Abstract
A wide variety of delivery systems have been developed and many products based on the drug delivery technology are commercially available. The development of controlled-release technologies accelerated new dosage form design by altering pharmacokinetic and pharmacodynamics profiles of given drugs, resulting in improved efficacy and safety. Various natural or synthetic polymers have been applied to make matrix, reservoir or implant forms due to the characteristics of polymers, especially ease of control for modifications of biocompatibility, biodegradation, porosity, charge, mechanical strength and hydrophobicity/hydrophilicity. Hydrogel is a hydrophilic, polymeric network capable of imbibing large amount of water and biological fluids. This review article introduces various applications of natural and synthetic polymer-based hydrogels from pharmaceutical, biomedical and bioengineering points of view.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recent advances in recombinant biotechnology have produced specific and efficient peptide and protein drug candidates in an economic process. However, several challenges still remain to overcome physicochemical and biological hurdles such as low stability, low permeability, short half-life, enzymatic susceptibility, targeting and immunogenicity. A wide variety of delivery systems have been developed and many products based on the drug delivery technology are commercially available. Conventional development of proteins followed parenteral injections of liquid formulations to detour first pass metabolism after oral administration. The development of controlled-release technologies accelerated new dosage form design by altering pharmacokinetic and pharmacodynamics profiles of given drugs, resulting in improved efficacy and safety. Various natural or synthetic polymers have been applied to make matrix, reservoir or implant forms due to the characteristics of polymers, especially ease of control for modifications of biocompatibility, biodegradation, porosity, charge, mechanical strength and hydrophobicity/hydrophilicity. The delivery method and route for a given drug should be screened based on biological efficacy, bioavailability, stability, toxicity, ease of manufacturing, etc.
Hydrogel is a three-dimensional, hydrophilic homo or copolymer network, which is capable of soaking up large amounts of water or biological fluids. Its affinity to absorb water is attributed to the presence of hydrophilic groups such as –OH, –COOH, –NH2, –CONH and –CONH2 in polymers forming hydrogel structures (Peppas and Khare 1993). The three-dimensional network of hydrogel can be formed by covalent bond, van der Waals interaction, hydrogen bonding and physical entanglement. Hydrogel has been used in the development of smart drug delivery systems. Hydrogel can protect a drug from enzyme and low pH, and enhance the bio-activity of drug. Hydrogel property can be controlled by the chemical or physical cross-linking of polymer. The gelation property of hydrogel depends on chemical composition, cross-linking density and hydrophobicity. Hydrogel has been widely used for numerous protein and gene delivery application. The bio-compatibility of hydrogel should be considered because hydrogel structure is similar to macromolecular based components in the body and encounters invasive administration to the body (Lee and Mooney 2001).
This review article introduces various applications of natural and synthetic polymer-based hydrogels from pharmaceutical, biomedical and bioengineering points of view. Special emphasis has been made on hydrogels based on natural polymers such as Fibrin, hyaluronic acid, alginate, chitosan, dextran, methylcellulose, gellan and guar gum, and synthetic polymers such as poly(ethylene oxide) (PEO), poly(acrylic acid) (PAA), poly(N-isopropylacrylamide), poly(vinyl alcohol) and poly(ethylene glycol) (PEG)-polyester copolymers.
Natural polymer-based hydrogels
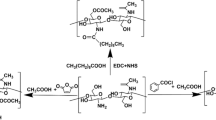
The selection of biomaterial is an important step in the development of drug delivery system. Biomaterials for drug delivery purpose should be bio-compatible, non-toxic and bio-eliminable. Natural polymers are produced by several living organisms and typical examples for the pharmaceutical uses are shown in Fig. 1.
Fibrin
Fibrin is a fibrous protein involved in the clotting of blood and wound healing (Laurens et al. 2006) and has been widely used as surgical operation and drug delivery devices. Fibrin can form a gel through enzymatic polymerization of fibrinogen in the presence of thrombin (Perka et al. 2000). As fibrin can be isolated from patient’s blood, fibrin gel has been tested as an autologous carrier for protein drug delivery, which has minimum immune response and toxic degradation. Fibrin gel can be eliminated by enzymatic degradation during wound healing (Ye et al. 2000). The epidermal growth factor and keratinocyte growth factor were loaded to fibrin hydrogel and applied for epidermal regeneration in athymic mice (Gwak et al. 2005). The bone morphogenetic protein-2-loaded fibrin hydrogel was transplanted to animal model and demonstrated significant increase in bone formation. Another growth factor protein, fibroblast growth factor-1, was loaded in fibrin and hydroxyapatite mixed hydrogel (Geer et al. 2005). This system showed site-specific delivery of growth factor and enhancement of angiogenesis and osteogenic cellular response. However, mechanical strength of fibrin hydrogel limits its clinical application.
Hyaluronic acid
Hyaluronic acid is a glycosaminoglycan distributed widely in connective, epithelial and neural tissues, which consists of β-1,4-linked dimer units of β-1,3-linked d-glucuronic acid and N-acetyl-d-glucosamine (Fig. 1a). Hyaluronic acid having diverse molecular weights has been widely used as a drug delivery device for ophthalmia (Saettone et al. 1994) and applied in fields of various surgery and wound healing (Luo et al. 2000; Arnold et al. 1995; Brown et al. 1999). Hyaluronic acid can form hydrogel by polyion complex formation (Arimura et al. 2005), covalent cross-linking (Shu et al. 2002), radical polymerization (Inukai et al. 2000) or photo-cross-linking (Leach and Schmidt 2005). Hyaluronic acid can also form hybrid gels with PEG diepoxide by a cross-linking method. This hybrid gel has 6–9 μm diameter pore and can be degraded by hyaluronidase and collagenase. Amino or aldehyde functional group could be introduced to hyaluronic acid by cross-linking to form hydrogel. This gel showed effective delivery of bone morphogenetic protein-2 for ectopic bone formation.
Methylcellulose
Cellulose is a natural polysaccharide consisting of linear chain of β-(1-4) linked d-glucose unit (Fig. 1b). Methylcellulose and hydroxypropyl methylcellulose and hydrophobically-modified cellulose derivatives are water soluble polymers that have been utilized in food, ceramic processing and pharmaceutical areas (Sarkar 1979; Hirrien et al. 1998). Methylcellulose solution transforms into gel over 50 °C, whereas hydroxypropyl methylcellulose shows the phase transition between 75 and 90 °C. Due to the high gelation temperature of methylcellulose, several challenges have been made to decrease gelling temperature by grafting with a synthetic polymer such as N-isopropylacrylamide or adding salting-out salts (Liu et al. 2004; Jin and Kim 2008). It was reported that the addition of methylcellulose decreases gelling temperature of N-isopropylacrylamide-based hydrogel and enhances mechanical strength. We have reported that the addition of ammonium sulfate to methylcellulose aqueous solution can reduce gelling temperature at which the phase remains a solution at room temperature, but changes into gel at body temperature, which becomes practical for clinical applications (Jin and Kim 2008). This thermo-reversible hydrogel provided sustained release of a therapeutic protein and long term therapeutic efficacy in a ligation/reperfusion rat myocardial infarction model (Won et al. 2010). Methylcellulose is capable of maintaining thermo-gelling property even after mixing with other polymers. Pluronic F127 was added into the methylcellulose solution to solubilize docetaxel, a hydrophobic anti-cancer drug, and homogeneously mix with the solution. This combination system showed slow release pattern of docetaxel and effectively reduced tumor growth in tumor xenograft mice model (Kim et al. 2012). Aqueous solution of ethyl (hydroxyl-ethyl) cellulose (EHEC) also exhibits the temperature sensitive behavior. However, the viscosity of EHEC decreases at high temperature, which is not appropriate for the preparation of injectable formulations. Several studies demonstrated that the addition of an ionic surfactant to the EHEC solution could modify its thermal behavior (Wittgren et al. 2005). This system showed a sol–gel transition upon heating from room to body temperature, resulting in the formation of clear gels. The surfactant was considered to interact with EHEC by a strong cooperative process forming micelle-like clusters on the polymer. The EHEC/surfactant system for local delivery of lidocaine and priocaine showed sustained release profiles over 60 min, making them effective for short term pain control.
Dextran
Dextran is a polysaccharide composed of branched glucan (Fig. 1c) and synthesized by bacteria and yeast. Due to dextran’s biocompatibility, dextran-based hydrogels have been widely used as a blood plasma substitute and delivery systems of various drugs. There are several types of dextran hydrogel including glycidyl methacrylate dextran, hydroxyethyl methacrylate dextran and hydroxyethyl methacrylate lactate dextran. The glycidyl methacrylate dextran was able to efficiently load several proteins such as lysozyme, albumin, BSA and immunoglobulin G using radical polymerization. The release rates of model proteins from hydrogel depended on the protein size and cross-link density. This study demonstrated that release of model protein can be modulated by changing cross-linking density, initial water content and amount of loaded dextranase (Franssen et al. 1999a). Another dextran derivative, hydroxyethyl methacrylated dextran, was prepared in forms of hydrogel and microsphere for immunoglobulin G delivery (Franssen et al. 1999b). Due to the presence of carbonate ester, this hydrogel was degradable under physiological condition. The immunoglobulin G-loaded hydrogel showed a biphasic release pattern resulting from the chain dissolution.
Alginate
Alginate is an anionic polysaccharide widely distributed in the cell walls of brown algae and provides a gelation property. Alginate is a linear polysaccharide that consisted of 1,4-linked β-d-mannuronic acid and α-l-guluronic acid (Fig. 1d). The mechanical and swelling properties of alginate hydrogel can be controlled by covalent cross-linking with various types of molecules. Because the molecular weights of commonly used alginates are larger than the renal clearance cut-off in kidney, low molecular weight alginate and its derivatives have been developed. The pH- and temperature-dependent hydrogels were made by mixing methylcellulose and alginate. This system can easily mix with drug and transform into the gel state by increasing temperature due to the thermo-gelation property of methylcellulose. This gel could transform its state under low pH condition due to the acidic property of alginate (Bouhadir et al. 2001). The temperature-sensitive hydrogel has been developed by hiding calcium ions into temperature-sensitive liposomes. The calcium liposome/alginate hybrid gel remained aqueous solution at 20 °C, gelation occurred when this system was heated above the liposome phase transition temperature due to the leakage of entrapped calcium (Ruel-Gari and Leroux 2004). Slightly oxidized alginate was introduced and showed gel degradation depending on the pH and temperature of aqueous media (Bouhadir et al. 2001). Alginate hydrogel has been widely utilized as injectable delivery systems of cells and proteins including insulin and growth factors. The osteoinductive growth factor-loaded poly(lactic-co-glycolic acid) microsphere was mixed with alginate solution in the presence of calcium carbonate. This delivery system showed effective delivery of growth factor and increase in the proliferation rate of MG-63 cells. It is also useful for conformal filling of bone defects (Luginbuehl et al. 2005).
Chitosan
Chitosan is prepared by N-deacetylation of chitin (Fig. 1e). Chitosan has been tested for biomedical applications including wound dressing, drug delivery and tissue engineering. Chitosan having similar structure with natural GAGs is biocompatible, non-toxic and degradable by chitosanase and lysozyme (Li et al. 2013). While chitosan is insoluble under neutral condition and in most organic solvents, it is soluble in acidic media due to the existence of amino groups and high crystallinity. Therefore, many studies have developed chitosan derivatives for the improvement enhancement of solubility and processing (Kurita 2006). Chitosan can form hydrogel by either physical cross-linking or chemical cross-linking with glutaraldehyde (Lin et al. 2005; Obara et al. 2003). The UV irradiation is another method for gelation of chitosan derivatives (Obara et al. 2003). Grafting PEG to chitosan resulted in temperature reversible property. This mixture solution is injectable liquid at low temperature, which transforms into a semi-solid gel at body temperature. Cross-linking BSA to this copolymer by genipin showed prolonged and quasi-linear release profile. This system is useful for sustained protein delivery and tissue engineering applications (Bhattarai et al. 2005). For effective insulin delivery, chitosan gel bead was prepared with copper (II) ion and glycine (Kofuji et al. 2003, 2005). The insulin-loaded chitosan gel bead was injected into diabetic mice model and consistent reduction of blood glucose level was observed. For the treatment of chronic myocardial infarctions, fibroblast growth factor was incorporated into chitosan gel and implanted near ischemic myocardium surface. Sustained release of growth factor from chitosan gel induced angiogenesis and improved blood circulation in the ischemic myocardium (Fujita et al. 2005).
Gellan
Gellan gum is a bacterial exo-polysaccharide prepared by the aerobic submerged fermentation. This polymer can form hydrogel that becomes mechanically strong when formed with divalent ions. Gellan hydrogel is temperature-reversible at about 50 °C, depending on the concentration and presence of cations. Gellan have been widely used as food ingredient and novel ophthalmic formulations due to biocompatibility. Gellan hydrogel can be formed in tear fluid and sodium proved to be the best gel promoting ion in vivo (Paulsson et al. 1999). Gellan hydrogel was developed for nasal delivery of fluorescein dextran used as a model molecule (Jansson et al. 2005). Gellan solution has a fluid like property, but it can transform into gel rapidly when exposed to cations. Therefore, this gel is suitable for nasal delivery with its low viscosity at room temperature and subsequent gelation upon contact with mucosa. It has been shown that 0.9 % NaCl contained gellan hydrogel forms strong hydrogel that remains in the nasal cavity for required time interval. The optimized gellan hydrogel showed slow clearance due to high local concentration of polymer. Recently, gellan bead was developed to evaluate the effect of various cations (Singh and Kim 2005). The gelation mechanism of gellan is divided into two steps, the first step is the formation of double helices from random coil chains and the second step is an aggregation of pairs of double helices. The electrostatic interaction with cations in solution affected coil-helix transition of gellan hydrogel. Other approach of gellan hydrogel is oral drug delivery (Miyazaki et al. 1999). The gellan solutions contained calcium chloride and sodium citrate showed release of a model drug in high acidic environment of the stomach. After oral administration of gellan hydrogel, the plasma level of drug demonstrated a sustained release profile representing higher bioavailability compared with commercial solution products.
Pullulan
Pullulan is a bacterial homo-polysaccharide produced by Aurebasidium pullulans. Pullulan is formed by glycosidic linkage of α(1-6) d-glucopyranose and α(1-4) d-glucopyranose unit. This polymer has been used in food processing as a filler, as a pharmaceutical coating agent and in manufacturing using its fiber forming property. Pullulan derivatives have been developed to introduce new physic-chemical property. These derivatives provided increased solubility in organic solvent and new reactive functional groups. Cholesterol-bearing pullulan has a tendency to form self-aggregates with hydrophobic model drugs (Lee and Akiyoshi 2004; Jiang et al. 2013). The cholesterol group could be introduced to the pullulan backbone by non-covalent cross-linking. This nanogel was stable so that the size did not change for a week at room temperature. The nanogel was capable of binding with various hydrophobic drugs and soluble proteins (Akiyoshi et al. 1998; Lee and Akiyoshi 2004; Kobayashi et al. 2009). Hydrophobically-modified pullulan has been applied for anti-cancer drug delivery. The cytotoxicity result of adriamycin-loaded gel in breast tumor cell line at different pH conditions showed increased cytotoxicity at pH 6.8. Recently, pullulan nanogel has been developed for gene delivery to mammalian cell (Gupta and Gupta 2004). Pullulan nanogel with a hydrophilic core was prepared using glutaraldehyde as a cross-linker, resulting system being able to encapsulate water soluble DNA for intracellular delivery. Anionic pullulan hydrogel was reported to be prepared using epichlorohydrine as cross-linking agent (Mocanu et al. 2002). This nanogel was able to protect loaded protein against acidic environment, thus it was thought to be useful for oral administration of gastro-sensitive drug.
Guar gum
Guar gum is widely distributed in nature and made up of mannose and galactose sugar units. They differ from each other in their mannose/galactose ratio and distribution pattern of the galactose residues along the mannan chain. This polymer has been studied for colon delivery because it can be degraded by enzymes present in the intestine (Sinha and Kumria 2001). Guar gum hydrogel can protect a drug in the stomach and small intestine, while delivering drug to colon where it undergoes assimilation by specific microorganisms or degradation by the enzymes could be achievable.
Synthetic polymer based hydrogel
Temperature-sensitive synthetic polymers
The hydrogels that have temperature sensitive properties are most studied hydrogel systems in drug deliver R&D. Temperature-sensitive polymers contain hydrophobic groups such as methyl, ethyl, propyl or aromatic groups. Poly(N-isopropylacrylamide) (PNIPAAm) is a classical synthetic temperature-sensitive polymer. Poly(N,N-diethylacrylamide)(PDEAAm) has been widely studied due to a low lower critical solution temperature (LCST) close to the body temperature. A series of block co-polymers composed of PEO and poly(propylene oxide) (PPO) showed inverse temperature sensitive property. Because its gelation temperature is at around body temperature, these co-polymers have been applied for controlled drug delivery.
PEO has been widely studied and used for diverse medical applications due to its low toxicity and bio-compatibility. PEO can be synthesized by cationic or anionic polymerization of ethylene. Many types of PEO derivatives can be prepared by irradiation and chemical cross-linking with genipin. Branched PEO and bi-functional PEG have been reported to form gel and demonstrated effectiveness for protein delivery (Andreopoulos et al. 1996; Zhao and Harris 1997). Star-shaped PEO was prepared by irradiation and modified with galactose for liver cell targeting. Also, heparin-binding peptide-conjugated star PEO showed sol–gel transition property. Various PEO-based co-polymers have been developed and used in drug delivery field (Gombotz and Pettit 1995; Harada and Kataoka 1999). A series of triblock copolymer of PEO and poly(propylene oxide) (PEO–PPO–PEO) (poloxamer) is one of representative copolymer known by the trade names: Synperonics, Pluronics and Kolliphor. This copolymer behaves thermo-reversible gelation without cross-linking reagents. Due to the enhancement of drug penetration and activity of anti-neoplastic agents against tumor, poloxamer has been widely used for drug delivery (Alakhov et al. 1996). Other protein drugs such as insulin, lysozyme and BSA can be encapsulated to copolymer-based hydrogel and released with controlled manners. Because the release rate of protein drug can be modified with temperature, it is also useful vehicle for protein drug delivery. Although poloxamers have various advantages for drug delivery, their non-biodegradability limits practical clinical application. Therefore, degradable copolymer was synthesized with poly(lactic acid)(PLA) which is degradable and applied in many medical applications (Lee and Lee 2002). PLA–PEO–PLA copolymer was synthesized by ring opening polymerization. This copolymer-based hydrogel was fully soft, so hydrogel could be injected using a syringe for delivery of protein drug. Degradable PLA–PEO–PLA multi-block copolymer can be made by condensation reaction of l-lactic acid. This copolymer hydrogel has a temperature dependent reversible sol–gel transition property, which can be easily formulated with protein drug at body temperature. By this reason, this system is useful in drug delivery and tissue engineering.
Poly(N-isopropylacrylamide) (PNIPAAm) is a temperature-sensitive polymer that can physically mix and be cross-linked with proteins, DNA and engineered recombinant proteins. The LCST of PNIPAAm hydrogel is approximately 32 °C so that this gel can be easily mixed with protein drug solution at room temperature and injected to desired site with invasive manner (Kim and Healy 2003). However, this hydrogel is not biodegradable and cross-linking molecule is mostly toxic and carcinogenic. To solve this problem, dextran-conjugated PNIPAAm copolymer was developed to modulate the degradation property with temperature (Huh et al. 2000). Lysozyme was loaded into this gel by polyelectrolyte complex and released from gel retaining its enzymatic activity. The release rate of lysozyme was controlled by the length of ethylene glycol chains and content of phosphate-carrying monomer units. Recent study reported a copolymer synthesized by reversible addition fragmentation transfer method using different PNIPAAm and PAA ratio (Yin et al. 2006). The release rate of loaded drug can be controlled by physiological temperature and local pH difference.
Several studies introduced a copolymer made of hydrophilic, bio-compatible PEG and bio-degradable polyester. Thermo-reversible property of hydrogel was given by adjustment of hydrophobic polyester block and PEG block length. The injectable PEG–PLGA–PEG triblock copolymer was developed (Jeong et al. 1997). This copolymer was bio-degradable, bio-compatible and had sol–gel transition property. A hydrogel quickly gelling at physiological temperature was made using a mixture of high molecular weight PLGA and low molecular weight PEG. This hydrophilic/hydrophobic combination provided a surfactant behavior of polymer in aqueous condition, which allowed solubilization of a hydrophobic drug. This hydrogel provided sufficient mechanical properties and maintained integrity for longer than a month (Jeong et al. 2000). For delivery of testosterone, tri-block PLGA–PEG–PLGA based hydrogel system was developed (Chen and Singh 2005). They demonstrated that loaded testosterone was slowly released than commercially used devices by slow degradation of hydrophobic units. Regel® is one of the Macromed’s delivery technologies and based on triblock copolymer, PLGA–PEG–PLGA. Oncogel® is a frozen formulation of paclitaxel in Regel®. Oncogel® was injected directly into the tumor and showed a continuous release of paclitaxel for up to 6 weeks. Cytoryn® is a novel, injectable depot system of interleukin-2 (IL-2) for cancer immunotherapy using Regel® delivery system. This system is liquid form below room temperature, which immediately forms a gel depot upon injection from which the drug is released in a controlled manner. Regel® system stabilized and released IL-2 in its bioactive form. The release rate of drug was controlled by diffusion and degradation of the depot. Another recent approach was based on multi-block copolymer with biodegradable polyester. PEG/poly(l-lactic acid)(PLLA) copolymer showed a sol–gel transition property depending on both total molecular weight and molecular weight of each building block. In vitro and in vivo gelation studies demonstrated that copolymer with an optimized molecular weight holds potential as an injectable carrier for biomedical application (Lee et al. 2006).
pH-sensitive synthetic polymers
The pH-sensitive polymers have acidic or basic groups that either accept or release protons in response to changes in environmental pH. The polymers with large number of ionic groups are named polyelectrolytes. Hydrogel made of cross-linked polyelectrolytes showed a big difference in swelling property depending on the pH. The swelling and pH-sensitivity of hydrogel can be modified using neutral co-monomers such as 2-hydroxyethyl methacrylate, methyl methacrylate and maleic anhydride (Falamarzian and Varshosaz 1998; Kou et al. 1988). Hydrogel-based poly(methacrylic acid) (PMA) grafted with PEG showed a unique pH-sensitive property. The acidic protons of carboxyl groups of PMA interacted with ether oxygen of PEG at low pH and formed hydrogel. Due to ionization of carboxyl groups of PMA at high pH, de-complexation led to swelling of hydrogel.
pH-sensitive polymer-based hydrogels have been widely used to develop controlled release formulations for oral administration. Hydrogel made of PAA or PMA could facilitate the release of drugs in a neutral pH environment. PAA-based hydrogel was studied for controlled release of proteins such as albumin, lysozyme, insulin and fibrinogen (Am Ende and Peppas 1997). Co-polymerization of acrylic acid and PEO constituted cross-linked network of PAA and PEO. This hydrogel was an efficient system for delivery of cationic protein and cytochrome c via polyion complex formation. The protein release rate from hydrogel can be controlled by addition of calcium ions or polycations (Oh et al. 2007). For oral delivery of insulin, cross-linked poly(methacrylic acid)–PEG and PAA–PEG were designed. These copolymer hydrogel showed low cytotoxicity in cultured cells and enhanced permeability of insulin released from hydrogel across the cell monolayer.
Poly(vinylacetaldiethylaminoacetate) (PVD) has pH-dependent aqueous solubility. The scanning electron microscope and turbidity observations demonstrated that PVD formed hydrogel in pH from 4 to 7.4 (Aikawa et al. 1998). The release property of PVD hydrogel was tested using chlorpheniramine maleate. While the drug was rapidly released from PVD solution, the release rate was significantly reduced with PVD hydrogel formation. In vivo study showed that the disappearance rate of chlorpheniramine maleate was decreased by increasing PVD concentration.
Other environment-sensitive synthetic polymers
Electro-sensitive hydrogels have been developed for drug delivery. Electro-sensitive hydrogels showed sol–gel transition properties in the presence of electric field. Poly(2-acrylamido-2-methylpropane sulfonic acid-co-n-butylmethacrylate)-based hydrogel was tested to release edrophonium chloride in a pulsatile manner (Kwon et al. 1991). The intensity of electric stimulation in media controlled release of drug with ‘on–off’ manner. The electric field-induced volume change of poly(dimethylaminopropyl acrylamide) hydrogel induced pulsatile release of insulin (Sawahata et al. 1990). Light-sensitive hydrogels were developed by conjugation of light-sensitive chromophore to poly(N-isopropylacrylamide) hydrogel. When the light applied to hydrogel, the chromophore, such as trisodium salt of copper chlorophyllin, absorbed light, local temperature of hydrogel increased, finally hydrogel formed. The change in temperature could be controlled by light intensity and concentration of chromophore. Several polymers can form in situ gels by solvent exchange method several products are already in the market. Eligard® was developed by the Sanofi-Aventis company and has an indication for the palliative treatment of advanced prostate cancer. This polymeric delivery system is composed of biodegradable poly(dl-lactide-co-glycolide) polymer dissolved in biocompatible solvent, N-methyl-2-pyrrolidone. A therapeutic agent, leuprolide acetate, is physically mixed with the polymer immediately before injection. The drug/polymer mixture is injected via needle into the subcutaneous tissue and forms solid drug depot. Then, leuprolide acetate is released at a controlled rate as the polymer is degraded by normal biologic processes. For the treatment of periodontal disease, the Tolmar incorporation has produced Atritox®, 10 % doxycycline hyclate in the ATRIGEL® delivery system. This product is composed of two syringe mixing system. The A syringe contains ATRIGEL®, 36.7 % poly(dl-lactide) dissolved in 63.3 % N-methyl-2-pyrrolidone, and B syringe contains 50 mg of doxycycline hyclate. This system is applied into the disease site after mixing A and B syringes. Upon contacting with crevicular fluid, viscose solution solidifies and allows controlled release of drug for a period of 7 days.
Conclusion
In this review, we summarized natural and synthetic polymers that have potentials for delivery of drugs in the forms of hydrogel. Hydrogels originated from natural polymers are generally bio-compatible and bio-degradable. However, the source of polymer and batch variation provides limitations in practical clinical applications. Although various synthetic polymer-based hydrogels were developed for drug delivery and tissue engineering, most of synthetic polymer-based hydrogels are not generally degradable in body and remaining chemical reagents such as monomer, cross-linker and organic solvent cause toxicity and irritation to body.
For the development of polymeric carriers for drug delivery, special concerns should be given on how appropriate polymer can be selected, synthesized, designed and tailored to effectively deliver drug in response to signals generated in body. Controlling release rate of drug corresponding to environmental sensitivity provided to polymer is critical factor in maintaining drug concentration within the therapeutic window. Although a number of intelligent systems using polymer have been developed, few studies reported effect of mechanical signals on release of drug from hydrogel. Mechanical signal is very useful stimuli for controlling drug release.
Design and synthesis of new polymeric system providing improved bio-compatibility, bio-degradability and targetability would be essential for practical clinical applications.
References
Aikawa, K., K. Matsumoto, H. Uda, S. Tanaka, H. Shimamura, Y. Aramaki, and S. Tsuchiya. 1998. Hydrogel formation of the pH response polymer polyvinylacetal diethylaminoacetate (AEA). International Journal of Pharmaceutics 167: 97–104.
Akiyoshi, K., S. Kobayashi, S. Shichibe, D. Mix, M. Baudys, S.W. Kim, and J. Sunamoto. 1998. Self-assembled hydrogel nanoparticle of cholesterol-bearing pullulan as a carrier of protein drugs: Complexation and stabilization of insulin. Journal of Controlled Release 54: 313–320.
Alakhov, V., E. Moskaleva, E.V. Batrakova, and A.V. Kabanov. 1996. Hypersensitization of multidrug resistant human ovarian carcinoma cells by pluronic P85 block copolymer. Bioconjugate Chemistry 7: 209–216.
Am Ende, M.T., and N.A. Peppas. 1997. Transport of ionizable drugs and proteins in crosslinked poly(acrylic acid) and poly(acrylic acid-co-2-hydroxyethyl methacrylate) hydrogels. II. Diffusion and release studies. Journal of Controlled Release 48: 47–56.
Andreopoulos, F.M., C.R. Deible, M.T. Stauffer, S.G. Weber, W.R. Wagner, E.J. Beckman, and A.J. Russell. 1996. Photoscissable hydrogel synthesis via rapid photopolymerization of novel PEG-based polymers in the absence of photoinitiators. Journal of the American Chemical Society 118: 6235–6240.
Arimura, H., T. Ouchi, A. Kishida, and Y. Ohya. 2005. Preparation of a hyaluronic acid hydrogel through polyion complex formation using cationic polylactide-based microspheres as a biodegradable cross-linking agent. Journal of Biomaterials Science Polymer Edition 16: 1347–1358.
Arnold, F., C. Jia, C. He, G.W. Cherry, B. Carbow, W. Meyer-Ingold, D. Bader, and D.C. West. 1995. Hyaluronan, heterogeneity, and healing: The effects of ultrapure hyaluronan of defined molecular size on the repair of full-thickness pig skin wounds. Wound Repair and Regeneration 3: 299–310.
Bhattarai, N., H.R. Ramay, J. Gunn, F.A. Matsen, and M. Zhang. 2005. PEG-grafted chitosan as an injectable thermosensitive hydrogel for sustained protein release. Journal of Controlled Release 103: 609–624.
Bouhadir, K.H., K.Y. Lee, E. Alsberg, K.L. Damm, K.W. Anderson, and D.J. Mooney. 2001. Degradation of partially oxidized alginate and its potential application for tissue engineering. Biotechnology Progress 17: 945–950.
Brown, T.J., D. Alcorn, and J.R. Fraser. 1999. Absorption of hyaluronan applied to the surface of intact skin. Journal of Investigative Dermatology 113: 740–746.
Chen, S.B., and J. Singh. 2005. Controlled delivery of testosterone from smart polymer solution based systems: In vitro evaluation. International Journal of Pharmaceutics 295: 183–190.
Falamarzian, M., and J. Varshosaz. 1998. The effect of structural changes on swelling kinetics of polybasic/hydrophobic pH-sensitive hydrogels. Drug Development and Industrial Pharmacy 24: 667–669.
Franssen, O., R.J. Stenekes, and W.E. Hennink. 1999a. Controlled release of a model protein from enzymatically degrading dextran microspheres. Journal of Controlled Release 59: 219–228.
Franssen, O., L. Vandervennet, P. Roders, and W.E. Hennink. 1999b. Degradable dextran hydrogels: Controlled release of a model protein from cylinders and microspheres. Journal of Controlled Release 60: 211–221.
Fujita, M., M. Ishihara, Y. Morimoto, M. Simizu, Y. Saito, H. Yura, T. Matsui, B. Takase, H. Hattori, Y. Kanatani, M. Kikuchi, and T. Maehara. 2005. Efficacy of photocrosslinkable chitosan hydrogel containing fibroblast growth factor-2 in a rabbit model of chronic myocardial infarction. Journal of Surgical Research 126: 27–33.
Geer, D.J., D.D. Swartz, and S.T. Andreadis. 2005. Biomimetic delivery of keratinocyte growth factor upon cellular demand for accelerated wound healing in vitro and in vivo. American Journal of Pathology 167: 1575–1586.
Gombotz, W.R., and D.K. Pettit. 1995. Biodegradable polymers for protein and peptide drug delivery. Bioconjugate Chemistry 6: 332–351.
Gupta, M., and A.K. Gupta. 2004. Hydrogel pullulan nanoparticles encapsulating pBUDLacZ plasmid as an efficient gene delivery carrier. Journal of Controlled Release 99: 157–166.
Gwak, S.J., S.S. Kim, K. Sung, J. Han, C.Y. Choi, and B.S. Kim. 2005. Synergistic effect of keratinocyte transplantation and epidermal growth factor delivery on epidermal regeneration. Cell Transplantation 14: 809–817.
Harada, A., and K. Kataoka. 1999. Chain length recognition: Core-shell supramolecular assembly from oppositely charged block copolymers. Science 283: 65–67.
Hirrien, M., C. Chevillard, J. Desbrieres, M.A.V. Axelos, and M. Rinaudo. 1998. Thermogelation of methylcelluloses: New evidence for understanding the gelation mechanism. Polymer 39: 6251–6259.
Huh, K.M., J. Hashi, T. Ooya, and N. Yui. 2000. Synthesis and characterization of dextran grafted with poly(N-isopropylacrylamide-co-N, N-dimethyl-acrylamide). Macromolecular Chemistry and Physics 201: 613–619.
Inukai, M., Y. Jin, C. Yomota, and M. Yonese. 2000. Preparation and characterization of hyaluronate-hydroxyethyl acrylate blend hydrogel for controlled release device. Chemical and Pharmaceutical Bulletin 48: 850–854.
Jansson, B., H. Hagerstrom, N. Fransen, K. Edsman, and E. Bjork. 2005. The influence of gellan gum on the transfer of fluorescein dextran across rat nasal epithelium in vivo. European Journal of Pharmaceutics and Biopharmaceutics 59: 557–564.
Jeong, B., Y.H. Bae, and S.W. Kim. 2000. In situ gelation of PEG-PLGA-PEG triblock copolymer aqueous solutions and degradation thereof. Journal of Biomedical Materials Research 50: 171–177.
Jeong, B., Y.H. Bae, D.S. Lee, and S.W. Kim. 1997. Biodegradable block copolymers as injectable drug-delivery systems. Nature 388: 860–862.
Jiang, L., X. Li, L. Liu, and Q. Zhang. 2013. Cellular uptake mechanism and intracellular fate of hydrophobically modified pullulan nanoparticles. International Journal of Nanomedicine 8: 1825–1834.
Jin, K.M., and Y.H. Kim. 2008. Injectable, thermo-reversible and complex coacervate combination gels for protein drug delivery. Journal of Controlled Release 127: 249–256.
Kim, J.K., Y.W. Won, K.S. Lim, and Y.H. Kim. 2012. Low-molecular-weight methylcellulose-based thermo-reversible gel/pluronic micelle combination system for local and sustained docetaxel delivery. Pharmaceutical Research 29: 525–534.
Kim, S., and K.E. Healy. 2003. Synthesis and characterization of injectable poly(N-isopropylacrylamide-co-acrylic acid) hydrogels with proteolytically degradable cross-links. Biomacromolecules 4: 1214–1223.
Kobayashi, H., O. Katakura, N. Morimoto, K. Akiyoshi, and S. Kasugai. 2009. Effects of cholesterol-bearing pullulan (CHP)-nanogels in combination with prostaglandin E1 on wound healing. Journal of Biomedical Materials Research 91: 55–60.
Kofuji, K., H. Akamine, H. Oshirabe, Y. Maeda, Y. Murata, and S. Kawashima. 2003. Retention and release behavior of insulin in chitosan gel beads. Journal of Biomaterials Science, Polymer Edition 14: 1243–1253.
Kofuji, K., Y. Murata, and S. Kawashima. 2005. Sustained insulin release with biodegradation of chitosan gel beads prepared by copper ions. International Journal of Pharmaceutics 303: 95–103.
Kou, J.H., G.L. Amidon, and P.I. Lee. 1988. pH-dependent swelling and solute diffusion characteristics of poly(hydroxyethyl methacrylate-co-methacrylic acid) hydrogels. Pharmaceutical Research 5: 592–597.
Kurita, K. 2006. Chitin and chitosan: Functional biopolymers from marine crustaceans. Mar Biotechnol (NY) 8: 203–226.
Kwon, I.C., Y.H. Bae, T. Okano, and S.W. Kim. 1991. Drug release from electric-current sensitive polymers. Journal of Controlled Release 17: 149–156.
Laurens, N., P. Koolwijk, and M.P. De Maat. 2006. Fibrin structure and wound healing. Journal of Thrombosis and Haemostasis 4: 932–939.
Leach, J.B., and C.E. Schmidt. 2005. Characterization of protein release from photocrosslinkable hyaluronic acid-polyethylene glycol hydrogel tissue engineering scaffolds. Biomaterials 26: 125–135.
Lee, H.T., and D.S. Lee. 2002. Thermoresponsive phase transitions of PLA-block-PEO-block-PLA triblock stereo-copolymers in aqueous solution. Macromolecular Research 10: 359–364.
Lee, I., and K. Akiyoshi. 2004. Single molecular mechanics of a cholesterol-bearing pullulan nanogel at the hydrophobic interfaces. Biomaterials 25: 2911–2918.
Lee, J., Y.H. Bae, Y.S. Sohn, and B. Jeong. 2006. Thermogelling aqueous solutions of alternating multiblock copolymers of poly(l-lactic acid) and poly(ethylene glycol). Biomacromolecules 7: 1729–1734.
Lee, K.Y., and D.J. Mooney. 2001. Hydrogels for tissue engineering. Chemical Reviews 101: 1869–1879.
Li, Z., S. Cho, I.C. Kwon, M.M. Janat-Amsbury, and K.M. Huh. 2013. Preparation and characterization of glycol chitin as a new thermogelling polymer for biomedical applications. Carbohydrate Polymers 92: 2267–2275.
Lin, Y.H., H.F. Liang, C.K. Chung, M.C. Chen, and H.W. Sung. 2005. Physically crosslinked alginate/N, O-carboxymethyl chitosan hydrogels with calcium for oral delivery of protein drugs. Biomaterials 26: 2105–2113.
Liu, W., B. Zhang, W.W. Lu, X. Li, D. Zhu, K. De Yao, Q. Wang, C. Zhao, and C. Wang. 2004. A rapid temperature-responsive sol–gel reversible poly(N-isopropylacrylamide)-g-methylcellulose copolymer hydrogel. Biomaterials 25: 3005–3012.
Luginbuehl, V., E. Wenk, A. Koch, B. Gander, H.P. Merkle, and L. Meinel. 2005. Insulin-like growth factor I-releasing alginate-tricalciumphosphate composites for bone regeneration. Pharmaceutical Research 22: 940–950.
Luo, Y., K.R. Kirker, and G.D. Prestwich. 2000. Cross-linked hyaluronic acid hydrogel films: New biomaterials for drug delivery. Journal of Controlled Release 69: 169–184.
Miyazaki, S., H. Aoyama, N. Kawasaki, W. Kubo, and D. Attwood. 1999. In situ-gelling gellan formulations as vehicles for oral drug delivery. Journal of Controlled Release 60: 287–295.
Mocanu, G., D. Mihai, L. Picton, D. Lecerf, and G. Muller. 2002. Associative pullulan gels and their interaction with biological active substances. Journal of Controlled Release 83: 41–51.
Obara, K., M. Ishihara, T. Ishizuka, M. Fujita, Y. Ozeki, T. Maehara, Y. Saito, H. Yura, T. Matsui, H. Hattori, M. Kikuchi, and A. Kurita. 2003. Photocrosslinkable chitosan hydrogel containing fibroblast growth factor-2 stimulates wound healing in healing-impaired db/db mice. Biomaterials 24: 3437–3444.
Oh, K.T., T.K. Bronich, V.A. Kabanov, and A.V. Kabanov. 2007. Block polyelectrolyte networks from poly(acrylic acid) and poly(ethylene oxide): Sorption and release of cytochrome C. Biomacromolecules 8: 490–497.
Paulsson, M., H. Hagerstrom, and K. Edsman. 1999. Rheological studies of the gelation of deacetylated gellan gum (Gelrite) in physiological conditions. European Journal of Pharmaceutical Sciences 9: 99–105.
Peppas, N.A., and A.R. Khare. 1993. Preparation, structure and diffusional behavior of hydrogels in controlled-release. Advanced Drug Delivery Reviews 11: 1–35.
Perka, C., R.S. Spitzer, K. Lindenhayn, M. Sittinger, and O. Schultz. 2000. Matrix-mixed culture: New methodology for chondrocyte culture and preparation of cartilage transplants. Journal of Biomedical Materials Research 49: 305–311.
Ruel-Gari, E., and J.-C. Leroux. 2004. In situ-forming hydrogels—review of temperature-sensitive systems. European Journal of Pharmaceutics and Biopharmaceutics 58: 409–426.
Saettone, M.F., D. Monti, M.T. Torracca, and P. Chetoni. 1994. Mucoadhesive ophthalmic vehicles—evaluation of polymeric low-viscosity formulations. Journal of Ocular Pharmacology 10: 83–92.
Sarkar, N. 1979. Thermal gelation properties of methyl and hydroxypropyl methylcellulose. Journal of Applied Polymer Science 24: 1073–1087.
Sawahata, K., M. Hara, H. Yasunaga, and Y. Osada. 1990. Electrically controlled drug delivery system using polyelectrolyte gels. Journal of Controlled Release 14: 253–262.
Shu, X.Z., Y.C. Liu, Y. Luo, M.C. Roberts, and G.D. Prestwich. 2002. Disulfide cross-linked hyaluronan hydrogels. Biomacromolecules 3: 1304–1311.
Singh, B.N., and K.H. Kim. 2005. Effects of divalent cations on drug encapsulation efficiency of deacylated gellan gum. Journal of Microencapsulation 22: 761–771.
Sinha, V.R., and R. Kumria. 2001. Polysaccharides in colon-specific drug delivery. International Journal of Pharmaceutics 224: 19–38.
Wittgren, B., M. Stefansson, and B. Porsch. 2005. Interactions between sodium dodecyl sulphate and non-ionic cellulose derivatives studied by size exclusion chromatography with online multi-angle light scattering and refractometric detection. Journal of Chromatography A 1082: 166–175.
Won, Y.-W., J.-K. Kim, M.-J. Cha, K.-C. Hwang, D. Choi, and Y.-H. Kim. 2010. Prolongation and enhancement of the anti-apoptotic effects of PTD-Hsp27 fusion proteins using an injectable thermo-reversible gel in a rat myocardial infarction model. Journal of Controlled Release 144: 181–189.
Ye, Q., G. Zund, P. Benedikt, S. Jockenhoevel, S.P. Hoerstrup, S. Sakyama, J.A. Hubbell, and M. Turina. 2000. Fibrin gel as a three dimensional matrix in cardiovascular tissue engineering. European Journal of Cardio-Thoracic Surgery 17: 587–591.
Yin, X., A.S. Hoffman, and P.S. Stayton. 2006. Poly(N-isopropylacrylamide-co-propylacrylic acid) copolymers that respond sharply to temperature and pH. Biomacromolecules 7: 1381–1385.
Zhao, X., and J.M. Harris. 1997. Novel degradable poly(ethylene glycol) esters for drug delivery. Poly(Ethylene Glycol) 680: 458–472.
Acknowledgments
This work was partially supported by the National Research Foundation of Korea Grant funded by the Korean government (2013030789) and the Brain Korea 21 plus (BK21 plus) program (22A20130011095).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, J.K., Kim, H.J., Chung, JY. et al. Natural and synthetic biomaterials for controlled drug delivery. Arch. Pharm. Res. 37, 60–68 (2014). https://doi.org/10.1007/s12272-013-0280-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-013-0280-6