Abstract
Alternative tumor necrosis factor-α (TNF-α) inhibitors and non-TNF biologics are available as treatment options for rheumatoid arthritis patients who exhibit inadequate response to TNF-α inhibitor (TNF-IR patients). These agents have considerable efficacy compared with placebo, but head-to-head comparisons among these agents have not been performed. The objective of this study was to use Bayesian approach to compare the effectiveness of cycling TNF-α inhibitors versus switching to non-TNF biologics in TNF-IR patients. A systematic review was conducted using MEDLINE and Cochrane library. Key endpoints were the American College of Rheumatology (ACR) responses of 20/50/70 and the health assessment questionnaire (HAQ) score change at six months. Bayesian outcomes were calculated as the probability that OR is greater than one and HAQ score change difference is less than zero. Compared with TNF-α inhibitors, non-TNF biologics were associated with higher ACR response rates; in ACR20, the OR was 1.639 for abatacept [P(OR > 1) = 90.7 %], 1.871 for rituximab [P(OR > 1) = 96.2 %] and 3.52 for tocilizumab [P(OR > 1) = 99.9 %]. Similar trends were shown in the HAQ change comparison; the median differences (MD) were −0.259 for abatacept [P(MD < 0) = 100 %], −0.160 for rituximab [P(MD < 0) = 98.2 %], and −0.200 for tocilizumab [P(MD < 0) = 99.3 %]. In conclusion, switching to non-TNF biologics was more effective than cycling TNF-α inhibitor in TNF-IR patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune disease characterized by inflammation of the synovial tissue, with an estimated prevalence of 1–2 % (DiPiro et al. 2005). RA results in pain, joint destruction and diminished function, which are associated with impaired health-related quality of life and work disability (Strand and Singh 2008; Smith et al. 2011; Odegård et al. 2005; Strand et al. 2012b). Usually, this chronic and destructive condition requires lifelong medication and, therefore, finding an effective treatment is important to reduce the significant burden not only to patients with RA but also society at large (Miranda et al. 2012).
Disease-modifying anti-rheumatic drugs (DMARDs) are defined as medications that slow or stop the progression of RA by rapid and sustained suppression of inflammation (O’dell 2004). Conventional DMARDs (cDMARDs) are generally used as a first-line treatment strategy, of which methotrexate is the most commonly used. For patients with inadequate response to cDMARDs, biologic DMARDs (bDMARDs) are available (Smolen et al. 2010; Singh et al. 2012). Based on the mode of action, bDMARDs are classified into tumor necrosis factor-α (TNF-α) inhibitors and non-TNF biologics. TNF-α inhibitors include adalimumab, etanercept, infliximab, certolizumab pegol, and golimumab. Non-TNF biologics include abatacept (T cell activation inhibitor), rituximab (B-cell depleting agent), and tocilizumab (interleukin-6 blocking agent) (Genovese et al. 2005; Cohen et al. 2006; Emery et al. 2008).
Most patients with inadequate response to cDMARDs use a TNF-α inhibitor as bDMARD treatment. For patients who fail to respond to the initial TNF-α inhibitor treatment, alternative TNF-α inhibitors (cycling in TNF-α inhibitors), or non-TNF biologics (switching to non-TNF biologics) are recommended (Smolen et al. 2010; Singh et al. 2012). However, there has been an ongoing debate as to which treatment option is more efficacious—cycling of TNF-α inhibitors or switching to non-TNF biologics—due to lack of clinical evidence. Meanwhile, the safety aspects of these agents have been assessed that they do not seem to be differences in several studies (Horton et al. 2010; Ruderman 2012; Smolen et al. 2013).
While randomized controlled trials (RCTs) have been performed to compare these agents with placebo, there have been no head-to-head trials directly comparing cycling of TNF-α inhibitors with switching to non-TNF biologics. In the absence of head-to-head RCTs, Bayesian network meta-analysis (NMA) allows for indirect comparisons of all relevant treatment options. NMA (also called multiple treatment comparison, or MTC) is a generalization of standard meta-analysis for pairwise trials to a simultaneous analysis of multiple pair-wise comparisons (Jansen et al. 2008). The Bayesian approach has an additional advantage because it allows the probabilities of the treatment options to be best to be computed. Therefore, Bayesian analyses may assist clinicians and reimbursement decision makers (Lu and Ades 2004; Jansen et al. 2008). The objective of this study was to compare the effectiveness of cycling in TNF-α inhibitors with switching to non-TNF biologics in patients who do not respond to treatment with a TNF-α inhibitor by conducting a systematic review of previous studies and performing Bayesian NMA.
Methods
Search strategy
We performed a systematic review of the literature to identify studies that have investigated the efficacy of bDMARDs approved to treat patients with RA who failed to respond to previous treatments with TNF-α inhibitors. The systematic review was conducted independently by two reviewers, using the MEDLINE and Cochrane library (CENTRAL: Cochrane central registration for controlled trials) databases, to identify studies published before and up to April 04, 2013. Searches included appropriate keywords and Medical Subject Headings (MeSH) for disease and bDMARD names, and were limited to human RCTs published in English.
Inclusion and exclusion criteria
According to European League Against Rheumatism (EULAR) and the American College of Rheumatology (ACR) guidelines, TNF-α inhibitors (adalimumab, certolizumab, etanercept, infliximab and golimumab) and non-TNF biologics (abatacept, rituximab, and tocilizumab) may be used in patients with inadequate response to a TNF-α inhibitor (Smolen et al. 2010; Singh et al. 2012). These biologic agents were included in the search. The inclusion criteria were adult patients with RA who showed inadequate response to a TNF-α inhibitor (TNF-IR patients), and who used a bDMARD with cDMARD combination therapy in double-blind RCTs with outcomes assessments at 6 months. Six months was chosen as the assessment period based on the ACR guidelines (Singh et al. 2012). Apart from RA, ankylosing spondylitis, psoriatic arthritis and connective tissue diseases and biologic mono-therapy were excluded from the search.
Data extraction and outcome measures
Two researchers extracted predefined data from studies included in the literature review, and quality assessment was performed using Cochrane’s Risk of Bias (Fig. 1). Disagreement was resolved through a third researcher’s assessment, to reach a consensus. Extracted data were reviewed and checked before analysis.
The main outcome measures chosen were the ACR20/50/70 and the health assessment questionnaire (HAQ) improvement. ACR20/50/70 are defined as a 20, 50, or 70 % improvement in tender and swollen joints, respectively, and the same level of improvement in three of the following five variables: patient and physician global assessments of overall disease activity, patient evaluation of pain, a score of physical disability, and blood acute-phase reactants (Orme et al. 2012). The HAQ disability index is one of the most widely used self-assessment instruments for measuring functional disability in patients with a variety of rheumatic diseases, and is expressed on a scale of 0–3 units (0: no functional disability, 3: severe functional disability) (Krishnan et al. 2004).
The total number of patients, number of respondents achieving the ACR response, and mean HAQ improvement from baseline and standard deviations (SDs) were extracted. For golimumab, this analysis used HAQ change from baseline from CADTH therapeutic review (2010) because the study selected for golimumab, GO-AFTER trial, did not provide the HAQ change value. Missing SD values were imputed by the average of SDs reported for all the other RCTs. Demographic data such as age and gender, mean disease duration, proportion of female patients, and baseline HAQ score were recorded.
Statistical analysis
A Bayesian NMA was performed to simultaneously conduct indirect comparisons between treatments that were not directly compared and to allow all pair-wise comparisons. This approach provides the flexibility of including a wide range of data and borrowing strength across the whole evidence network, and therefore makes the best use of available data (Schmitz et al. 2012; Jang et al. 2013). Evidence structures for the analysis are shown in a network diagram (Fig. 2). All analyses were performed with WinBUGS 1.4.3 statistical software. The first 10,000 simulations were discarded to allow for model convergence and the subsequent 50,000 simulations were used to estimate posterior probabilities.
In our analysis, treatment effect parameters were modeled by using non-informative (vague) prior distributions that were normally distributed with a mean of 0 and a variance of 10,000. For the dichotomous ACR20/50/70 endpoints, NMA calculated odds ratios (ORs) and treatment effect as a percentage of patients who experienced an event. For the continuous HAQ score data, an NMA model was fitted to differences in HAQ improvement among various treatments. Posterior medians with 95 % credible intervals (CrIs) were reported as the best estimate for the central value, since means may be overly influenced by outliers. Additionally, posterior probabilities were calculated using posterior probability distributions for hypothesis testing. A probability of 90 % suggests that a treatment is more effective than the comparator. The probability of OR greater than one for ACR response was also computed. Bayesian methodology allows conclusions to be drawn regarding the probability that the ORs are greater than one in cases in which the associated 95 % CrI includes one (Moran et al. 2010). Similarly, the probability of a difference in HAQ score change of less than zero was computed and interpreted.
Results
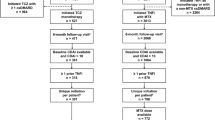
The initial search yielded a total of 853 studies. Of these, 28 remained after 261 duplicates and 564 studies that did not meet the inclusion criteria were excluded based on a review of titles and abstracts (Fig. 3). After the review of full articles, another 22 studies were removed; 10 studies did not provide the main outcome measures of interest, six were duplicate studies, three did not evaluate the intervention of interest, and the remainder did not have the targeting study population, study objective, or outcome assessment time. As a result, a total of 6 studies were selected for analysis; one study of golimumab (Smolen et al. 2009), one study of rituximab (Cohen et al. 2006), two studies of abatacept (Genovese et al. 2005; Westhovens et al. 2006), and two studies of tocilizumab (Emery et al. 2008; Strand et al. 2012a). Two of the abatacept studies analyzed data from the ATTAIN trial. Genovese et al. (2005) included data for the ACR response but did not provide data for the HAQ change from baseline. Therefore, we included Westhovens et al. (2006) to provide the latter. For the same reason, we selected two tocilizumab studies that were based on the RADIATE trial. Golimumab was the only TNF-α inhibitor and the rest were non-TNF biologics.
All of the included studies were conducted in samples of patients who failed initial TNF-α inhibitor treatment, and baseline characteristics were similar across all studies (Table 1). The mean age of patients was 53.6 years. All of the studies had high percentages of females (74–84 %) and the mean disease duration was over 9 years. The baseline HAQ score ranged from 1.6 to 1.9. Based on these characteristics, we conducted analyses using the fixed-effect model.
ACR20/50/70 results are shown in Tables 2 and 3. The proportion of patients who achieved ACR20 was highest for tocilizumab (62.4 %; 95 % CrI 49.9–74.0 %), followed by rituximab (47.0 %; 95 % CrI 37.7–56.6 %), abatacept (43.7 %; 95 % CrI 32.9–55.4 %) and golimumab (32.1 %; 95 % CrI 22.3–44.0 %), and lowest for placebo (15.5 %; 95 % CrI 12.8–18.5 %). Similarly, the ACR50 effectiveness measure was highest for tocilizumab and lowest for placebo. Rituximab had the highest proportion of patients who achieved ACR70.
Table 3, derived from the results in Table 2, summarizes ORs for non-TNF biologics in comparison to golimumab, a TNF-α inhibitor. For ACR20, abatacept had OR of 1.639 [95 % CrI 0.786–3.408; P(OR > 1) = 90.7 %], rituximab 1.871 [95 % CrI 0.937–3.725; P(OR > 1) = 96.2 %], and tocilizumab 3.52 (95 % CrI 1.567–7.946; P(OR > 1) = 99.9 %). The posterior probabilities (OR > 1) of all non-TNF biologics were over 90 %, suggesting that these agents were more effective. For ACR50, ORs were 1.623 [95 % CrI 0.454–6.247; P(OR > 1) = 72.2 %], 1.702 [95 % CrI 0.558–5.087; P(OR > 1) = 83.0 %], and 2.552 [95 % CrI 0.752–9.1; P(OR > 1) = 93.3 %] for abatacept, rituximab and tocilizumab, respectively. Based on the probability of OR > 1, tocilizumab has clear benefits in comparison to golimumab, a TNF-α inhibitor. For ACR70, ORs were 2.048 [95 % CrI 0.361–16.47; P(OR > 1) = 78.4 %], 3.876 [95 % CrI 0.685–35.37; P(OR > 1) = 93.5 %], and 3.107 [95 % CrI 0.532–25.49; P(OR > 1) = 89.2 %] for abatacept, rituximab and tocilizumab, respectively. In this case, rituximab was shown to be more effective than the TNF-α inhibitor, based on the probability of OR > 1.
HAQ score change results are shown in Tables 4 and 5. The magnitude of change was lowest for placebo at −0.078 (95 % CrI −0.115 to −0.042) and increased from golimumab (−0.218; 95 % CrI −0.325 to −0.112), rituximab (−0.378; 95 % CrI −0.467 to −0.29), tocilizumab (−0.418; 95 % CrI −0.525 to −0.311), and abatacept (−0.478; 95 % CrI −0.575 to −0.381). The probability of being best among five treatments was highest for abatacept at 74.4 %. Comparisons of each bDMARD with placebo showed that the magnitude of the change was highest for abatacept [−0.400; 95 % CrI −0.499 to −0.299; P(difference < 0) = 100 %] followed by tocilizumab [−0.340; 95 % CrI −0.453 to −0.227; P(difference < 0) = 100 %] and rituximab [−0.300; 95 % CrI −0.397 to −0.203; P(difference < 0) = 100 %], and lowest for golimumab [−0.140; 95 % CrI −0.255 to −0.026; P(difference < 0) = 99.2 %].
Table 5, derived from Table 4, presents the results of comparing each non-TNF biologic drug to golimumab, a TNF-α inhibitor. The magnitude of the difference in HAQ change was highest for abatacept [−0.260; 95 % CrI −0.411 to −0.107; P(difference < 0) = 100 %], followed by tocilizumab [−0.200; 95 % CrI −0.36 to −0.039; P(difference < 0) = 99.3 %]. It was lowest for rituximab [−0.160; 95 % CrI −0.31 to −0.01; P(difference < 0) = 98.2 %]. Based on posterior probabilities, non-TNF biologics improved HAQ scores compared with the TNF-α inhibitor.
Discussion
We found that the ACR responses of switching to non-TNF biologics were higher than those with cycling of alternative TNF-α inhibitors, even though the superior agent identified based on the probability of being best was different for each category. According to calculations of posterior probabilities of OR > 1, all three non-TNF biologics had higher proportions of patients who achieved ACR20 response than the TNF-α inhibitor. Among all non-TNF biologics, tocilizumab showed superior benefits in ACR50 and rituximab had the best effectiveness in ACR70, compared with the TNF-α inhibitor. Similarly, switching to non-TNF biologics resulted in greater changes in HAQ outcomes, compared with alternative TNF-α inhibitor cycling. In addition, in evaluations of posterior probabilities of differences less than zero, all of the non-TNF biologics were also shown to be more effective than the TNF-α inhibitor.
Our findings are consistent with those of several previous studies. The CADTH therapeutic review comparing three bDMARDs (abatacept, rituximab, and golimumab) concluded that based on RCTs, there are benefits associated with switching to non-TNF biologics rather than cycling of TNF-α inhibitors in TNF-IR patients (CADTH therapeutic review 2010). Several observational studies also are in good accord with our results (Chatzidionysiou and Van Vollenhoven 2013; Soliman et al. 2012; Gomez-Reino et al. 2012). Chatzidionysiou and Van Vollenhoven (2013) concluded that TNF-IR patients showed better overall results when treated with rituximab than with another TNF-α inhibitor. Soliman et al. (2012) also suggested that switching to rituximab may be more beneficial than cycling to an alternative TNF-α inhibitor in TNF-IR patients.
Although a number of indirect comparisons of bDMARDs have been performed, most previous studies dealt with inadequate response to cDMARDs, and only a few studies have been performed in TNF-IR patients (Guyot et al. 2012; Jansen et al. 2008; Lee et al. 2008; CADTH therapeutic review 2010; Devine et al. 2011; Launois et al. 2011; Salliot et al. 2011; Orme et al. 2012; Schmitz et al. 2012). Salliot et al. (2011) compared the efficacy of bDMARDs in TNF-IR patients using meta-analysis and indirect comparisons. This approach is useful for comparing two treatment strategies that have a common comparator, but is not appropriate for simultaneously comparing multiple treatment strategies. The CADTH therapeutic review (2010) could not include all recommended treatments because of the timing of the analysis, nor the HAQ change from baseline as an efficacy outcome measure, which recently began to be reported in the majority of clinical studies (Schmitz et al. 2012). The present study is the first NMA to analyze ACR response and HAQ score change for currently available agents in TNF-IR patients with RA.
This study has some limitations. First, there was limited clinical evidence for the efficacy of biologic agents in TNF-IR patients although a comprehensive search strategy was conducted to identify all relevant evidence. There was only one RCT available for each variable. Furthermore, there was no RCT regarding cycling in other TNF-α inhibitors such as etanercept, infliximab, and adalimumab in TNF-IR patients. Analysis of cycling in TNF-α inhibitors was restricted to only golimumab, for which such data are available. Therefore, the results of this analysis should be interpreted with caution and further studies on the other TNF-α inhibitors are needed. Second, the durations of included trials were too short to assess long-term benefits and harms in RA patients. The delayed and rare effects of biologic agents would not have been detected in the short-duration trials. Since RA is a chronic disease requiring prolonged treatment, further studies to evaluate long-term efficacy and safety are needed.
According to the clinical evidence to date, our findings suggest that non-TNF biologic agents such as rituximab, abatacept, and tocilizumab are more effective than TNF-α inhibitors for the treatment of RA patients after failure of initial treatment with a TNF-α inhibitor. However, more clinical evidence is needed in the future to recommend the best treatment options.
References
CADTH therapeutic review. 2010. Clinical and economic overview: biological response modifier agents for adults with rheumatoid arthritis. Canadian Agency for Drugs and Technologies in Health: Ottawa.
Chatzidionysiou, K., and R.F. Van Vollenhoven. 2013. Rituximab versus anti-TNF in patients who previously failed one TNF inhibitor in an observational cohort. Scandinavian Journal of Rheumatology 42: 190–195.
Cohen, S.B., P. Emery, M.W. Greenwald, M. Dougados, R.A. Furie, M.C. Genovese, E.C. Keystone, J.E. Loveless, G.R. Burmester, M.W. Cravets, E.W. Hessey, T. Shaw, and M.C. Totoritis. 2006. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: Results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis and Rheumatism 54: 2793–2806.
Devine, E.B., R. Alfonso-Cristancho, and S.D. Sullivan. 2011. Effectiveness of biologic therapies for rheumatoid arthritis: an indirect comparisons approach. Pharmacotherapy 31: 39–51.
Dipiro, J.T., R.L. Talbert, G.C. Yee, G.R. Matzke, B.G. Wells, and L.M. Posey. 2005. Pharmacotherapy: a pathophysiologic approach. New York: McGraw-Hill.
Emery, P., E. Keystone, H.P. Tony, A. Cantagrel, R. Van Vollenhoven, A. Sanchez, E. Alecock, J. Lee, and J. Kremer. 2008. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Annals of the Rheumatic Diseases 67: 1516–1523.
Genovese, M.C., J.-C. Becker, M. Schiff, M. Luggen, Y. Sherrer, J. Kremer, C. Birbara, J. Box, K. Natarajan, I. Nuamah, T. Li, R. Aranda, D.T. Hagerty, and M. Dougados. 2005. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor α inhibition. New England Journal of Medicine 353: 1114–1123.
Gomez-Reino, J.J., C. Rodriguez-Lozano, C. Campos-Fernandez, M. Montoro, M.A. Descalzo, and L. Carmona. 2012. Change in the discontinuation pattern of tumour necrosis factor antagonists in rheumatoid arthritis over 10 years: data from the Spanish registry BIOBADASER 2.0. Annals of the Rheumatic Diseases 71: 382–385.
Guyot, P., P.C. Taylor, R. Christensen, L. Pericleous, P. Drost, I. Eijgelshoven, G. Bergman, and M. Lebmeier. 2012. Indirect treatment comparison of abatacept with methotrexate versus other biologic agents for active rheumatoid arthritis despite methotrexate therapy in the United Kingdom. Journal of Rheumatology 39: 1198–1206.
Horton, S., M.H. Buch, and P. Emery. 2010. Efficacy, tolerability and safety of biologic therapy in rheumatoid disease: patient considerations. Drug Healthcare and Patient Safety 2: 101–119.
Jang, E.J., D.H. Kim, J. Ahn, B.H. Jang, and S.M. Choi. 2013. Methods for Bayesian meta-analysis. Seoul: National Evidence-based Healthcare Collaborating Agency.
Jansen, J.P., B. Crawford, G. Bergman, and W. Stam. 2008. Bayesian meta-analysis of multiple treatment comparisons: an introduction to mixed treatment comparisons. Value Health 11: 956–964.
Krishnan, E., T. Sokka, A. Hakkinen, H. Hubert, and P. Hannonen. 2004. Normative values for the health assessment questionnaire disability index: benchmarking disability in the general population. Arthritis and Rheumatism 50: 953–960.
Launois, R., B. Avouac, F. Berenbaum, O. Blin, I. Bru, B. Fautrel, J.M. Joubert, J. Sibilia, and B. Combe. 2011. Comparison of certolizumab pegol with other anticytokine agents for treatment of rheumatoid arthritis: a multiple-treatment Bayesian metaanalysis. Journal of Rheumatology 38: 835–845.
Lee, Y.H., J.H. Woo, Y.H. Rho, S.J. Choi, J.D. Ji, and G.G. Song. 2008. Meta-analysis of the combination of TNF inhibitors plus MTX compared to MTX monotherapy, and the adjusted indirect comparison of TNF inhibitors in patients suffering from active rheumatoid arthritis. Rheumatology International 28: 553–559.
Lu, G., and A.E. Ades. 2004. Combination of direct and indirect evidence in mixed treatment comparisons. Statistics in Medicine 23: 3105–3124.
Miranda, L.C., H. Santos, J. Ferreira, P. Coelho, C. Silva, and J. Saraiva-Ribeiro. 2012. Finding rheumatoid arthritis impact on life (FRAIL Study): economic burden. Acta Reumatologica Portuguesa 37: 134–142.
Moran, J.L., P.L. Graham, S. Rockliff, and A.D. Bersten. 2010. Updating the evidence for the role of corticosteroids in severe sepsis and septic shock: a Bayesian meta-analytic perspective. Critical Care 14: R134.
O’dell, J.R. 2004. Therapeutic strategies for rheumatoid arthritis. New England Journal of Medicine 350: 2591–2602.
Odegård, S., A. Finset, T.K. Kvien, P. Mowinckel, and T. Uhlig. 2005. Work disability in rheumatoid arthritis is predicted by physical and psychological health status: a 7-year study from the Oslo RA register. Scandinavian Journal of Rheumatology 34: 441–447.
Orme, M.E., K.S. Macgilchrist, S. Mitchell, D. Spurden, and A. Bird. 2012. Systematic review and network meta-analysis of combination and monotherapy treatments in disease-modifying antirheumatic drug-experienced patients with rheumatoid arthritis: analysis of American College of Rheumatology criteria scores 20, 50, and 70. Biologics 6: 429–464.
Ruderman, E.M. 2012. Overview of safety of non-biologic and biologic DMARDs. Rheumatology 51(Suppl 6): vi37–vi43.
Salliot, C., A. Finckh, W. Katchamart, Y. Lu, Y. Sun, C. Bombardier, and E. Keystone. 2011. Indirect comparisons of the efficacy of biological antirheumatic agents in rheumatoid arthritis in patients with an inadequate response to conventional disease-modifying antirheumatic drugs or to an anti-tumour necrosis factor agent: a meta-analysis. Annals of the Rheumatic Diseases 70: 266–271.
Schmitz, S., R. Adams, C.D. Walsh, M. Barry, and O. Fitzgerald. 2012. A mixed treatment comparison of the efficacy of anti-TNF agents in rheumatoid arthritis for methotrexate non-responders demonstrates differences between treatments: a Bayesian approach. Annals of the Rheumatic Diseases 71: 225–230.
Singh, J.A., D.E. Furst, A. Bharat, J.R. Curtis, A.F. Kavanaugh, J.M. Kremer, L.W. Moreland, J. O’dell, K.L. Winthrop, T. Beukelman, S.L. Bridges Jr, W.W. Chatham, H.E. Paulus, M. Suarez-Almazor, C. Bombardier, M. Dougados, D. Khanna, C.M. King, A.L. Leong, E.L. Matteson, J.T. Schousboe, E. Moynihan, K.S. Kolba, A. Jain, E.R. Volkmann, H. Agrawal, S. Bae, A.S. Mudano, N.M. Patkar, and K.G. Saag. 2012. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care & Research 64: 625–639.
Smith, H.S., A.R. Smith, and P. Seidner. 2011. Painful rheumatoid arthritis. Pain Physician 14: E427–E458.
Smolen, J.S., J. Kay, M.K. Doyle, R. Landewe, E.L. Matteson, J. Wollenhaupt, N. Gaylis, F.T. Murphy, J.S. Neal, Y. Zhou, S. Visvanathan, E.C. Hsia, and M.U. Rahman. 2009. Golimumab in patients with active rheumatoid arthritis after treatment with tumour necrosis factor alpha inhibitors (GO-AFTER study): a multicentre, randomised, double-blind, placebo-controlled, phase III trial. Lancet 374: 210–221.
Smolen, J.S., R. Landewe, F.C. Breedveld, M. Buch, G. Burmester, M. Dougados, P. Emery, C. Gaujoux-Viala, L. Gossec, J. Nam, S. Ramiro, K. Winthrop, M. De Wit, D. Aletaha, N. Betteridge, J.W. Bijlsma, M. Boers, F. Buttgereit, B. Combe, M. Cutolo, N. Damjanov, J.M. Hazes, M. Kouloumas, T.K. Kvien, X. Mariette, K. Pavelka, P.L. Van Riel, A. Rubbert-Roth, M. Scholte-Voshaar, D.L. Scott, T. Sokka-Isler, J.B. Wong, and D. Van Der Heijde. 2013. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Annals of the Rheumatic Diseases. doi:10.1136/annrheumdis-2013-204573.
Smolen, J.S., R. Landewe, F.C. Breedveld, M. Dougados, P. Emery, C. Gaujoux-Viala, S. Gorter, R. Knevel, J. Nam, M. Schoels, D. Aletaha, M. Buch, L. Gossec, T. Huizinga, J.W. Bijlsma, G. Burmester, B. Combe, M. Cutolo, C. Gabay, J. Gomez-Reino, M. Kouloumas, T.K. Kvien, E. Martin-Mola, I. Mcinnes, K. Pavelka, P. Van Riel, M. Scholte, D.L. Scott, T. Sokka, G. Valesini, R. Van Vollenhoven, K.L. Winthrop, J. Wong, A. Zink, and D. Van Der Heijde. 2010. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Annals of the Rheumatic Diseases 69: 964–975.
Soliman, M.M., K.L. Hyrich, M. Lunt, K.D. Watson, D.P.M. Symmons, and D.M. Ashcroft. 2012. On Behalf of the British Society for Rheumatology Biologics, R. Rituximab or a second anti-tumor necrosis factor therapy for rheumatoid arthritis patients who have failed their first anti-tumor necrosis factor therapy? Comparative analysis from the British Society for Rheumatology Biologics Register. Arthritis Care & Research 64: 1108–1115.
Strand, V., G.R. Burmester, S. Ogale, J. Devenport, A. John, and P. Emery. 2012a. Improvements in health-related quality of life after treatment with tocilizumab in patients with rheumatoid arthritis refractory to tumour necrosis factor inhibitors: results from the 24-week randomized controlled RADIATE study. Rheumatology 51: 1860–1869.
Strand, V., V. Sharp, A.S. Koenig, G. Park, Y. Shi, B. Wang, D.J. Zack, and D. Fiorentino. 2012b. Comparison of health-related quality of life in rheumatoid arthritis, psoriatic arthritis and psoriasis and effects of etanercept treatment. Annals of the Rheumatic Diseases 71: 1143–1150.
Strand, V., and J.A. Singh. 2008. Improved health-related quality of life with effective disease-modifying antirheumatic drugs: evidence from randomized controlled trials. American Journal of Managed Care 14: 234–254.
Westhovens, R., J.C. Cole, T. Li, M. Martin, R. Maclean, P. Lin, B. Blaisdell, G.V. Wallenstein, R. Aranda, and Y. Sherrer. 2006. Improved health-related quality of life for rheumatoid arthritis patients treated with abatacept who have inadequate response to anti-TNF therapy in a double-blind, placebo-controlled, multicentre randomized clinical trial. Rheumatology 45: 1238–1246.
Acknowledgments
This study was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare, Republic of Korea. (HI10C2020).
Conflict of interest
All authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, HL., Lee, MY., Park, SY. et al. Comparative effectiveness of cycling of tumor necrosis factor-α (TNF-α) inhibitors versus switching to non-TNF biologics in rheumatoid arthritis patients with inadequate response to TNF-α inhibitor using a Bayesian approach. Arch. Pharm. Res. 37, 662–670 (2014). https://doi.org/10.1007/s12272-014-0337-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-014-0337-1