Abstract
These experiments were performed to investigate whether 3,4,5-trimethoxycinnamic acid (TMCA), one of the constituents derived from Polygalae Radix, enhances pentobarbital-induced sleeping behaviors, and to alter sleep architecture through the γ-aminobutyric acid (GABA)ergic systems in mice. TMCA decreased the locomotor activity. TMCA prolonged total sleep time, and reduced sleep latency induced by pentobarbital, similar to muscimol, a GABAA agonist. From the electrocencephalogram recording for 6 h after TMCA administration, the number of sleep/wake cycles were reduced by TMCA. TMCA also increased the total sleep time and non-rapid eye movement (NREM) sleep. In addition, TMCA increased Cl− influx in primary cultured cerebellar granule cells of mice. TMCA increased the activation of glutamic acid decarboxylase (GAD) and the expressions of γ-subunit of GABAA receptors in the cerebellar granule cells. However, α- and β-subunits proteins of GABAA receptors were not increased. Therefore, TMCA would increase pentobarbital induced-sleep and NREM sleep in mice. These results indicate that TMCA may enhance sleep and alter sleep architecture through GABAAergic systyems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In traditional Chinese medicine, Onji (Polygalae Radix, roots of Polygala tenuifolia WILLD) has been used for the treatment of insomnia, palpitations with anxiety, restlessness and disorientation (Yao et al. 2010; Shin et al. 2009). Accumulating evidence showed that Onji had antipsychotic, cognitive improving/neuroprotective, and anti-inflammatory effects on the central nervous system (CNS) (Chung et al. 2002; Jang et al. 1997; Kim et al. 1998; Zhang et al. 2008). Polygalasaponins, the well-known main constituents of Onji, could improve learning and memory abilities of dementia-like rats induced by combined injection of α-amyloid peptide. Polygalasaponins also ameliorated learning and memory impairments in aged and dysmnesic mice, which were associated with increases of norepinephrine (NE) and dopamine (DA) levels in the hippocampus, and decrease of acetylcholinesterase (AChE) activity in the cortex of mice (Zhang et al. 2008). On the other hand, previous study reported that oral administration of 3,4,5-trimethoxycinnamic acid (TMCA), another constituent in Onji, prolonged sleeping time induced by hexobarbital in mice to exhibit sedative action (Kawashima et al. 2004).
The search for alternative compounds for the treatment of insomnia has progressed in the past decade. In western countries, many sedatives, anxiolytics, and hypnotics have been developed from natural products. The therapeutic value of these agents has been well established, and sedative herbs have played an important role in managing anxiety-symptoms in the context of both psychiatric disorders and medical illness. In Asian countries, especially in China, Japan and Korea, there have been numerous herbal medicines exerting anxiolytic, sedative, and hypnotic effects with few side effects. Fructus schisandrae, Ziziphi Semen, Flower of Chrysanthemum morifolium, Panax ginseng, Poria cocos, and so on. Polygalae Radix, as a traditional Chinese medicine, also has extensively been used in clinics for the treatment of insomnia. (Huang et al. 2007; Wu 2007; Zhang et al. 2007; Jiang et al. 2007; Rhee et al. 1990; Kim et al. 2011; Chung-Il et al. 2012; Chung-Soo et al. 2011; Shu-Long et al. 2010).
However, there is still limited information on the pharmacological studies of TMCA, one of constituents of Polygala Radix used for insomnia. Therefore, the present study was to evaluate the hypnotic effects of TMCA on pentobarbital-induced sleep behaviors, and to investigate possible mechanism. The increase in pentobarbital-induced sleeping time could be useful tool for examining influences on GABAAergic systems. In order to find out the possible mechanisms, Cl− channel activation and expression of GABAA receptors were measured. We also analyzed sleep architecture after administration of TMCA to understand sleep patterns.
Materials and methods
Reagents and chemicals
N-(ethoxycarbonylmethyl)-6-methoxyquinolinium bromide (MQAE), TMCA and muscimol were purchased from Sigma-Aldrich Co. (St Louis, MO, USA). Pentobarbital sodium was purchased from Hanlim Pharm. Co., Ltd. (Seoul, Korea). Fetal bovine serum (FBS) and DMEM were obtained from GIBCO (Grand Island, NY, USA). The specific rabbit polyclonal antibodies against GABAA receptor subunits or GAD65/67 and the corresponding conjugated anti-rabbit immunoglobulin G-horseradish peroxidase were obtained from Abcam plc (Cambridge, UK). Chemiluminescent HRP substrate was purchased from Millipore Co. (Billerica, MA, USA).
Animals
ICR male mice (purchased from Samtako, Korea), weighing 25–28 g, were used in groups of 10–12. Mice were housed in acrylic cages (45 × 60 × 23 cm) with water and food available ad libitum under an artificial 12 h light/dark cycle (light on at 7:00 am), and at the relative humidity (50–52 %) and a constant temperature (22 + 2 °C). To ensure adaptation to the new environment, mice were kept in the departmental holding room for 1 week before testing. All of the behavioral experiments were performed between 10:00 and 17:00. All of the experiments involving animals were carried out in accordance with the National Institute of Health Guide for Care and Use of Laboratory Animals (NIH publication No. 85–23, revised 1985), and the Institutional Animal Care and Use Committee of Chungbuk National University approved the protocol.
Locomotor activity
Spontaneous locomotor activity was measured automatically with a tilting-type ambulometer (AMB-10, O’Hara, Japan). Each mouse was placed in the activity cage (20 cm in diameter, 18 cm in height) after an adaptation period of 10 min (Park et al. 2005). Diazepam (2.0 mg/kg, i.p.) and TMCA (2.0, 5.0 and 10 mg/kg, p.o.) were administered to the mouse 30 and 60 min prior to the experiment, respectively. The ambulatory activity was measured for 1 h after the administration of the agents (Morton et al. 2011).
Pentobarbital-induced sleep
Mice (10–12 in each group) were used for in this experiment. All experiments were carried out between 1:00 and 5:00 pm. Mice were food-deprived for 24 h, prior to the experiment. Pentobarbital sodium (42 mg/kg) and muscimol were diluted in 0.9 % physiological saline, and administered intraperitoneally (i.p.) to each mouse to induce sleep. TMCA was suspended in dissolved in 0.01 % DMSO, and was administered orally (p.o.) to the mice. Muscimol (0.2 mg/kg) as a reference drug, and TMCA (2, 5 and 10 mg/kg) were administered 15 min and 1 h prior to pentobarbital injection, respectively. Mice were placed in a box after the administration of test drugs. Those animals that stopped moving in the box after pentobarbital injection were immediately transferred to another box. The time elapsed between pentobarbital injection and the loss of righting reflex was recorded as the sleep latency. The time elapsed between the loss and recovery of the righting reflex was recorded as sleeping time. Animals that failed to fall asleep within 5 min after pentobarbital administration were excluded from the experiments (Wolfman et al. 1996; Darias et al. 1998).
Surgery
Each mouse was implanted with a transmitter (TL10M3-F50-EEE; Data Sciences International, St. Paul, MN, USA) for recording EEG and activity via telemetry as described previously (Sanford et al. 2006). The body of transmitter was implanted subcutaneously off the midline and posterior to the scapula. It was attached to the skin with three sutures for stabilization. After midline incision of the skin, holes drilled in the appropriate locations in the skull with a dental drill, and the coordinates for the leading electrodes of transmitters were measured relative to brgma according to a stereotaxic altlas (Lesnikov and Tsvetkova 1985). The leading electrodes of transmitters were placed in contact with the dura through holes of the skull in the hypothalamus (A: 0.1, P: 5.0, L: 0.15). The electrodes were anchored to the skull with screws and dental cement. Surgical anesthesia was achieved with pentobarbital (50 mg/kg, i.p.). After 1-week recovery, they were used for the EEG recording.
Data collection
Telemetric recording of cortical EEG and activity were conducted using procedures similar to previous reports (Sanford et al. 2006). For the EEG signal, the gain of transmitters was set at −0.5/+ 0.5 volts per/units × 2 and the raw signals generated from the transmitter were in the range of 0.5–20.0 Hz. The signals were processed by a Data Sciences International analog converter and routed to an AD converter (Eagle PC30, USA) housed in a PC class computer. The AD converter digitized the EEG and activity signals at 128 Hz. The digitized data were transferred to the computer and displayed graphically by the program on the computer monitor. An on-line fast Fourier transformation (FFT) was performed on the EEG data at 2 s intervals during data acquisition (256 samples) after a Hanning window treatment. The FFT analysis generated power density values from 0.0 to 20.0 Hz at a resolution of 0.5 Hz. The FFT data were further averaged in the range of 0.0–20 Hz for every 10 s. The sleep data and FFT results were saved to the hard disk every 10 s for additional off-line analysis. Movement of the animal in relation to the telemetry receiver generated transistor–transistor logic (TTL) pulses that were collected and counted as a measure of activity. Oral administration of TMCA was loaded 1 h before the EEG recording. Recording began at 7:00 am and the 24 h EEG and activity were recorded in each mouse.
Data analysis
The amount of time in wakefulness, NREM and REM sleep were determined from the digitized data at 10 s intervals using professional animal sleep analysis software SleepSign 2.1 (KISSEI Comtec Co Ltd, Matsumoto, Japan). Briefly, the software discriminates wakefulness as high-frequency low-amplitude EEG. NREM was scored based on the presence of spindles interspersed with slow waves in the EEG. EEG power during REM is significantly reduced in lower frequency δ-wave (0.75–4.0 Hz) and increased in the range of θ-wave activity (5.0–9.0 Hz, peak at 7.5 Hz). The time spent (min) in NREM, REM, total sleep time (NREM + REM) and numbers of sleep-wake cycle were processed to obtain 6 h period totals for each mouse. We further calculated the time spent in each sleep–wake state (wake, NREM, and REM) in each recording. The absolute EEG power were calculated during wakefulness, NREM, and REM. Data were calculated in 0.5 Hz bins from 0.5 to 20 Hz for the entire 6 h recording. Subsequently, EEG power density was evaluated in three selected frequency bands for wakefulness, NREM, and REM (δ-wave, θ-wave and α-wave) were subsequently evaluated.
Cell culture
Primary cultures of cerebellar neurons enriched in granule cells were prepared from cerebella of 8-day-old ICR mouse as previously described (Chung-Il et al. 2012). After 8 days in culture, these cells expressed functional GABAA receptors, with an expression pattern similar to that of the cerebellum during postnatal development, but different from the pattern observed in the adult mouse cerebellum. Briefly, cells were plated (1.0 × 105 cells per well) in 96 well-microplates that had been coated with poly-l-lysine (50 μg/mL; Sigma, St. Louis, MO, USA) and were cultured DMEM nutrient and neurobasal A media supplemented with 10 % heat-inactivated fetal bovine serum, glutamine (4 mM), gentamicin (100 μg/mL), antibiotic–antimycotic solution (10 μg/mL), and potassium chloride (25 mM); a high concentration of potassium was necessary to induce persistent depolarization, which promotes the survival of granule cells. Cytosine arabinofuranoside (final concentration, 10 μM) was added to cultures 18–24 h after plating, to inhibit the proliferation of nonneuronal cells.
Measurement of intracellular Cl− influx
The intracellular chloride ion (Cl−) concentration [(Cl−) i ] of cerebellar granule cells of mouse was estimated using Cl− sensitive fluorescence probe MQAE according to the method of West and Molloy, with a slight modification (West and Molloy 1996). The buffer (pH 7.4) contained the following components: 2.4 mM HPO4 2−, 0.6 mM H2PO4 −, 10 mM HEPES, 10 mM d-glucose, and 1 mM MgSO4. A variety of MQAE-loading conditions were assessed. The cells were incubated overnight in medium containing 10 mM MQAE (Sigma, St Louis, MO, USA). After loading, the cells were washed three times in the appropriate Cl− containing buffer or Cl− free buffer. The buffer was replaced with buffer with or without the compounds or Cl− free buffer. Repetitive fluorescence measurements were initiated immediately using a FLUOstar plate reader (Excitation wavelength: 320 nm, emission wavelength: 460 nm; BMG LabTechnology, Germany). The data is presented as the relative fluorescence F0/F, where F0 is the fluorescence without Cl− ions and F is the fluorescence as a function of time. The F0/F values were directly proportional to [Cl−] i .
Western blot of GABAA receptors subunits and GAD expression
The primary cells of cerebellum of mouse were cultured. TMCA was dissolved in dissolved in 0.01 % DMSO, and diluted sequentially in culture medium to final concentrations of 1.0, 3.0, 5.0 and 10 μg/ml. The control group was treated with solvent alone at the same dilution as that used for drug treatment. The culture medium was completely replaced with fresh medium containing the appropriate drug. After treatment of TMCA, the cells were harvested and treated with lysis buffer. The extracts were centrifuged at 13,000×g at 4 °C for 10 min, and the supernatant was recovered. The concentration of protein in the supernatant was determined, and then the supernatant was used for western blot analysis. The concentration of total protein was determined by the modified Lowry method using bovine serum albumin as a standard (Lowry et al. 1951). The samples were stored at −20 °C.
Twenty-five micrograms of protein was added to each lane, and sodium dodecyl sulfate polyacrylamide gel electrophoresis was performed using 12 % polyacrylamide gels. Proteins were transferred to PVDF membranes (Hybond-P, GE Healthcare, Amersham, UK) using a semidry transfer system. The blots were blocked for 2 h at room temperature with 5 % (w/v) BSA [applied to all primary antibodies excepting for glyceraldehyde 3-phosphate dehydrogenase (GADPH)] and 5 % (w/v) skim milk (only applied to GADPH) in Tris-buffered saline solution (TBS) containing 0.1 % Tween 20. Immunoblots were incubated with one of the following primary antibodies: rabbit anti-GABAA α1 polyclonal antibody (diluted 1:2,500 in TBS containing 0.1 % Tween 20, 5 % BSA Abcam plc, Cambridge, UK), rabbit anti-GABAA β1 polyclonal antibody, rabbit anti-GABAA γ1 polyclonal antibody and rabbit anti-glutamic acid decarboxylase (GAD) polyclonal antibody. Blots were then washed and incubated with the horseradish peroxidase-conjugated second antibodies: goat anti-rabbit IgG. Immunoreactive bands were developed with a BM chemiluminescence detection kit (Roche Diagnostics, Mannheim, Germany). The quantitative analysis of detected bands were performed with densitometric scanning, and all values were normalized to the amount of GADPH in the sample, which was measured as follows. All of the immunoblots were stripped, incubated with sheep anti-GADPH, subsequently incubated with rabbit anti-rabbit IgG, and developed to confirm equal protein loading.
Statistical analysis
All statistical analysis were conducted using SigmaStat software (SPSS Inc., Chicago, IL, USA). Measures of analysis of variance (ANOVA) were used in the data analysis. After extensive ANOVA testing, posthoc comparisons of means were performed using Holm-sidak test. A p value of less than 0.05 was considered to be significant. The values are expressed as mean ± SE.
Results
Effects of TMCA on locomotor activity
The locomotor activity of mice treated with different dosages of TMCA or diazepam was compared with that of mice treated with vehicle. Locomotor activity was significantly decreased by TMCA (10 mg/kg) and diazepam (2.0 mg/kg). However, TMCA at the doses of 2.0 and 5.0 mg/kg did not affect locomotor activity in tested animals (Fig. 1).
Effects of TMCA and diazepam on locomotor activity test in mice. Ambulation activity was measured for 1 h, 30 min after oral administration of diazepam and 1 h after the administration of TMCA. Each column represents mean ± SE. The significance of the effects of the compounds was assessed using analysis of variance (ANOVA) followed by Holm-sidak test. * p < 0.05, *** p < 0.005, compared to control
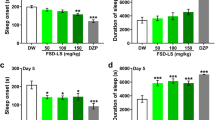
Onset and duration of sleep by TMCA
TMCA increased the sleep time in pentobarbital-induced sleep. TMCA produced a dose dependent prolongation of pentobarbital-induced sleep time at dosages of 2.0, 5.0 and 10 mg/kg. However, TMCA did not only affect the latency of sleep at dosages of 2.0 mg/kg. Pretreatment with muscimol (0.2 mg/kg, i.p.) as a positive control, 15 min before the administration of pentobarbital (42 mg/kg), produced an increase in total sleep time and a decrease in the latency of sleep (Fig. 2).
Effects of TMCA on onset and duration of sleep in pentobarbital-treated mice. Mice were food-depriced for 24 h before the experiment. Pentobarbital (42 mg/kg, i.p.) was administered to mice following administration of muscimol or TMCA. The sleep time (a) and sleep latency (b) were recorded. Each column represents mean ± SE. The significance of the effects of the compounds was assessed using analysis of variance (ANOVA) followed by Holm-sidak test. * p < 0.05, ** p < 0.01, *** p < 0.005, compared to control
Effects of TMCA on the number of sleep–wake cycles
As shown in Fig. 3, after TMCA 10 mg/kg dose treatment, sleep–wake cycles were reduced (p < 0.05).
Effects of TMCA on sleep architecture
After TMCA treatment, no significant changes in REM sleep architecture were shown during a 6 h recording, TMCA treatment increased NREM and total sleep, but decreased wakefulness (p < 0.05) (Fig. 4).
Effects of TMCA on influx of Cl− in primary cultured cerebellar granule cells
Intracellular Cl− influx in primary cultured cerebellar granule cells was measured. The measured data is presented as the relative fluorescence F0/F, where F0 is the fluorescence without Cl− and directly proportional to intracellular Cl− concentration. Treatment of granule cells with TMCA (1.0, 3.0, 5.0, 10 μg/ml) produced a significant increase in Cl− influx. Pentobarbital 10 μM also increased the influx of Cl− in primary cultured cerebellar granule cells (Fig. 5).
Effects of TMCA on chloride influx. After the culture of cerebellar granule cells for 8 days. the cells were incubated with MQAE overnight, and then TMCA (1, 3, 5, 10 μg/ml) and pentobarbital (PENT, 10 μM) were added 1 h prior to measurement. Each column represents mean ± SE. The significance of the effects of the compounds was assessed using analysis of variance (ANOVA) followed by Holm-sidak test. ** p < 0.01, *** p < 0.005, compared to control
GAD and GABAA receptors expressions by TMCA
Primary cultured cerebellar granule cells were treated TMCA (10 μg/ml) or pentobarbital (10 μM) for 1 h and they were sacrificed to examine the effect of these drugs on the abundance of GAD and GABAA receptors subunits in the cerebellum (Figs. 6, 7). TMCA treatment increased expression of GAD65 and γ-subunit, but did not influence the amounts of α-, β-subunits in the GABAA receptors. However, pentobarbital significantly decreased amounts of the α-subunit and increased γ-subunit, but did not affect the abundance of β-subunit.
Effects on TMCA on expression of GAD. Immunoblots of lysed cerebellar granule cells which were treated for 1 h following administration of TMCA or pentobarbital are shown. GADPH levels were need for the normalization of the protein expression. The experiment was repeated many times. The significance of the effects of the compounds was assessed using analysis of variance (ANOVA) followed by Holm-sidak test. Each column represents mean ± SE. * p < 0.05, compared to control. GAD glutamic acid decarboxylase, PENT pentobarbital
Effects on TMCA on expression of GABAA receptors subunits. GADPH levels were need for the normalization of the protein expression. The experiments were repeated for three times. The significance of the effects of the compounds was assessed using analysis of variance (ANOVA) followed by Holm-sidak test. Each column represents mean ± SE. * p < 0.05, compared to control. GAD glutamic acid decarboxylase, PENT pentobarbital
Discussion
TMCA decreaed locomotor activity in mice. It can be a useful tool for examining the stimulatory or inhibitory effects on CNS. TMCA also potentiated pentobarbital-induced sleep time. Pentobarbital potentiates the effects of GABA, acting at the GABA/benzodiazepine receptor–ionophore complex (Olsen 1981; Ticku and Maksay 1983). Many hypnotics, anti-anxiety and anti-epilepsy drugs have been shown to cause prolongation pentobarbital-induced sleeping time (Han et al. 2009; Ma et al. 2008; Martinez et al. 2006). It was interesting that TMCA prolonged pentobarbital-induced sleep behaviors and interacted with pentobarbital in the CNS via the GABAAergic systems, in agreement with previous experiments (Dimova et al. 1994). In addition, we found that TMCA and muscimol interacted with pentobarbital, as enhancing sleep behaviors in rodents. TMCA potentiated pentobarbital-induced sleep, and these results were similar to those of muscimol, a GABAA receptor agonist. This indicates that the hypnotic effects of TMCA may be due to interaction with GABAAergic systems. GABAA receptors possess various binding sites, including binding sites for GABA, benzodiazepine and barbiturates. GABAA receptors form heteromeric GABA-gated Cl−channels, which are assembled from a large family of subunits genes. GABAA receptors Cl− channel are opened after binding GABA to give a net inward flux of negative Cl− (outward current), hyperpolarizing the membrane and reducing neuronal firing (Macdonald and Olsen 1994). Muscimol and other GABAA receptors agonists cause potentiation of Cl− influx when administered with pentobarbital or other agonists (Chistina Grobin et al. 1999). TMCA produced a significant increase in Cl− influx. This increase was similar to that of pentobarbital. It is suggested that TMCA may act to induce Cl− channel opening of GABAA receptors.
Researchers have demonstrated that the pharmacological profile and different drug-induced behaviors of GABAA receptors depend upon its subunit composition (Rudolph and Mohler 2006). In addition, GAD, the rate liminting enzyme in GABA biosynthesis, also plays an important role in maintaining GABA levels in the brain (Tillakaratne et al. 1995). Hence, the alteration of expression levels of this enzyme may change GABA transmission in the brain. GAD65 is responsible for vesicular GABA production. We determined GAD proteins and GABAA receptors subunits expression levels at the effective dosage of TMCA. The expression levels of GABAA receptors α-, β- and γ-subunits also were investigated to know the possible site of action by which TMCA exerted its sleep-potentiating effects. It was reported that these isoforms were directly involved in GABA transmission at the synapse (Buddhala et al. 2009). The most abundant GABAA receptors subunits composition, α1β2γ2, is present in most brain regions, including the cerebellum, and these subunits are related to the hypnotic/sedative effect of GABAA receptor ligands (Rudolph and Mohler 2006). Our results showed that the increased levels of γ-subunit and GAD. However, TMCA did not affect expression of α- and β-subunits of GABAA receptors.
Sleep can be divided into two major stages; REM and NREM sleep. The most essential form of sleep is believed to be NREM sleep. Sleep has been described as consisting of four stages. Stages 1–4 increase the depth of sleep as the subject progresses through these stages (Rechtschaffen et al. 1966; Chung-Soo et al. 2011). After oral administration of TMCA, sleep architecture were analyzed using the EEG recording for 6 h in mice. We found that the total percentage of wakefulness was decreased, and the number of sleep/wake cycles were reduced by TMCA. TMCA also increased the percentage of NREM sleep..
Many herbal preparations and a diversity of drugs used to promote sleep are known to act on GABAA receptors. Drugs acting on GABAA receptors mainly act to increase synaptic inhibition either by directly activating GABAA receptors, or more usually, by enhancing the action of other ligands on GABAA receptors. These results suggest that TMCA has sleep-potentiaing effects, which may be mediated by Cl− channel opening.
The search for novel plant-derived pharmacotherapies for psychiatric illness has progressed significantly in the past decade. A considerable number of herbal constituents whose behavioral effects and pharmacological actions have been well characterized, may be good candidates for further investigations that may ultimately lead to clinical use of these constituents. The potential benefits of herbal remedies such as St. John’s wort and Kava–kava in psychiatric practice have been addressed previously. TMCA may be another good candidate for the treatment of psychiatric illnesses, such as sleep disorders.
In conclusion, TMCA enhances hypnotic effects in pentobarbital-treated mice. This enhancement may result from Cl− channel activation and GABAergic transmission. Further investigation is needed to determine the effects of other TMCA derivatives with strong pharmacological action. However, the toxic effects of TMNCA have also been established, including various withdrawal syndromes, dependence, tolerance, and impairment of cognitive function, memory, and general daytime performance.
References
Buddhala, C., C.C. Hsu, and J.Y. Wu. 2009. A novel mechanism for GABA synthesis and packaging into synaptic vesicles. Neurochemistry International 55: 9–12.
Chistina Grobin, A., J.R. Inglefield, R.D. Schwartz-Bloom, L.L. Devaud, and A.L. Morrow. 1999. Fluorescence imaging of GABAA receptor-mediated intracellular [Cl−] in P19-N cells reveals unique pharmacological properties. Brain Research 827: 1–11.
Chung-Il, L., C.-S. Kim, H. Jin-Yi, O. Eun-Hye, O. Ki-Wan, and J.S. Eun. 2012. Repeated administration of Korea red ginseng extract increases non-rapid-eye movement sleep via GABAAergic systems. Journal of Ginseng Research 36: 403–410.
Chung-Soo, K., H. Ji-Yi, K. Seunghwan, J.T. Hong, and O. Ki-Wan. 2011. Herbs for the treatment of insomnia. Biomolecules & Therapeutics 19: 274–281.
Chung, I.W., N.A. Moore, W.K. Oh, M.F. O’neill, J.S. Ahn, J.B. Park, U.G. Kang, and Y.S. Kim. 2002. Behavioural pharmacology of polygalasaponins indicates potential antipsychotic efficacy. Pharmacology, Biochemistry and Behavior 71: 191–195.
Darias, V., S. Abdala, D. Martin-Herrera, M.L. Tello, and S. Vega. 1998. CNS effects of a series of 1,2,4-triazolyl heterocarboxylic derivatives. Die Pharmazie 53: 477–481.
Dimova, S., M. Koleva, D. Rangelova, and T. Stoytchev. 1994. Liver enzyme activities after multiple administration of nifedipine in mice. Pharmacology and Toxicology 75: 315–318.
Han, H., Y. Ma, J.S. Eun, R. Li, J.T. Hong, M.K. Lee, and K.W. Oh. 2009. Anxiolytic-like effects of sanjoinine A isolated from Zizyphi Spinosi Semen: possible involvement of GABAergic transmission. Pharmacology, Biochemistry and Behavior 92: 206–213.
Huang, F., Y. Xiong, L. Xu, S. Ma, and C. Dou. 2007. Sedative and hypnotic activities of the ethanol fraction from Fructus Schisandrae in mice and rats. Journal of Ethnopharmacology 110: 471–475.
Jang, K.J., K.H. Lee, S.L. Kim, D.Y. Choi, B.K. Park, D.H. Im, Y.J. Cho, W.K. Jhoo, and H.C. Kim. 1997. Chongmyungtang attenuates kainic acid-induced seizure and mortal effect in the mouse. Archives of Pharmacal Research 20: 375–378.
Jiang, J.G., X.J. Huang, J. Chen, and Q.S. Lin. 2007. Comparison of the sedative and hypnotic effects of flavonoids, saponins, and polysaccharides extracted from Semen Ziziphus jujube. Natural Product Research 21: 310–320.
Kawashima, K., D. Miyako, Y. Ishino, T. Makino, K. Saito, and Y. Kano. 2004. Anti-stress effects of 3,4,5-trimethoxycinnamic acid, an active constituent of roots of Polygala tenuifolia (Onji). Biological and Pharmaceutical Bulletin 27: 1317–1319.
Kim, H.M., E.H. Lee, H.J. Na, S.B. Lee, T.Y. Shin, Y.S. Lyu, N.S. Kim, and S. Nomura. 1998. Effect of Polygala tenuifolia root extract on the tumor necrosis factor-alpha secretion from mouse astrocytes. Journal of Ethnopharmacology 61: 201–208.
Kim, J.W., J.Y. Han, J.T. Hong, R. Li, J.S. Eun, and K.W. Oh. 2011. Ethanol extract of the flower chrysanthemum morifolium augments pentobarbital-induced sleep behaviors: involvement of Cl channel activation. eCAM 2011: 109164.
Lesnikov, V.A., and I.P. Tsvetkova. 1985. Stereotaxic coordinates of the mouse hypothalamus. Fiziologicheskiĭ zhurnal SSSR imeni I. M. Sechenova 71: 798–804.
Lowry, O.H., N.J. Rosebrough, A.L. Farr, and R.J. Randall. 1951. Protein measurement with the folin phenol reagent. Journal of Biological Chemistry 193: 265–275.
Ma, Y., H. Ma, J. Jo, D.S. Kim, S.S. Woo, R. Lk, J.T. Hong, D.C. Moon, K.W. Oh, and J.S. Eun. 2008. Honokiol potentiates pentobarbital-induced sleeping behaviors through GABAA receptor Cl− channel activation. Biomolecules & Therapeutics 16: 328–335.
Macdonald, R.L., and R.W. Olsen. 1994. GABAA receptor channels. Annual Review of Neuroscience 17: 569–602.
Martinez, A.L., F. Dominguez, S. Orozco, M. Chavez, H. Salgado, M. Gonzalez, and M.E. Gonzalez-Trujano. 2006. Neuropharmacological effects of an ethanol extract of the Magnolia dealbata Zucc. leaves in mice. Journal of Ethnopharmacology 106: 250–255.
Morton, G.J., K.J. Kaiyala, J.D. Fisher, K. Ogimoto, M.W. Schwartz, and B.E. Wisse. 2011. Identification of a physiological role for leptin in the regulation of ambulatory activity and wheel running in mice. American journal of physiology. Endocrinology and metabolism 300: E392–E401.
Olsen, R.W. 1981. GABA-benzodiazepine-barbiturate receptor interactions. Journal of Neurochemistry 37: 1–13.
Park, J.H., H.Y. Cha, J.J. Seo, J.T. Hong, K. Han, and K.W. Oh. 2005. Anxiolytic-like effects of ginseng in the elevated plus-maze model: comparison of red ginseng and sun ginseng. Progress in Neuro-psychopharmacology and Biological Psychiatry 29: 895–900.
Rechtschaffen, A., P. Hauri, and M. Zeitlin. 1966. Auditory awakening thresholds in REM and NREM sleep stages. Perceptual and Motor Skills 22: 927–942.
Rhee, Y.H., S.P. Lee, K. Honda, and S. Inoue. 1990. Panax ginseng extract modulates sleep in unrestrained rats. Psychopharmacology (Berl) 101: 486–488.
Rudolph, U., and H. Mohler. 2006. GABA-based therapeutic approaches: GABAA receptor subtype functions. Current Opinion in Pharmacology 6: 18–23.
Sanford, L.D., L. Yang, X. Liu, and X. Tang. 2006. Effects of tetrodotoxin (TTX) inactivation of the central nucleus of the amygdala (CNA) on dark period sleep and activity. Brain Research 1084: 80–88.
Shin, K.Y., B.Y. Won, C. Heo, H.J. Kim, D.P. Jang, C.H. Park, S. Kim, H.S. Kim, Y.B. Kim, H.G. Lee, S.H. Lee, Z.H. Cho, and Y.H. Suh. 2009. BT-11 improves stress-induced memory impairments through increment of glucose utilization and total neural cell adhesion molecule levels in rat brains. Journal of Neuroscience Research 87: 260–268.
Shu-Long, Y., N. Sang-Yoon, H. Jin-Yi, K. Jun-Cheol, L. Kinam, H. Jin Tae, O. Ki-Wan, and E. Jae Soon. 2010. Alterations of spontaneous sleep architecture and cortical electroencephalogram power spectra by red ginseng extract via GABAAergic systemss. Journal of Ginseng Research 34: 304–313.
Ticku, M.K., and G. Maksay. 1983. Convulsant/depressant site of action at the allosteric benzodiazepine-GABA receptor–ionophore complex. Life Sciences 33: 2363–2375.
Tillakaratne, N.J., L. Medina-Kauwe, and K.M. Gibson. 1995. Gamma-aminobutyric acid (GABA) metabolism in mammalian neural and nonneural tissues. Comparative Biochemistry and Physiology Part A 112: 247–263.
West, M.R., and C.R. Molloy. 1996. A microplate assay measuring chloride ion channel activity. Analytical Biochemistry 241: 51–58.
Wolfman, C., H. Viola, M. Marder, C. Wasowski, P. Ardenghi, I. Izquierdo, A.C. Paladini, and J.H. Medina. 1996. Anxioselective properties of 6,3′-dinitroflavone, a high-affinity benzodiazepine receptor ligand. European Journal of Pharmacology 318: 23–30.
Wu, J.W. 2007. Clinical observation on the effects of Chinese herbal drugs and acupuncture plus traditional Chinese herbal medicine in treatment of 295 patients with insomnia. Zhong Xi Yi Jie He Xue Bao. Journal of Chinese Integrative Medicine 5: 592–593.
Yao, Y., M. Jia, J.G. Wu, H. Zhang, L.N. Sun, W.S. Chen, and K. Rahman. 2010. Anxiolytic and sedative-hypnotic activities of polygalasaponins from Polygala tenuifolia in mice. Pharmaceutical Biology 48: 801–807.
Zhang, H., T. Han, C.H. Yu, K. Rahman, L.P. Qin, and C. Peng. 2007. Ameliorating effects of essential oil from Acori graminei rhizoma on learning and memory in aged rats and mice. Journal of Pharmacy and Pharmacology 59: 301–309.
Zhang, H., T. Han, L. Zhang, C.H. Yu, D.G. Wan, K. Rahman, L.P. Qin, and C. Peng. 2008. Effects of tenuifolin extracted from radix polygalae on learning and memory: a behavioral and biochemical study on aged and amnesic mice. Phytomedicine 15: 587–594.
Acknowledgments
This work was suported by Priority Research Centers Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2009-0094034).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, CI., Han, JY., Hong, J.T. et al. 3,4,5-Trimethoxycinnamic acid (TMCA), one of the constituents of Polygalae Radix enhances pentobarbital-induced sleeping behaviors via GABAAergic systems in mice. Arch. Pharm. Res. 36, 1244–1251 (2013). https://doi.org/10.1007/s12272-013-0167-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-013-0167-6