Abstract
Bifidobacteria are considered one of the most beneficial probiotics and have been widely studied for their effects in preventing and treating specific pathological conditions. The present study explored the antiviral activity of Bifidobacterium adolescentis SPM0212 isolated from healthy Koreans against hepatitis B virus (HBV) and its mechanism of action. To determine the effect of B. adolescentis SPM0212 against HBV, the level of HBV surface antigen (HBsAg) in the culture medium and the levels of viral transcripts in HepG2.2.15 cells were measured by enzyme-linked immunosorbent assay and reverse transcription-quantitative PCR (RT-qPCR), respectively. To clarify the mechanism, we performed RT-qPCR using specific primers for genes encoding Interferon (IFN)-signaling components and IFN-inducible antiviral effectors. The cell extract of B. adolescentis SPM0212 dose-dependently decreased the extracellular HBsAg level by up to 50 %. Its gene expression in HepG2.2.15 cells was also inhibited by 40 %. This extract significantly increased the expression level of myxovirus resistance A, which is an IFN-inducible antiviral effector. Furthermore, the antiviral activity was observed in the fraction of compound(s) with molecular weights under 30 kDa. Thus, the cell extract of B. adolescentis SPM0212 inhibits HBV and its antiviral mechanism is associated with the Mx GTPase pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatitis B virus (HBV) infection is one of the major public health problems in the world. Worldwide, about 2 billion people have been infected by HBV and approximately 350 million people are chronic carriers of HBV (Kao and Chen 2002; Lavanchy 2004). These carriers are at high risk for developing HBV-associated diseases, including liver cirrhosis, liver failure and hepatocellular carcinoma (HCC) (Im and Sung 2006; Wang et al. 2009). The agents that are currently available for the treatment of chronic HBV infection are interferon-α and nucleoside analogs, such as lamivudine [2′,3′-dideoxy-3′-thiacytidene (3TC)] (Buti and Esteban 2005). However, their clinical utility is limited by some defects: interferon-α has adverse effects and nucleoside derivatives, although relatively safe and efficacious, can induce the emergence of resistant mutants, recurrence after long-term treatment, and drug withdrawal (Perrillo 2005; Kim et al. 2009). Furthermore, none of these therapeutic agents can effectively eradicate covalent closed circular DNA (cccDNA) of HBV (Jia et al. 2007). Therefore, novel strategies to treat this disease are urgently needed.
Over the past decades, studies on the beneficial health effects of probiotics or the substances they naturally produce have been actively progressing in various fields. Probiotic bacteria such as Lactobacillus spp. and Bifidobacterium spp. have multiple beneficial health effects, including (i) improving gastrointestinal tract (GIT) health; (ii) synthesizing and enhancing the bioavailability of nutrients; (iii) enhancing the immune system; (iv) lowering serum cholesterol; (v) blocking and eliminating pathogens; and (vi) reducing the risk of certain cancers (Gill and Guarner 2004; Parvez et al. 2006). Studies on probiotic bacteria have mainly focused on intestinal diseases (e.g., acute viral diarrhea, constipation, ulcerative colitis, and colon cancer) since they are normal flora in GIT of humans and are expected to show beneficial effects on intestinal diseases (Gill and Guarner 2004; Parvez et al. 2006). However, according to recent studies, probiotic bacteria are also effective for hepatoprotection. One study reported that bifidobacteria have substantial alcohol dehydrogenase activity and therefore exhibit a beneficial regulatory effect on acetaldehyde levels (Nosova et al. 2000). According to Han et al. (2005b), Lactobacillus brevis HY7401, Lactobacillus acidophilus CSG and Bifidobacterium longum HY8001 inhibit β-glucuronidase, which causes liver damage, and show potent hepatoprotective effects against CCl4− or t-BHP-induced liver injury in mice. The probiotics Streptococcus thermophilus, bifidobacteria, L. acidophilus, Lactobacillus plantarum, Lactobacillus casei, and Lactobacillus delbrueckii bulgaricus have multiple mechanisms of action that could disrupt the pathogenesis of hepatic encephalopathy (HE) (De Santis et al., 2000; Solga, 2003). Thus, they may be useful probiotic bacteria for the improvement of liver disease, including hepatitis B. In this study, we therefore investigated the antiviral activity of B. adolescentis SPM0212 against HBV and analyzed its mechanism through the pathways of the IFN-mediated antiviral response.
The HepG2.2.15 cell line is a derivative of the human HepG2 hepatoma cell line, which contains integrated HBV DNA of HBV serotype ayw (genotype D). This cell line secretes HBsAg, HBeAg and complete Dane particles (Sells et al. 1987; Korba and Milman 1991). HepG2.2.15 cells are now widely used as an in vitro model for screening of novel antiviral agents with activity against HBV (Jansen et al. 1993; Kruining et al. 1995). In our experiment, we chose the HepG2.2.15 cell line as an in vitro cellular model. The health-improving effects of probiotic bacteria have also been found in various sample types such as live-, heated-, sonicated-bacteria and their culture supernatants, and these effects can be shown even with dead probiotic bacteria through absorption of physiologically active components (Han et al. 2005a; Lee et al. 2008; Shin et al. 2010). Thus, we investigated the anti-HBV effects of sonicated cell extract of Bifidobacterium as intestinal microflora that may benefit the liver.
Materials and methods
Preparation of Bifidobacteria samples
For the isolation of bifidobacteria, fecal samples were collected from healthy Koreans (20–30 years old). Fecal samples were diluted and seeded onto selective blood liver agar (Nissui Pharm, Japan) containing 5 % sheep blood. After 48-hr incubation in anaerobic conditions (90 % N2, 5 % H2, 5 % CO2) using Bactron Anaerobic Chamber (Sheldon Manufacturing Inc., USA) at 37 °C, brown or reddish-brown colonies 2–3 mm in diameter were selected for further identification. A fructose-6-phosphate phosphoketolase (F6PPK) test was performed to ensure that the colonies selected were bifidobacteria (Cha et al. 2012). To identify the isolated Bifidobacterium spp. at the species level, 16S rRNA sequencing was performed by Bio leaders (Daejeon, Korea). Bifidobacterium spp. isolates were cultured at 37 °C for 48 h in general anaerobic medium (GAM) broth (Nissui Pharm. Co. Ltd., Japan) under anaerobic conditions. For the preparation of bifidobacteria cell extracts, cells were harvested during the exponential growth phase by centrifugation at 4,000 rpm for 10 min, washed with PBS, and resuspended in the same buffer. These bacterial suspensions were then adjusted to a final concentration of 9.0 log colony forming unit (CFU)/ml and sonicated for 6 min (amplitude 100 %). Cell extracts were used for further experiments after filtration (0.2 μm) and serial dilution (two-fold from 2−1 to 2−5).
Written informed consent was obtained from all volunteers who provided samples, and the protocol was approved by the Institution Review Board of the Office of Research Development, Sahmyook University.
Cell culture
HepG2.2.15 cells were cultured in RPMI 1640 (Hyclone®, USA) supplemented with 10 % fetal bovine serum (Sigma Chemical Co., USA) and 1 % (v/v) penicillin (10,000 U/ml)/streptomycin (10,000 U/ml) (GIBCO, USA) under 5 % CO2 atmosphere at 37 °C. Subconfluent monolayer cells were detached from the culture dishes by trypsin treatment and then transferred to new cell culture dishes. Cell number was assessed by the trypan blue dye-exclusion method (Strober 2001).
Cytotoxicity assay
The cytotoxicity of bifidobacteria samples to the culture cells was assessed by the MTT [3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide] (Sigma Chemical Co., USA) assay. HepG2.2.15 cells were seeded on 96-well microplates (NunC, Denmark) at a density of 1 × 104 cells per well with bifidobacteria samples and incubated for 24 h. The cells with media alone were used as the untreated control. The incubation medium was removed and 200 μl of fresh medium containing MTT reagent was added to each well. After incubation for 4 h at 37 °C, the culture medium containing MTT reagent was removed and 100 μl of dimethylsulfoxide (DMSO) was added to each well. Viable cells were then detected by measuring absorbance at 570 nm. The percentage of cell viability was calculated as (OD of treated samples/OD of untreated control) × 100.
Measurement of secreted HBsAg
The levels of HBsAg in the culture medium of HepG2.2.15 were measured by using GENEDIA® HBsAg ELISA 3.0 (Green Cross Medical Science Corp. Korea) according to the manufacturer’s protocol. The HepG2.2.15 cells were plated on 12-well microplates (NunC, Denmark) at a density of 2 × 105 cells per well and incubated for 3 days. The incubation medium was then removed and 1 ml of fresh medium containing 100 μl of diluted bifidobacteria samples was added to each well. After incubation for 24 h, the supernatants were collected and used for ELISA. The absorbance was measured at 450/620 nm using an ELISA reader (Molecular Devices, USA). As positive and negative controls, HepG2.2.15 cells were treated with 3TC (125 μg/ml) and PBS, respectively.
Real-time quantitative PCR analysis
HepG2.2.15 cells were treated with the samples as described above. For intracellular assays, total DNA and RNA from each of the cells were extracted with QIAamp® DNA Mini and Blood Mini kit (Qiagen, Germany) and RNeasy® Mini Kit (Qiagen, Germany), respectively. cDNA was synthesized with the Omniscript® Reverse Transcription kit (Qiagen, Germany) in accordance with the manufacturer’s instructions. For extracellular assays, 2 μl of the culture medium was used as a template (Lee et al. 2009b). Real-time quantitative PCR was conducted in a LightCycler system (Roche, Germany) with a LightCycler FastStart DNA Master SYBR Green I kit (Roche, Germany), and the data were analyzed with LightCycler software version 4.5. The primer sequences are shown in Table 1. GAPDH and β-Actin, which are housekeeping genes, were used as internal controls. At least three independent assays were performed. Relative quantification of the target gene was calculated using the ΔΔCT method (Livak and Schmittgen 2001).
Characterization of antiviral compound(s) in cell extract
To examine the characteristics of antiviral compound(s), we heated the cell extract for 10 min at 50 °C and separated it by a standard of molecular weight of 30 kDa in Amicon® Ultra-0.5 Centrifugal Filter Devices (Millipore, USA). We then investigated the effect of heated or separated sample on HBsAg secretion as described above. The protein in the cell extract was quantified by the Bradford method.
Statistical analysis
Results were expressed as mean ± standard deviation (SD). Significant differences were separated using Duncan’s multiple range test and commercial statistical analysis software, version 9.0 (SAS Institute, Cary, NC). All data were considered significant at p < 0.05.
Results
Cytotoxicity assessment of B. adolescentis SPM0212
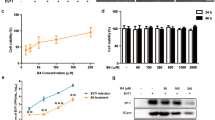
The results from the MTT assay showed that bifidobacteria samples had no cytotoxicity (Fig. 1).
Cytotoxicity of B adolecentis SPM0212 cell extract on HepG2.2.15. Experiments were performed by means of an MTT enzyme assay. HepG2.2.15 were incubated in the presence of various concentrations of B. adolescentis SPM0212 cell extract for 24 h. 6.25: cell extract of B. adolescentis SPM0212 at 7.8 CFU/ml; 12.5: 8.1 log CFU/ml; 25: 8.4 log CFU/ml; 50: 8.7 log CFU/ml; 100: 9.0 log CFU/ml. Each column represents mean ± SD with respect to 100 % control
Effect of B. adolescentis SPM0212 on HBsAg secretion
To select the Bifidobacterium isolate with the highest inhibitory effect against HBV, we measured the level of HBsAg in culture supernatants of HepG2.2.15 cells. Among all tested bifidobacteria samples at 7.0 log CFU/ml, cell extract of B. adolescentis SPM0212 showed the highest inhibitory effect and its effect was not a species-specific but a strain-specific (Table 2). Furthermore, this cell extract dose-dependently inhibited HBsAg secretion (Fig. 2). The cell extract of this strain at 9.0 log CFU/ml showed a ~ 50 % stronger inhibitory effect. The combined effect of this cell extract and 3TC (125 μg/ml) was better than that of 3TC only, but at the midrange concentrations, a single treatment of the cell extract showed better effect (Fig. 2).
Effects of a combination of B. adolescentis SPM0212 cell extract and 3TC on HBsAg production. HepG2.2.15 cells were treated with various concentrations of B. adolescentis SPM0212 cell extract or B. adolescentis SPM0212 in combination with 3TC (125 μg/ml) for 24 h and the level of HBsAg in the media was analyzed with ELISA assay. Data were presented as the %rate of HBsAg production. 3TC: lamivudine (125 μg/ml); 3.1: cell extract of B. adolescentis SPM0212 at 7.5 CFU/ml; 6.25: 7.8 log CFU/ml; 12.5: 8.1 log CFU/ml; 25: 8.4 log CFU/ml; 50: 8.7 log CFU/ml; 100: 9.0 log CFU/ml. a–iMeans with different superscripts differ significantly (p < 0.05)
Effect of B. adolescentis SPM0212 on HBV replication
To determine the effect of B. adolescentis SPM0212 cell extract on HBV replication, we performed real-time quantitative PCR analysis using specific primers for HBV genes. The results showed that the extracellular level of HBV DNA was significantly decreased by treatment with B. adolescentis SPM0212 cell extract (Fig. 3). In addition, this effect of B. adolescentis SPM0212 cell extract was similar to that of 3TC (125 μg/ml; positive control). However, this cell extract did not have such inhibitory effect on the intracellular HBV DNA level (Fig. 3).
Effects of B. adolescentis SPM0212 cell extract on HBV replication. HepG2.2.15 cells were treated with the cell extract of B. adolescentis SPM0212 at 9.0 CFU/ml for 24 h and the levels of extracellular and intracellular HBV DNA were analyzed with real-time qPCR. 3TC: lamivudine (125 μg/ml). *p < 0.05
The mRNA transcript level of HBsAg was reduced significantly by B. adolescentis SPM0212 cell extract at 9.0 log CFU/ml, which had better effect than 3TC (Fig. 4). However, there were no significant differences in the gene expression levels of HBcAg and HBeAg (Fig. 4).
Mechanism of anti-HBV activity by B. adolescentis SPM0212
To determine the antiviral mechanism of B. adolescentis SPM0212 cell extract, we measured the expression of genes encoding IFN-signaling components [IFN-α receptor (IFNAR), signal transducer and activator of transcription 1 (STAT1) and 6-16] and IFN-inducible antiviral effectors [myxovirus resistance A (MxA), 2′,5′-oligoadenylate synthetase (OAS) and protein kinase R (PKR)] in HepG2.2.15 cells. The cell extract of B. adolescentis SPM0212 at 9.0 log CFU/ml significantly increased STAT1 and 6-16 gene expression, but did not affect IFNAR gene expression (Fig. 5). The expression level of MxA, which is one of the antiviral effectors and is produced by STAT1 activation, was significantly increased (Fig. 6). The expression level of PKR was increased, but no significant difference was observed. There was no significant increase in OAS expression either (Fig. 6).
Effects of B. adolescentis SPM0212 cell extract on gene expression of IFN-signaling components. HepG2.2.15 were treated with the cell extract of B. adolescentis SPM0212 at 9.0 log CFU/ml for 24 h and the mRNA levels of IFNAR, STAT1 and 6-16 were analyzed with RT-qPCR. 3TC: lamivudine (125 μg/ml). *p < 0.05
Effects of B. adolescentis SPM0212 cell extract on gene expression of IFN-inducible antiviral effectors. HepG2.2.15 cells were treated with the cell extract of B. adolescentis SPM0212 at 9.0 log CFU/ml for 24 h and the mRNA levels of MxA, PKR and OAS were analyzed with RT-qPCR. 3TC: lamivudine (125 μg/ml). *p < 0.05
Characteristics of antiviral compound(s) in cell extract
The cell extract of B. adolescentis SPM0212 at 9.0 log CFU/ml contained 330.7 μg/ml of protein. The anti-HBV activity of B. adolescentis SPM0212 was observed in the fraction of compound(s) with molecular weights under 30 kDa, and this compound(s) lost activity when heated at 50 °C for 10 min (Fig. 7).
Characterization of antiviral compound(s) from B. adolescentis SPM0212 cell extract. SPM0212: untreated sample of B. adolescentis SPM0212 cell extract, Heated-SPM0212: heat-treated sample of B. adolescentis SPM0212 cell extract, F-1: the fraction with molecular masses higher than 30 kDa, F-2: the fraction with molecular masses lower than 30 kDa. *p < 0.05
Discussion
There are presently two therapeutic options for HBV treatment: one is direct antiviral therapy to inhibit replication of HBV and the other is indirect immunomodulatory therapy to enhance cellular immunity to destroy the virus-infected hepatocytes (Buti and Esteban 2005; Perrillo 2005).
The HBV-DNA level is an important indicator when evaluating the effect of a treatment. The increase of serum HBV-DNA level is associated with increased development of liver cirrhosis and HCC, as well as mortality risk (Chen et al. 2004; Iloeje et al. 2007; Cho and Song 2011). In this study, cell extract of B. adolescentis SPM0212 at 9.0 log CFU/ml significantly reduced the HBV-DNA level in the culture media of HepG2.2.15 cells by about 30 %. In addition, this inhibitory effect was similar to that of 3TC (125 μg/ml), a positive control. HBsAg is the main structural protein of HBV that helps the virus to attach and infect the host cell. The reduction of HBsAg level in serum is strongly correlated with decreased cccDNA level in the cell (Chen et al. 2004; Su et al. 2010). In the case of HBsAg seroclearance, there was almost no recurrence and progression to liver cirrhosis and HCC (Yuen et al. 2004; Arase et al. 2006). In this study, B. adolescentis SPM0212 cell extract dose-dependently decreased the extracellular HBsAg level by up to 50 %. The gene expression of HBsAg in HepG2.2.15 cells was also inhibited by 40 %. According to Lin et al. (2003), cholesterol and lipid in the host cell are necessary for the secretion of HBsAg. In addition, previous studies showed that many Bifidobacterium isolates have a cholesterol-lowering effect (Lee et al. 2009a; Shin et al. 2010; Lee et al. 2011). Therefore, B. adolescentis SPM0212 cell extract may interfere with both HBsAg secretion and its gene expression. Another antigen of HBV, HBeAg is also secreted from HepG2.2.15 during cell culture (Sells et al. 1987; Korba and Milman 1991). HBsAg level increases time dependently, but the HBeAg level reaches a peak 24 h later and there are no changes thereafter (Zhao et al. 2011). Therefore, in this study, no significant differences were observed in gene expression of HBeAg because HepG2.2.15 cells were treated with B. adolescentis SPM0212 cell extract after 3 days in culture.
Viable or heat-killed Bifidobacterium species, as well as certain of their cell components, can stimulate the production of hydrogen peroxide, nitric oxide (NO), and cytokines, such as interleukin (IL)-6 and tumor necrosis factor (TNF)-α, in macrophage cell lines (Miettinen et al. 1996; Han et al. 2005a). In our previous study, the butanol extract of B. adolescentis SPM0212 dose-dependently increased TNF-α and NO production (Lee et al. 2008). So, we investigated the antiviral mechanism of B. adolescentis SPM0212 cell extract through the pathways of the IFN-mediated antiviral response. The IFN family of cytokines is now recognized as a key component of the innate immune response and as the first line of defense against viral infection (Sadler and Williams 2008). Gene targeting studies have distinguished four main effector pathways of the IFN-mediated antiviral response: the Mx GTPase pathway, the 2′,5′-oligoadenylate-synthetase-directed ribonuclease L pathway, the PKR pathway and the ISG15 ubiquitin-like pathway. These effector pathways individually block viral transcription, degrade viral RNA, inhibit translation and modify protein function to control all steps of viral replication (Sadler and Williams 2008). The cell extract of B. adolescentis SPM0212 significantly increased gene expression of IFN-signaling components (STAT1 and 6-16 gene). Consequently, MxA gene expression was significantly increased. MxA protein binds viral nucleocapsids or other viral components and then degrades them. Viruses that are susceptible to the activities of MxA include coxsackievirus, orthobunyavirus, hantavirus, phlebovirus and dugbe virus as well as HBV (Chieux et al. 2001; Gordien et al. 2001; Andersson et al. 2004). These results indicate that B. adolescentis SPM0212 could have potential applications as antiviral agents against clinically significant viruses such as HBV and coxsackievirus. We suggest that this Bifidobacterium strain may be a useful probiotic microorganism for the prevention or alternative therapy of hepatitis B, as well as provide hepatoprotection. However, more information is needed regarding this effect in various clinical conditions.
References
Andersson, I., L. Bladh, M. Mousavi-Jazi, K.E. Maqnusson, A. Lundkvist, O. Haller, and A. Mirazimi. 2004. Human MxA protein inhibits the replication of Crimean-Congo hemorrhagic fever virus. Journal of Virology 78: 4323–4329.
Arase, Y., K. Ikeda, F. Suzuki, Y. Suzuki, S. Saitoh, M. Kobayashi, N. Akuta, T. Somey, T. Hosaka, H. Sezaki, M. Kobayashi, and H. Kumada. 2006. Long-term outcome after hepatitis B surface antigen seroclearance in patients with chronic hepatitis B. American Journal of Medicine 119: 71.e9-16.
Buti, M., and R. Esteban. 2005. Drugs in development for hepatitis B. Drugs 65: 1451–1460.
Cha, M.K., D.K. Lee, H.M. An, S.W. Lee, S.H. Shin, J.H. Kwon, and K.J. Kim. 2012. Antiviral activity of Bifidobacterium adolescentis SPM 1005-A on human papillomavirus type 16. BMC Medicine 10: 72. doi:10.1186/1741-7015-10-72.
Chen, C.H., C.M. Lee, J.H. Wang, H.D. Tung, C.H. Hung, and S.N. Lu. 2004. Correlation of quantitative assay of hepatitis B surface antigen and HBV DNA levels in asymptomatic hepatitis B virus carriers. European Journal of Gastroenterology and Hepatology 16: 1213–1218.
Chieux, V., W. Chehadeh, J. Harvev, O. Haller, P. Wattre, and D. Hober. 2001. Inhibition of coxsackievirus B4 replication in stably transfected cells expressing human MxA protein. Virology 283: 84–92.
Cho, Y.K., and B.C. Song. 2011. New insight for HBV DNA HBsAg quantitation during antiviral therapy in patients with chronic hepatitis B. Korean Journal of Gastroenterology 57: 144–149.
De Santis, A., G. Famularo, and C. De Simone. 2000. Probiotics for the hemodynamic alterations of patients with liver cirrhosis. American Journal of Gastroenterology 95: 323–324.
Gill, H.S., and F. Guarner. 2004. Probiotics and human health: a clinical perspective. Postgraduate Medical Journal 80: 516–526.
Gordien, E., O. Rosmorduc, C. Peltekian, F. Garreau, C. Brechot, and D. Kremsdorf. 2001. Inhinbition of hepatitis B virus replication by the interferon-inducible MxA protein. Journal of Virology 75: 2684–2691.
Han, S.H., K.H. Cho, C.K. Lee, Y.C. Song, S.H. Park, N.J. Ha, and K.J. Kim. 2005a. Enhancement of antigen presentation capability of dendritic cells and activation of macrophages by the components of Bifidobacterium pseudocatenulatum SPM1204. Journal of Applied Pharmacology 13: 174–180.
Han, S.Y., C.S. Huh, Y.T. Ahn, K.S. Lim, Y.J. Baek, and D.H. Kim. 2005b. Hepatoprotective effect of lactic acid bacteria. Journal of Microbiology and Biotechnology 15: 887–890.
Iloeje, U.H., H.I. Yang, C.L. Jen, J. Su, L.Y. Wang, S.L. You, and C.J. Chen. 2007. Risk and predictors of mortality associated with chronic hepatitis B infection. Clinical Gastroenterology and Hepatology 5: 921–931.
Im, S.J., and Y.C. Sung. 2006. Action mechanism in immunopathogenesis and clearance of HBV. Korean Journal of Gastroenterology 12: 154–162.
Jansen, R.W., L.C. Johnson, and D.R. Averett. 1993. High-capacity in vitro assessment of anti-hepatitis B virus compound selectivity by a virion-specific polymerase chain reaction assay. Antimicrobial Agents and Chemotherapy 37: 441–447.
Jia, F., Y.Z. Zhang, and C.M. Liu. 2007. Stable inhibition of hepatitis B virus expression and replication in HeptG2.2.15 cells by RNA interference based on retrovirus delivery. Journal of Biotechnology 128: 32–40.
Kao, J.H., and D.S. Chen. 2002. Global control of hepatitis B virus infection. Lancet Infectious Diseases 2: 395–403.
Kim, B.K., S.Y. Kwon, C.H. Lee, W.H. Choe, H.M. Choi, and H.W. Koo. 2009. Analysis of the cost-effectiveness of antiviral therapies in chronic hepatitis B patients in Korea. Korean Journal of Gastroenterology 15: 25–41.
Korba, B.E., and G. Milman. 1991. A cell culture assay for compounds which inhibit hepatitis B virus replication. Antiviral Research 15: 217–228.
Kruining, J., R.A. Heijtink, and S.W. Schalm. 1995. Antiviral agents in hepatitis B virus transfected cell lines: inhibitory and cytotoxic effect related to time of treatment. Journal of Hepatology 22: 263–267.
Lavanchy, D. 2004. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. Journal of Viral Hepatitis 11: 97–107.
Lee, D.K., S. Jang, E.H. Baek, M.J. Kim, K.S. Lee, H.S. Shin, M.J. Chung, J.E. Kim, and K.O. Lee. 2009a. Lactic acid bacteria affect serum cholesterol levels, harmful fecal enzyme activity, and fecal water content. Lipids in Health and Disease 8: 21. doi:10.1186/1476-511X-8-21.
Lee, Y.H., L.J. Kang, and S.G. Lee. 2009b. Nelumbo nucifera leaves extract reduced the production of hepatitis B surface antigen on HepG2.2.15. Korean Journal of Medicinal Crop Science 17: 133–138.
Lee, D.K., S. Jang, M.J. Kim, J.H. Kim, M.J. Chung, K.J. Kim, and N.J. Ha. 2008. Anti-proliferative effects of Bifidobacterium adolescentis SPM0212 extract on human colon cancer cell lines. BMC Cancer 8: 310. doi:10.1186/1471-2407-8-310.
Lee, D.K., S.Y. Park, S. Jang, E.H. Baek, M.J. Kim, S.M. Huh, K.S. Choi, M.J. Chung, J.E. Kim, K.O. Lee, and N.J. Ha. 2011. The combination of mixed lactic acid bacteria and dietary fiber lowers serum cholesterol levels and fecal harmful enzyme activities in rats. Archives of Pharmacal Research 34: 23–29.
Lin, Y.L., M.S. Shiao, C. Mettling, and C.K. Chou. 2003. Cholesterol requirement of hepatitis B surface antigen (HBsAg) secretion. Virology 314: 253–260.
Livak, K.J., and T.D. Schmittgen. 2001. Analysis of relative gene expression data using real time quantitative PCR and the 2−ΔΔCT method. Methods 25: 402–408.
Miettinen, M., J. Vuopio-Varkila, and K. Varkila. 1996. Production of human tumor necrosis factor alpha, interleukin-6, and interleukin-10 is induced by lactic acid bacteria. Infection and Immunity 64: 5403–5405.
Nosova, T., H. Jousimies-Somer, K. Jokelainen, R. Hein, and M. Salaspuro. 2000. Acetaldehyde production and metabolism by human indigenous and probiotic Lactobacillus and Bifidobacterium strains. Alcohol and Alcoholism 35: 561–568.
Parvez, S., K.A. Malik, S. Ah Kang, and H.Y. Kim. 2006. Probiotics and their fermented food products are beneficial for health. Journal of Applied Microbiology 100: 1171–1185.
Perrillo, R.P. 2005. Current treatment of chronic hepatitis B: benefits and limitations. Seminars in Liver Disease 25: 20–28.
Sadler, A.J., and B.R. Williams. 2008. Interferon-inducible antiviral effectors. Nature Reviews Immunology 8: 559–568.
Sells, M.A., M.L. Chen, and G. Acs. 1987. Production of hepatitis B virus particles in HepG2 cells transfected with cloned hepatitis B virus DNA. Proceedings of the National Academy of Sciences of the United States of America 84: 1005–1009.
Shin, H.S., S.Y. Park, D.K. Lee, S.A. Kim, H.M. An, J.R. Kim, M.J. Kim, M.G. Cha, S.W. Lee, K.J. Kim, K.O. Lee, and N.J. Ha. 2010. Hypocholesterolemic effect of sonication-killed Bifidobacterium longum isolated from healthy adult Koreans in high cholesterol fed rats. Archives of Pharmacal Research 33: 1425–1431.
Solga, S.F. 2003. Probiotics can treat hepatic encephalopathy. Medical Hypotheses 61: 307–313.
Strober, W. (2001) Trypan blue exclusion test of cell viability. Current Protocols in Immunology, Appendix 3, Appendix 3B doi:10.1002/0471142735.ima03bs21.
Su, T.H., C.S. Hsu, C.L. Chen, C.H. Liu, Y.W. Huang, T.C. Tseng, C.J. Liu, P.J. Chen, M.Y. Lai, D.S. Chen, and J.H. Kao. 2010. Serum hepatitis B surface antigen concentration correlates with HBV DNA level in patients with chronic hepatitis B. Antiviral Therapy 15: 1133–1139.
Wang, G.F., L.P. Shi, Y.D. Ren, Q.F. Liu, H.F. Liu, R.J. Zhang, Z. Li, F.H. Zhu, P.L. He, W. Tang, P.Z. Tao, C. Li, W.M. Zhao, and J.P. Zuo. 2009. Anti-hepatitis B virus activity of chlorogenic acid, quinic acid and caffeic acid in vivo and in vitro. Antiviral Research 83: 186–190.
Yuen, M.F., D.K. Wong, E. Sablon, E. Tse, I.O. Ng, H.J. Yuan, C.W. Siu, T.J. Sander, E.J. Bourne, J.G. Hall, L.D. Condreay, and C.L. Lai. 2004. HBsAg seroclearance in chronic hepatitis B in the Chinese: virological, histological and clinical aspects. Hepatology 39: 1694–1701.
Zhao, R., T.Z. Wang, D. Kong, L. Zhang, H.X. Meng, Y. Jiang, Y.Q. Wu, Z.X. Yu, and X.M. Jin. 2011. Hepatoma cell line HepG2.2.15 demonstrates distinct biological features compared with parental HepG2. World Journal of Gastroenterology 17: 1152–1159.
Acknowledgments
The authors are grateful to Professor Yun Gyu Park for providing the HepG2.2.15 cell lines and to Sahmyook University for the financial support. This work was supported by Business for Cooperative R&D between Industry, Academy, and Research Institute funded by the Korea Small and Medium Business Administration (Grants No. 000449270111).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, D.K., Kang, J.Y., Shin, H.S. et al. Antiviral activity of Bifidobacterium adolescentis SPM0212 against Hepatitis B virus. Arch. Pharm. Res. 36, 1525–1532 (2013). https://doi.org/10.1007/s12272-013-0141-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-013-0141-3