Abstract
Rheum palmatum, Chinese traditional herb, exhibits a great variety of anti-cancer and anti-viruses properties. This study rates antiviral activity of R. palmatum extracts and its components against Japanese encephalitis virus (JEV) in vitro. Methanol extract of R. palmatum contained higher levels of aloe emodin, chrysophanol, rhein, emodin and physcion than water extract. Methanol extract (IC50 = 15.04 μg/ml) exhibited more potent inhibitory effects on JEV plaque reduction than water extract (IC50 = 51.41 μg/ml). Meanwhile, IC50 values determined by plaque reduction assay were 15.82 μg/ml for chrysophanol and 17.39 μg/ml for aloe-emodin, respectively. Virucidal activity of agents correlated with anti-JEV activity, while virucidal IC50 values were 7.58 μg/ml for methanol extract, 17.36 μg/ml for water extract, 0.75 μg/ml for chrysophanol and 0.46 μg/ml for aloe-emodin, respectively. In addition, 10 μg/ml of extract, chrysophanol or aloe emodin caused 90 % inhibition of JEV yields in cells and significantly activated gamma activated sequence-driven promoters. Hence, methanol extract of R. palmatum and chrysophanol with high therapeutic index might be useful for development of antiviral agents against JEV.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheum palmatum is a traditional Chinese medicine widely used in treatment of gasteroenteritic and liver diseases (Li et al. 2007). R. palmatum and related components are associated with a great variety of anti-cancer and anti-virus properties (Li et al. 2007; Huang et al. 2007; Lin et al. 2008; Semple et al. 2001; Sydiskis et al. 1991; Wohlfarth and Efferth 2009). Many natural compounds have been identified from R. palmatum, including chrysophanol, rhein, emodin, aloe-emodin, sennoside A and physcion (Li et al. 2007). Aloe-emodin displays potent anti-virus activities and anti-cancer properties (Lin et al. 2006; Mijatovic et al. 2005; Kuo et al. 2002). Aloe-emodin exhibits multiple anti-viral effects (Huang et al. 2007; Sydiskis et al. 1991) against herpes simplex, influenza (Sydiskis et al. 1991), human cytomegalovirus (Barnard et al. 1992), and polio viruses (Semple et al. 2001). Both emodin and chrysophanol show antiviral activity against hepatitis B (Shuangsuo et al. 2006), polio (Semple et al. 2001), human immunodeficiency and hepatitis C (Kubin et al. 2005).
Japanese encephalitis virus (JEV), a mosquito-borne virus, belongs to the genus Flavivirus of the family Flaviviridae. JEV causes severe central nerve system diseases such as poliomyelitis-like acute flaccid paralysis, aseptic meningitis and encephalitis, the latter still a leading cause of high morbidity and mortality (Unni et al. 2011). JEV vaccines are currently accessible and effective, but zoonotic and occasional infections still occur in Southeast Asia and the Western Pacific region (Chung et al. 2007). Among an estimated 35,000–50,000 annual cases, 30–50 % of JEV patients develop permanent neuropsychiatric sequelae and 20–30 % result in death (Kaur and Vrati 2003). Extensive studies aimed at developing new antiviral therapeutic strategies may also be needed.
In this study, methanol and water extracts of R. palmatum and its related natural compounds were rated for inhibiting JEV replication in vitro. We demonstrated methanol and water extracts of R. palmatum reducing JEV plaques and virus yields in vitro. Derived components chrysophanol and aloe-emodin were less cytotoxic and exhibited IC50 values less than 20 μg/ml against JEV. We proved that anti-JEV ability of those extracts correlates with potent virucidal effects on JEV infectivity and activates interferon-γ-activated site (GAS)-driven promoter. We first reported antiviral activity of chrysophanol and aloe-emodin against JEV via virucidal efficacy and GAS promoter activation.
Materials and methods
Viruses and cells
JEV strain T1P1 kindly provided by Prof. Wei-June Chen at Chang Gung University (Taoyuan, Taiwan) was used as previously described (Hsiao et al. 2010). Vero cells (African green monkey kidney cells, ATCC No. CCL-81) for JEV amplification were maintained in Dulbecco’s modified Eagle’s medium (DMEM), BHK-21 cells (baby hamster kidney cells, ATCC No. CCL-10) used to determine JEV plaques, with virus yields grown in minimum essential medium (MEM) supplemented with 10 % fetal bovine serum (FBS).
Chemicals and R. palmatum extracts
Crude R. palmatum extract powder was obtained from Sun Ten Pharmaceutical Co., Ltd. For each tested extract, 1 g of powder was dissolved in 40 ml methanol or distilled water and gently shaken overnight at room temperature. Solution samples were filtered with Whatman No. 1 filter paper, lyophilized in an IWAKI FDR-50P freeze dryer, then sterilized by a 0.44 μm syringe filter and stored at −80 °C until used (100 % stock solution). Also, filtered extract was injected directly into the HPLC instrument with C-18 reverse phase column. Separation was conducted with gradient elution using acetonitrile and 0.1 % phosphoric acid at a flow rate of 1 ml/min, eluent detected at 250 nm. Chrysophanol, rhein, emodin, aloe-emodin, and physcion were purchased from Sigma Chemical Co. of St. Louis, serving as external standards for comparing chromatographic peaks of methanol and water extracts with the retention time of these marker compounds.
Cell viability assay
For cell viability assay, BHK-21 cells were cultured overnight on 96-well plates. Medium containing various concentrations of R. palmatum extract, chrysophanol, rhein, emodin, aloe-emodin, or physcion was added and incubated for another 72 h, followed by MTT assay. Survival rate of cells was calculated as ratio of optical density at 570–630 nm (OD570–630) of treated cells to OD570–630 of untreated cells. Cytotoxic rate (%) = [(A control − A experiment)/A control] × 100 %. Cytotoxic concentration giving 50 % (CC50) was determined as using a computer program (provided by John Spouge, NCBI, NIH).
Plaque reduction assay
To determine viral plaque reduction effect, medium containing various concentrations of R. palmatum extract, chrysophanol, or aloe-emodin was added along with JEV at 100 pfu, added into the well of BHK-21 cell monolayer at 37 °C for 1 h and then overlaid with MEM medium containing 1.1 % methylcellulose. Viral plaques were stained with naphthol blue-black dye after three days of incubation. The data represent mean ± SD of three independent experiments. Inhibitory concentration showing 50 % JEV plaque reduction (IC50) was determined using a computer program (provided by John Spouge, National Institutes of Health).
Virucidal activity assays
Virucidal assay was modified as a prior study (Cheng et al. 2008). JEV (105 pfu) was mixed with medium containing R. palmatum extract, chrysophanol, or aloe-emodin (0, 1, 10, 100 μg/ml), then incubated for 60 min at room temperature. A 1,000-fold dilution of each extract/virus or compound/virus mixture was added onto BHK-21 cell monolayer in 6-well plates for plaque assays. Residual infectivity and inhibitory concentration showing 50 % JEV plaque reduction (IC50) were performed as described in the plaque assay.
Quantitative assay of virus yields using real-time RT-PCR assay
To determine inhibitory effect on virus yields, R. palmatum extract, chrysophanol, or aloe-emodin was mixed with JEV at a MOI of 1, each mixture forthwith added into BHK-21 cells for 72 h. Cultured supernatants were harvested for viral genome extraction, using QIAamp Viral RNA Mini Kit (Qiagen). Real-time RT-PCR is performed with specific primers, SYBR green PCR Master Mix and SYBR green I dsDNA binding dye. Oligonucleotides for JEV E protein were JEV D3-F 5′-GGGAGTGATGGCCCCTGCAAAATT-3′ and JEV D3-R 5′-TCCAATGGAGCCAAAGTCCCAGGC-3′ as described previously (Huang et al. 2008). PCR product level was monitored with an ABI PRISM 7000 sequence detection system (Applied Biosystems).
GAS-driven promoter assay
BHK-21 cells were co-trasfected with a cis-reporter plasmid pGAS-Luc (Agilent Technologies, Catalog NO. 219091) and an internal control reporter pRluc-C1 (BioSignal Packard) in 6-well plates, using GenePorter reagent. Transfected cells were seeded into 24-well plates with DMEM containing 10 % FBS and treated with chrysophanol, or aloe-emodin at a concentration of 10 μg/ml. After 4-h incubation, enzyme activity of experimental firefly luciferase and control Renilla luciferase in cells was measured with dual Luciferase Reporter Assay System (Promega) and Luminometer TROPIX TR-717 (Applied Biosystems). Relative firefly luciferase activity of GAS-driven reporter was normalized by Renilla luciferase, then compared to untreated cells. Assays were performed in triplicate, the results expressed as mean ± SD.
Statistical analysis
ANOVA analysis using SPSS program (version 10.1, SPSS Inc., IL, USA) or Student t test analyzed all data. P < 0.05 was considered statistically significant.
Results
HPLC and cytotoxic analyses of R. palmatum extracts
To determine fingerprints of R. palmatum extracts, methanol and water extracts were analyzed using HPLC (Fig. 1). Chrysophanol, rhein, emodin, aloe-emodin, and physcion served as external standards, while retention time was 9 min for aloe-emodin, 10 min for rhein, 18 min for emodin, 30 min for chrysophanol, and 40 min for physcion (Fig. 1a).Comparison of chromatographic peaks of extracts with retention time of these marker compounds showed methanol extract containing higher amounts of the five marker components than water extract (Figs 1b, c). R. palmatum extracts and these five marker compounds were further evaluated their cytotoxicity to BHK-21 cells. In vitro cytotoxicity assay indicated CC50 values of both R. palmatum extract and related marker compounds varying from 3.47 μg/ml (rhein) to 54.80 μg/ml (R. palmatum water extract) 72 h post treatment (Table 1). Rhein and emodin showed high toxicity to BHK-21 cells (CC50 < 10 μg/ml); chrysophanol and aloe-emodin were less toxic.
JEV plaque reduction by R. palmatum extracts, chrysophanol and aloe-emodin
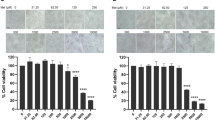
To test antiviral effects of R. palmatum against JEV, both extracts were tested by plaque reduction assay in BHK-21 cells (Fig. 2; Table 1). R. palmatum methanol extract manifested potent anti-JEV activity (IC50 = 15.04 μg/ml). Also, water extract containing a low amount of chrysophanol or aloe-emodin had low anti-JEV activity (IC50 = 51.41 μg/ml). Still, both extracts showed therapeutic index (CC50/IC50) > 10. Chrysophanol and aloe-emodin also had concentration-dependently inhibitory effects on JEV plaque reduction; IC50 values were 15.82 and 17.39 μg/ml, respectively. Therapeutic index of chrysophanol was higher than aloe-emodin.
Plaque reduction of JEV by R. palmatum extracts (a) and major components (b). Methanol and water extracts of R. palmatum and its components chrysophanol and aloe-emodin were serial diluted and mixed with JEV (100 pfu), and then each mixture was immediately added into the well of BHK-21 cell monolayer at 37 °C for 1 h and then overlaid with MEM medium containing 1.1 % methylcellulose. Viral plaques were stained with naphthol blue-black dye after 3 days of incubation
Virucidal activity of R. palmatum extracts, chrysophanol and aloe-emodin
To ascertain whether virucidal effect correlates with R. palmatum activity against JEV, R. palmatum extracts, chrysophanol and aloe-emodin were mixed along with JEV, incubated at 4 °C for 1 h and residual infectivity was examined by plaque assay (Fig. 3; Table 1). Results indicate both extracts, chrysophanol and aloe-emodin, exhibiting concentration-dependent virucidal activity as well as significant inhibitory effects on residual infectivity compared to controls. Virucidal IC50 values on JEV infectivity were 7.58 μg/ml of methanol extract, 17.36 μg/ml of water extract, 0.75 μg/ml of chrysophanol, and 0.46 μg/ml of aloe-emodin, respectively.
Virucidal activities of R. palmatum extracts (a) and major components (b). JEV (105 pfu) was mixed with indicated concentrations of extracts, chrysophanol, or aloe-emodin for a 60-min incubation at room temperature. Serial dilution of each extract/virus or compound/virus mixture was added onto BHK-21 cell monolayer in 6-well plates for plaque assays. The residual infectivity was performed as described in the plaque assay
Inhibition of JEV yields by chrysophanol and aloe-emodin
To detect R. palmatum inhibition of virus yields in vitro, viral loads in cultured supernatants for JEV-infected cells with or without treatment were plotted three days post infection by quantitative real-time RT-PCR (Table 2). Real-time RT-PCR assay indicated RNA levels of JEV in supernatant of infected cells treated with 10 μg/ml of R. palmatum extracts, chrysophanol and aloe-emodin as significantly lower than those of untreated infected cells. Subtracting value of average threshold RT-PCR cycle for viral load in cultured supernatants of infected cells treated with R. palmatum extracts, chrysophanol and aloe-emodin from that in untreated infected cells rose above 3.3, indicating 10 μg/ml of extracts, chrysophanol or aloe-emodin had more than 1-log reduction (equal to 90 % effective concentration [EC90]) in virus RNA loads. Results indicated both extracts, chrysophanol and aloe-emodin, inhibiting JEV yields in vitro.
Activation of GAS-driven promoter activity by chrysophanol and aloe-emodin
To examine other possible antiviral mechanism like induction of IFN response, in vivo signalling pathways in BHK-21 cells treated with R. palmatum extracts, chrysophanol and aloe-emodin were further detected by dual-luciferase reporter assay (Fig. 4). Cells were co-transfected with a pGAS-Luc plasmid containing GAS-driven cis-reporter (firefly luciferase) and internal control reporter (Renilla luciferase). After treatment for 4 h, relative expression of firefly luciferase driven from the indicated GAS-driven firefly luciferase activity was normalized by Renilla luciferase. Ratio of firefly luciferase intensity revealed that both extracts of R. palmatum, chrysophanol and aloe-emodin significantly increased activity of GAS-driven promoter. Aloe-emodin showed the highest ability to activate the GAS-driven promoter among them.
Discussion
The R. palmatum methanol extract showed more potent anti-JEV activity with IC50 less than 20 μg/ml (Fig. 1a; Table 1) than water extract, being associated higher level of chrysophanol and aloe-emodin in methanol extract (Figs. 1–2; Table 1). Related components chrysophanol (IC50 = 15.82 μg/ml) and aloe-emodin (IC50 = 17.39 μg/ml) significantly inhibited JEV replication in vitro, implying anti-JEV activity being associated with amount of chrysophanol and aloe-emodin in the extract. Results concurred with our prior studies, in that aloe-emodin definitely inhibited JEV and EV71 yields in human HL-CZ promonocyte cells and TE-671 medulloblastoma cells (Lin et al. 2008). Aloe-emodin also exhibits multiple antiviral effects: e.g., herpes simplex Types 1 and 2, varicella-zoster, pseudorabies and influenza (Sydiskis et al. 1991). Similar effect of chrysophanol derivatives has been reported in HBV, HCV, poliovirus Types 2 and 3 (Li et al. 2007; Semple et al. 2001; Wohlfarth and Efferth 2009). To our knowledge, this study was first to report the antiviral activity of R. palmatum methanol extract and chrysophanol against JEV.
Rheum palmatum extracts exhibited virucidal IC50 value below 20 μg/mL, indicating their direct action against JEV (Fig. 3; Table 1). Chrysophanol and aloe-emodin also had potent virucidal activity, with virucidal IC50 value less than 1 μg/mL, linked with virucidal activity of R. palmatum extracts against JEV. The result was similar to the prior study, in that aloe emodin exhibited the virucidal activity against herpes simplex Types 1 and 2, varicella-zoster, pseudorabies, influenza, but not adenovirus, and rhinovirus (Sydiskis et al. 1991). Moreover, the other anthraquinone derivative hypericin indicated their antiviral and virucidal activities against vesicular stomatitis, herpes simplex types 1 and 2, parainfluenza, and vaccinia viruses (Andersen et al. 1991). Thus, we first demonstrated R. palmatum extracts, chrysophanol and aloe-emodin exhibiting virucidal activity against JEV. Electron microscopic examination revealed anthraquinones partially disrupting the envelopes of herpes simplex viruses, suggesting the action as one of virucidal mechanisms by anthraquinones (Sydiskis et al. 1991). Since anthraquinones has been reported to inactivate non-enveloped viruses including enterovirus 71, poliovirus Types 2 and 3 (Lin et al. 2008; Semple et al. 2001), broad-spectrum antiviral and virucidal activities of R. palmatum extracts, chrysophanol and aloe-emodin against JEV and other viruses are still required to deeply elucidate mechanisms.
JEV yield assays indicated that R. palmatum extracts, chrysophanol and aloe-emodin had a more significant reduction (Table 2). Our results indicated that anti-JEV action of aloe-emodin showed higher inhibitory effect on JEV yields than chrysophanol as well as water extract higher than methanol extract. Except virucidal activity, the result implied that water extract and aloe-emodin could exhibit different anti-JEV actions. R. palmatum water extract has been demonstrated antiviral activities against herpes simplex virus type 1, poliovirus type 1, measles virus and SARS coronavirus (Kurokawa et al. 1993; Xu et al. 2005). Interestingly, R. palmatum extract had anti-SARS-3CL protease activity (Luo et al. 2009). R. palmatum water extract could contain some potent components directly acting against JEV. We previously showed aloe-emodin significantly activated ISRE and GAS-driven cis-reporting systems and induced NO production in human monocytes and medulloblastoma cells (Lin et al. 2008). This study indicated induction activity of GAS-driven promoter was aloe-emodin > chrysophanol > methanol extract > water extract (Fig. 4). The GAS element exists in the genes deriving a variety of antiviral actions, such as responses to IFN α/β, antigen processing and presentation, immune effector action and apoptosis (Cheney et al. 2002; Larkin et al. 2003; Moraes et al. 2007; Mossel et al. 2006; Peng et al. 2008; Scagnolari et al. 2007). Therefore, chrysophanol and aloe-emodin could activate GAS-driven genes as the indrect actions of IFN-γ triggering host innate immune responses against JEV infection.
Rheum palmatum methanol extract containing high amount of chrysophanol and aloe-emodin significantly showed dose-dependent antiviral activities against JEV. Chrysophanol with a higher therapeutic index than aloe-emodin exhibited potent virucidal activity at concentrations of less than 1 μg/ml, reducing 90 % virus yields in vitro at a concentration of 10 μg/ml, and inducing GAS-driven promoter activation as type II IFN-inducers. R. palmatum methanol extract and chrysophanol could exhibit multiple antiviral action against JEV infection, being promising for development of potential antiviral drugs.
References
Andersen, D.O., N.D. Weber, S.G. Wood, B.G. Hughes, B.K. Murray, and J.A. North. 1991. In vitro virucidal activity of selected anthraquinones and anthraquinone derivatives. Antiviral Research 16: 185–196.
Barnard, D.L., J.H. Huffman, J.L. Morris, S.G. Wood, B.G. Hughes, and R.W. Sidwell. 1992. Evaluation of the antiviral activity of anthraquinones, anthrones and anthraquinone derivatives against human cytomegalovirus. Antiviral Research 17: 63–77.
Cheney, I.W., V.C. Lai, W. Zhong, T. Brodhag, S. Dempsey, C. Lim, Z. Hong, J.Y. Lau, and R.C. Tam. 2002. Comparative analysis of anti-hepatitis C virus activity and gene expression mediated by alpha, beta, and gamma interferons. Journal of Virology 76: 11148–11154.
Cheng, H.Y., L.T. Lin, H.H. Huang, C.M. Yang, and C.C. Lin. 2008. Yin Chen Hao Tang, a Chinese prescription, inhibits both herpes simplex virus type-1 and type-2 infections in vitro. Antiviral Research 77: 14–19.
Chung, C.C., S.S. Lee, Y.S. Chen, H.C. Tsai, S.R. Wann, C.H. Kao, and Y.C. Liu. 2007. Acute flaccid paralysis as an unusual presenting symptom of Japanese encephalitis: a case report and review of the literature. Infection 35: 30–32.
Hsiao, N.W., J.W. Chen, T.C. Yang, G.M. Orloff, Y.Y. Wu, C.H. Lai, Y.C. Lan, and C.W. Lin. 2010. ISG15 over-expression inhibits replication of the Japanese encephalitis virus in human medulloblastoma cells. Antiviral Research 85: 504–511.
Huang, Q., G. Lu, H.M. Shen, M.C. Chung, and C.N. Ong. 2007. Anti-cancer properties of anthraquinones from rhubarb. Medicinal Research Reviews 27: 609–630.
Huang, S.H., T.C. Yang, M.H. Tsai, I.S. Tsai, H.C. Lu, P.H. Chuang, L. Wan, Y.J. Lin, C.H. Lai, and C.W. Lin. 2008. Gold nanoparticle-based RT-PCR and real-time quantitative RT-PCR assays for detection of Japanese encephalitis virus. Nanotechnology 19: 405101.
Kaur, R., and S. Vrati. 2003. Development of a recombinant vaccine against Japanese encephalitis. Journal of Neurovirology 9: 421–431.
Kubin, A., F. Wierrani, U. Burner, G. Alth, and W. Grünberger. 2005. Hypericin—The facts about a controversial agent. Current Pharmaceutical Design 11: 233–253.
Kuo, P.L., T.C. Lin, and C.C. Lin. 2002. The anti-proliferative activity of aloe-emodin is through p53-dependent and p21-dependent apoptotic pathway in human hepatoma cell lines. Life Sciences 71: 1879–1892.
Kurokawa, M., H. Ochiai, K. Nagasaka, M. Neki, H. Xu, S. Kadota, S. Sutardjo, T. Matsumoto, T. Namba, and K. Shiraki. 1993. Antiviral traditional medicines against herpes simplex virus (HSV-1), poliovirus, and measles virus in vitro and their therapeutic efficacies for HSV-1 infection in mice. Antiviral Research 22: 175–188.
Larkin, J., L. Jin, M. Farmen, D. Venable, Y. Huang, S.L. Tan, and J.I. Glass. 2003. Synergistic antiviral activity of human interferon combinations in the hepatitis C virus replicon system. Journal of Interferon and Cytokine Research 23: 247–257.
Li, Z., L.J. Li, Y. Sun, and J. Li. 2007. Identification of natural compounds with anti-hepatitis B virus activity from Rheum palmatum L. ethanol extract. Chemotherapy 53: 320–326.
Lin, J.G., G.W. Chen, T.M. Li, S.T. Chouh, T.W. Tan, and J.G. Chung. 2006. Aloe-emodin induces apoptosis in T24 human bladder cancer cells through the p53 dependent apoptotic pathway. The Journal of Urology 175: 343–347.
Lin, C.W., C.F. Wu, N.W. Hsiao, C.Y. Chang, S.W. Li, L. Wan, Y.J. Lin, and W.Y. Lin. 2008. Aloe-emodin is an interferon-inducing agent with antiviral activity against Japanese encephalitis virus and enterovirus 71. International Journal of Antimicrobial Agents 32: 355–359.
Luo, W., X. Su, S. Gong, Y. Qin, W. Liu, J. Li, H. Yu, and Q. Xu. 2009. Anti-SARS coronavirus 3C-like protease effects of Rheum palmatum L. extracts. Bioscience Trends 3: 124–126.
Mijatovic, S., D. Maksimovic-Ivanic, J. Radovic, D.J. Miljkovic, L.J. Harhaji, O. Vuckovic, S. Stosic-Grujicic, Mostarica, M. Mostarica, and V. Trajkovic. 2005. Anti-glioma action of aloe emodin: The role of ERK inhibition. Cellular and Molecular Life Sciences 62: 589–598.
Moraes, M.P., T. de Los Santos, M. Koster, T. Turecek, H. Wang, V.G. Andreyev, M.J. Grubman, V.J. Andreyev, and M.J. Grubman. 2007. Enhanced antiviral activity against foot-and-mouth disease virus by a combination of type I and II porcine interferons. Journal of Virology 81: 7124–7135.
Mossel, E.C., B. Sainz Jr, R.F. Garry, and C.J. Peters. 2006. Synergistic inhibition of SARS-coronavirus replication by type I and type II IFN. Advances in Experimental Medicine and Biology 581: 503–506.
Peng, T., J. Zhu, Y. Hwangbo, L. Corey, and R.E. Bumgarner. 2008. Independent and cooperative antiviral actions of beta interferon and gamma interferon against herpes simplex virus replication in primary human fibroblasts. Journal of Virology 82: 1934–1945.
Scagnolari, C., S. Trombetti, A. Alberelli, S. Cicetti, D. Bellarosa, R. Longo, A. Spanò, E. Riva, M. Clementi, and G. Antonelli. 2007. The synergistic interaction of interferon types I and II leads to marked reduction in severe acute respiratory syndrome-associated coronavirus replication and increase in the expression of mRNAs for interferon-induced proteins. Intervirology 50: 156–160.
Semple, S.J., S.M. Pyke, G.D. Reynolds, and R.L. Flower. 2001. In vitro antiviral activity of the anthraquinone chrysophanic acid against poliovirus. Antiviral Research 49: 169–178.
Shuangsuo, D., Z. Zhengguo, C. Yunru, Z. Xin, W. Baofeng, Y. Lichao, and C. Yan’an. 2006. Inhibition of the replication of hepatitis B virus in vitro by emodin. Medical Science Monitor 12: 3026.
Sydiskis, R.J., D.G. Owen, J.L. Lohr, K.H. Rosler, and R.N. Blomster. 1991. Inactivation of enveloped viruses by anthraquinones extracted from plants. Antimicrobial Agents and Chemotherapy 35: 2463–2466.
Unni, S.K., D. Růžek, C. Chhatbar, R. Mishra, M.K. Johri, and S.K. Singh. 2011. Japanese encephalitis virus: From genome to infectome. Microbes and Infection 13: 312–321.
Xu, X., L. Li, and J. Cong. 2005. An overview of antiviral research of Chinese medicine Rhubarb. China Pharmacist 8: 70–72.
Wohlfarth, C., and T. Efferth. 2009. Natural products as promising drug candidates for the treatment of hepatitis B and C. Acta Pharmacologic Sinica 30: 25–30.
Acknowledgments
This project was supported by grants from China Medical University (CMU101-S-24, CMU101-ASIA-05), and the Republic of China National Science Council (NSC 102-2320-B-039-044-MY3).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chang, SJ., Huang, SH., Lin, YJ. et al. Antiviral activity of Rheum palmatum methanol extract and chrysophanol against Japanese encephalitis virus. Arch. Pharm. Res. 37, 1117–1123 (2014). https://doi.org/10.1007/s12272-013-0325-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-013-0325-x