demonstrated the ability of sappanchalcone to attenuate arthritis progression in CIA mice, thereby providing protection against bone loss and cartilage destruction. The decreased BMD in control group could be attributable to the increase in the eroded surface and decreased trabecular thickness revealed by micro-CT. The increasing pool of pro-inflammatory cytokines may also decrease BMD indirectly by promoting osteoclast differentiation (Szekanecz et al. 2000). BMD in MTX and sappanchalcone-administered mice was higher compared to the control group, indicating that sappanchalcone attenuates BMD decreases near joints, and maintains bone architecture without inhibiting mineralization. Our results are in accord with a previous study of BMD using micro-CT, with joint protection observed following therapeutic and prophylactic administration of biphosphonate-microdronic acid (Yamane et al. 2003). We are currently investigating whether sappanchalcone is the primary active compound of the methanolic C. sappan extract responsible for attenuating chronic RA progression.
In summary, our data demonstrate that sappanchalcone show anti-inflammatory effects in collagen-sensitized mice by down-regulating the pro-inflammatory serum cytokines and decreasing subsequent bone loss. Our findings suggest that sappanchalcone can be used as an anti-inflammatory and bone protective agent for the treatment of RA.
References
Agarwal, R.B., and V.D. Rangari. 2003. Anti-inflammatory and antiarthritic activities of Lupeol and 19α-H Lupeol isolated from Strobilanthus callosus and Strobilanthus ixiocephala roots. Indian Journal of Pharmacology 35: 384–387.
Ahmed, S., J. Anuntiyo, C.J. Malemud, and T.M. Haqqi. 2005. Biological basis for the use of botanicals in osteoarthritis and rheumatoid arthritis: A review. Evidence Based Complementary Alternative Medicine 2: 301–308.
Baek, N.I., S.G. Jeon, E.M. Ahn, J.T. Hahn, J.H. Bahn, J.S. Jang, S.W. Cho, J.K. Park, and S.Y. Choi. 2000. Anticonvulsant compounds from the wood of Caesalpinia sappan L. Archives of Pharmacol Research 23: 344–348.
Chamundeeswari, D., J. Vasantha, S. Gopalakrishnan, and E. Sukumar. 2003. Free radical scavenging activity of the alcoholic extract of Trewia polycarpa roots in arthritic rats. Journal of Ethnopharmacology 88: 51–56.
Choudhury, R.C., S.K. Ghosh, and A.K. Palo. 2000. Cytogenetic toxicity of methotrexate in mouse bone marrow. Environmental Toxicology Bulletin 8: 191–196.
Cohen, A., and J. Goldman. 1996. Bromelain therapy in rheumatoid arthritis. Pennsylvania Medical Journal 67: 27–30.
David, D.B., A.L. Kary, and F.R. Edward. 2007. Collagen-induced arthritis. Nature Protocols 2: 1269–1275.
Deodhar, S.D., R. Sethi, and R.C. Srimal. 1980. Preliminary studies on antirheumatic activity of curcumin. Indian Journal of Medical Research 71: 632–634.
Fu, L.C., X.A. Huang, Z.Y. Lai, Y.J. Hu, H.J. Liu, and X.L. Cai. 2008. A new 3-benzylchroman derivative from Sappan Ligum (Caesalpinia sappan). Molecules 28: 1923–1930.
Go, M.L., X. Wu, and X.L. Liu. 2005. Chalcones: An update on cytotoxic and chemoprotective properties. Current Medicinal Chemistry 12: 483–499.
Hosseinzadeh, H., and H.M. Younesi. 2002. Antinociceptive and anti-inflammatory effects of Crocus sativus L. stigma and petal extracts in mice. BMC Pharmacology 2: 7.
Hughes, S.L., D. Dunlop, P. Edelman, R.W. Chang, and R.H. Singer. 1994. Impact of joint impairment on longitudinal disability in elderly persons. Journal of Gerontology 49: 291–300.
Jeon, J., J.H. Lee, K.A. Park, H.S. Byun, H. Lee, Y. Lee, T. Zhang, K. Kang, J.H. Seok, H.J. Kwon, M.D. Han, S.W. Kang, J.H. Hong, and G.M. Hur. 2014. Brazilin selectively disrupts proximal IL-1 receptor signalling complex formation by targeting an IKK-upstream signalling components. Biochemical Pharmacology 89: 515–525.
Jeong, G.S., D.S. Lee, B. Li, H.J. Lee, E.C. Kim, and Y.C. Kim. 2011. Effects of sappanchalcone on the cytoprotection and anti-inflammation via heme oxygenase-1 in human pulp and periodontal ligament cells. European Journal of Pharmacology 644: 230–237.
Jung, H.W., J.K. Jung, K.H. Son, D.H. Lee, T.M. Kang, Y.S. Kim, and Y.K. Park. 2011. Inhibitory effects of the root extract of Dipsacus asper Wall on collagen-induced arthritis in mice. Journal of Ethnopharmacology 6: 98–103.
Kim, M.S., J.M. Yi, S.H. Kim, S.H. Hong, and H.M. Kim. 2004. Madimadi, Korean folk medicine, blocks TNF-α, IL-1β, and IL-8 production by activated human immune cells. Cytokine 25: 179–186.
Kumar, I.V., B.N. Paul, R. Asthana, A. Saxena, S. Mehrotra, and G. Rajan. 2003. Swertia chirata mediated modulation of interleukin-1 beta, interleukin-6, interleukin-10, interferon-gamma, and tumor necrosis factor-alpha in arthritic mice. Immunopharmacology and Immunotoxicology 25: 573–583.
Lee, J.D., J.E. Huh, Y.H. Baek, K.C. Cho, D.Y. Choi, and D.S. Park. 2009. The efficacy and mechanism action of RvCSd, a new herbal agent, on immune suppression and cartilage protection in a mouse model of rheumatoid arthritis. Journal of Pharmacology Sciences 109: 211–221.
Lee, Y.M., Y.C. Kim, B.J. Choi, D.W. Lee, J.H. Yoon, and E.C. Kim. 2011. Mechanism of sappanchalcone-induced growth inhibition and apoptosis in human oral cancer cells. Toxicology in Vitro 25: 1782–1788.
Liu, A.L., S.H. Shu, H.L. Qin, S.M.Y. Lee, Y.T. Wang, and G.H. Du. 2009. In vitro anti-influenza viral activities of constituents from Caesalpinia sappan. Planta Medica 75: 337–339.
Lubberts, E., L.A. Joosten, B. Oppers, L. van den Bersselaar, C.J. Coenen-de Roo, J.K. Kolls, P. Schwarzenberger, F.A. van de Loo, and W.B. van den Berg. 2001. IL-1 independent role of IL-17 in synovial inflammation and joint destruction during collagen-induced arthritis. Journal of Immunology 167: 1004–1013.
Moon, H.I., I.M. Chung, S.H. Seo, and E.Y. Kang. 2010. Protective effects of 3′-deoxy-4-O-methylsappanol from Caesalpinia sappan against glutamate-induced neurotoxicity in primary cultured rat cortical cells. Phytotherapy Research 24: 463–465.
Myers, L.K., E.F. Rosloniec, M.A. Cremer, and A.H. Kang. 1997. Collagen-induced arthritis, an animal model of autoimmunity. Life Sciences 61: 1861–1878.
Neurath, M.F., K. Hildner, C. Becker, J.F. Schlaak, K. Barbulescu, T. Germann, E. Schmitt, P. Schirmacher, S. Haralambous, M. Pasparakis, K.-H. Meyer Zum BÜschenfelde, G. Kollias, and E. MÄrker-Hermann. 1999. Methotrexate specifically modulates cytokine production by T cells and macrophages in murine collagen-induced arthritis (CIA): A mechanism for methotrexate-mediated immunosuppression. Clinical and Experimental Immunology 115: 42–55.
Nowakowska, Z. 2007. A review of anti-infective and anti-inflammatory chalcones. European Journal of Medicinal Chemistry 42: 125–137.
Paul, B.N., and A.K. Saxena. 1997. Depletion of tumor-necrosis factor-alpha in mice by Nyctanthes arbor-tristis. Journal of Ethnopharmacology 56: 153–158.
Rathore, B., A.A. Mahdi, B.N. Paul, P.N. Saxena, and S.K. Das. 2007. Indian herbal medicines: Possible potent therapeutic agents for rheumatoid arthritis. Journal of Clinical Biochemistry and Nutrition 41: 12–17.
Sakaguchi, Y., H. Shirahase, A. Ichikawa, M. Kanda, Y. Nozaki, and Y. Uehara. 2004. Effects of selective iNOS inhibition on type II collagen-induced arthritis in mice. Life Sciences 75: 2257–2267.
Shen, J., H. Zhang, H. Lin, H. Su, D. Xing, and L. Du. 2007. Brazilein protects the brain against focal cerebral ischemia reperfusion injury correlating to inflammatory response suppression. European Journal of Pharmacology 558: 88–95.
Smolen, J.S., and G. Steiner. 2003. Therapeutic strategies for rheumatoid arthritis. Nature Reviews Drug Discovery 2: 473–488.
Smolen, J.S., K. Redlich, J. Zwerina, D. Aletaha, G. Steiner, and G. Schett. 2005. Pro-inflammatory cytokines in rheumatoid arthritis: pathogenetic and therapeutic aspects. Clinical Reviews in Allergy and Immunology 28: 239–248.
Stuart, J.M., M.A. Cremer, A.H. Kang, and A.S. Townes. 1979. Collagen-induced arthritis in rats: Evaluation of early immunogenic agents. Arthritis Rheumatism 22: 1344–1351.
Sylvester, J., A. Liacini, W.Q. Li, F. Dehnade, and M. Zafarullah. 2001. Tripterygium wilfordii Hook F extract suppresses proinflammatory cytokine induced expression of matrix metalloproteinase genes in articular chondrocytes by inhibiting activating protein-1 and nuclear factor-κB activities. Molecular Pharmacology 59: 1196–1205.
Szekanecz, Z., M.M. Halloran, M.V. Volin, J.M. Woods, R.M. Strieter, G.H. Kenneth, S.L. Kunkel, M.D. Burdick, and A.E. Koch. 2000. Temporal expression of inflammatory cytokines and chemokines in rat adjuvant-induced arthritis. Arthritis Rheumatism 43: 1266–1277.
Tanno, K., T. Nakajima, T. Shoji, O. Nakagawasai, F. Niijima, M. Ishikawa, Y. Endo, T. Sato, S. Satoh, and T. Tadano. 2006. Anti-inflammatory effect of propolis through inhibition of nitric oxide production on carrageenan-induced mouse paw edema. Biological Pharmaceutical Bulletin 29: 96–99.
Ueki, Y., S. Miyake, Y. Tominaga, and K. Eguchi. 1964. Increased nitric oxide levels in patients with rheumatoid arthritis. Journal of Rheumatology 23: 230–236.
Van der Berg, W.B., L.A.B. Joosten, G. Kollias, and F.A.J. Van der Loo. 1999. Role of TNF-α in experimental arthritis: Separate activity of interleukin 1β in chronicity and cartilage destruction. Annals of Rheumatic Diseases 58(suppl I): 140–148.
Washiyama, M., Y. Sasaki, T. Hosokawa, and S. Nagumo. 2009. Anti-inflammatory constituents of Sappan Lignum. Biological and Pharmaceutical Bulletin 32: 941–944.
Wu, S.Q., M. Otero, F.M. Unger, M.B. Goldring, A. Phrutivorapongkul, C. Chiari, A. Kolb, H. Viernstein, and S. Toegel. 2011. Anti-inflammatory activity of an ethanolic Caesalpinia sappan extract in human chondrocytes and macrophages. Journal of Ethnopharmacology 138: 364–372.
Yamane, I., H. Hagino, T. Okano, M. Enokida, D. Yamasaki, and R. Teshima. 2003. Effect of minodronic acid (ONO-5920) on bone mineral density and arthritis in adult rats with collagen-induced arthritis. Arthritis and Rheumatism 48: 1732–1741.
Yodsaoue, O., S. Cheenpracha, C. Karalai, C. Ponglimanont, and S. Tewtrakul. 2009. Anti-allergic activity of principles from the roots and heartwood of Caesalpinia sappan on antigen-induced β-hexosaminidase release. Phytotherapy Research 23: 1028–1031.
Zhao, H.X., H. Bai, and Y.S. Wang. 2008. A new homoisoflavan from Caesalpinia sappan. Journal of Natural Medicine 62: 325–327.
Zimmerman, D.H., P. Taylor, A. Bendele, R. Carambula, Y. Duzant, V. Lowe, S.P. O’Neill, E. Talor, and K.S. Rosenthal. 2010. CEL-2000: A therapeutic vaccine for rheumatoid arthritis arrests disease development and alters serum cytokine/chemokine patterns in the bovine collagen type II induced arthritis in the DBA mouse model. International Immunopharmacology 10: 412–421.
Acknowledgments
This study was supported in part by the Soonchunhyang University Research Fund.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jung, EG., Han, KI., Kwon, HJ. et al. Anti-inflammatory activity of sappanchalcone isolated from Caesalpinia sappan L. in a collagen-induced arthritis mouse model. Arch. Pharm. Res. 38, 973–983 (2015). https://doi.org/10.1007/s12272-015-0557-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-015-0557-z