Abstract
In the present study, the anti-inflammatory effect of salicylideneamino-2-thiophenol (SAL-2), a derivative of salicylate, on a potent oxidant 4-hydroxynonenal (HNE)-induced oxidative stress was investigated using rat prostate endothelial (YPEN-1) cells. We focused on anti-inflammatory activity of SAL-2 which was determined by its ability to suppress COX-2 and iNOS gene expression through suppression of NF-κB and redox regulation. We found that SAL-2 effectively inhibited HNE-induced reactive species generation, while upregulated GSH/GSSG ratio. Prostagrandin (PG) E2 production stimulated by arachidonic acid was suppressed by SAL-2. SAL-2 also downregulated COX-2 and iNOS expression induced by HNE, but salicylate did not. We found that SAL-2 inhibited HNE-mediated IKK phosphorylation, IκBα degradation and nuclear translocation of p65 which are linked to NF-κB activation. Furthermore, SAL-2 inhibited HNE-induced activation of mitogen-activated protein kinases. Collectively, SAL-2 inhibited COX-2 and iNOS gene expression through suppression of NF-κB leading to the inhibition of PGE2 synthesis. Based on these data, we propose that with its combined effect on strong anti-oxidant and anti-inflammatory action, SAL-2 can be a potent anti-inflammatory agent for treatment of inflammatory-related diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Salicylate (SAL) has been used as an effective remedy for the pain relief, fever, and rheumatic disease for decades, although its underlying mechanisms were not well defined until recently. Numerous researches established that SAL has potent efficacies that can selectively inhibit pro-inflammatory enzyme, cyclooxygenase (COX). It is interesting to note that inflammation is frequently elicited by various free radicals (Topper et al. 1996; Wheaton et al. 1996) but SAL has no scavenging effect on reactive species (RS) contrasting to its derivative like, salicylideneamino-2-thiophenol (SAL-2) (Chung et al. 2008; Kwon and Chae 2003).
The major differences and the specificity of SAL-2 may relate to the structural, and the presence of thiols (see the structure formula in Fig. 1). For instance, the basic structure of SAL-2 consisting of 2 benzene rings is similar to that found in well-known natural anti-oxidants, piceatannol, resveratrol, and oxyresveratrol. The thiol moiety seems to be requisite for the strong anti-oxidant action as found in physiologically versatile glutathione (GSH) and a potent N-acetylcysteine (NAC) both of which have shown to be important in reducing oxidation and preventing oxidative damage to various cells (Seo et al. 2002).
Based on the structural characteristic of thiol containing SAL-2, it is expected that SAL-2 might have anti-oxidative efficacies toward to RS, and at the same time, has anti-inflammatory action. This expectation was our rationale on which the present investigation was launched. To verify our hypothesis, we investigated SAL-2’s actions on the putative anti-oxidative effect, and also the anti-inflammatory effects at molecular level compared with its structure derivatives, SAL and 2-SAL.
Because much are known on inhibitory action of SAL such as NF-κB, c-Jun activation (Kopp and Ghosh 1994; Dong et al. 1997) and COX-2 protein or mRNA (Xu et al. 1999), we were interested in knowing whether SAL-2 might have a similar action on the modulation of NF-κB signaling pathway as SAL does. The involvement of the redox-sensitive NF-κB is widespread in which this most versatile transcription factor is known to modulator of inflammation, and its activation is responsive to various oxidant stimuli (Wheaton et al. 1996; Seo et al. 2002). For the inflammatory process, NF-κB regulates the gene expression of several pro-inflammatory cytokines, such as interleukin-1β (IL-1β), IL-6, and tumor necrosis factor-α, and inflammatory enzymes, including COX-2 and inducible nitric oxide synthase (iNOS), through κB sites located in their promoter region.
For better appreciation of aforementioned COX in inflammation, a brief description about its versatile roles in various pathophysiological situations is in order. COX that is a specific target site of many non-steroidal anti-inflammatory drugs catalyzes the first step in the conversion process of arachidonic acid to prostaglandin (PG). At present, two isoforms are known: COX-1 is expressed constitutively at constant levels, however the other isoform, COX-2, is readily inducible by many factors including tumor promoters, growth factors and cytokines (Crofford et al. 1994; Inoue et al. 1995; Xie et al. 1991). Also COX-derived PGs and reactive oxygen species are major contributor to the cell inflammation, proliferation, apoptosis, angiogenesis, and immune surveillance (Sheng et al. 1998; Stolina et al. 2000; Tsujii et al. 1998).
Among several factors involved in inflammation, pro-inflammatory iNOS should be mentioned (Nathan 1997). Since iNOS is widely distributes throughout tissues including macrophages, smooth muscle cells, and hepatocytes, quantitatively its activation has far reaching effects as seen in sepsis, and stroke (Nathan 1992; Marletta 1993). In our previous study, it was reported that SAL-2 inhibited genes expression of RANTES, MCP-1, and IL-8 in rat peritoneal macrophages (Chung et al. 2000).
In this present study, we attempt to obtain evidence on the SAL-2’s anti-inflammatory action by showing the inhibition of COX-2 and iNOS, and at the same time, to gain molecular insights on the underlying mechanisms of SAL-2 by exploring the status of NF-κB levels in YPEN-1 that are oxidatively stimulated by a potent oxidant 4-hydroxynonenal (HNE). HNE, a reactive by-product of arachidonic acid and linoleic acid, is generated in relatively large amounts (up to mM ranges) in response to oxidative insult and is believed to be largely responsible for the cytotoxic effects associated with oxidative stress (Esterbauer et al. 1991). The investigation was conducted in comparison with SAL-2’s well-known analogs, SAL and 2-SAL.
Materials and methods
Materials
YPEN-1 cells (a rat prostatic endothelial cell line) were obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA). This cell line is responsive to oxidative stress, its well-characterized redox-sensitive NF-κB modulation by various agents and our laboratory has experienced with in vitro samples for the molecular work on oxidative stress. Cells were cultured in Dulbecco’s Modified Eagle Media (DMEM) (Nissui Co., Tokyo) supplemented with 5 % heat-inactivated fetal bovine serum (Gibco, Grand Island, NY).
Salicylideneamino-2-thiophenol (SAL-2) and 2-salicylideneaminophenol (2-SAL) were obtained from Tokyo Kasei Kogyo Co. (Tokyo, Japan). Salicylate (SAL), DL-penicillamine, trolox, amphotericin B, and monoclonal antibody to β-actin were purchased from Sigma-Aldrich (St. Louis, MO, USA). Dihydrorhodamine 123 (DHR 123), 2,7-dichlorodihydrofluorescein diacetate (H2DCF-DA) were obtained from Invitrogen (Eugene, OR, USA). Enzyme immunoassay kit, HNE and peroxynitrite were obtained from Cayman Chemical (Ann Arbor, MI, USA). Polyvinylidene fluoride (PVDF) membrane (Immobilon-P) was obtained from Millipore (Bedford, MA, USA). Antibodies against COX-2, iNOS, p65, IκBα, phospho-NF-κB inducing kinase (NIK), and phospho-IκB kinase (IKK) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Enhanced chemiluminescence (ECL) Western blotting detection reagents were from GE Healthcare (Arlington Heights, IL, USA).
pNFκB-Luc vector that contains a specific binding sequence for NF-κB was obtained from Clontech (CA, USA), and FuGENE 6 Reagent was purchased from Roche (Indianapolis, IN, USA). Steady-Glo Luciferase Assay System was obtained from Promega (Madison, WI, USA. All other chemicals were of the highest purity available from either Sigma Chemical Co. or Junsei Chemical Co. (Tokyo, Japan).
Cells and cell culture conditions
YPEN-1, rat prostate endothelial cells were grown in DMEM with 5 % fetal bovine serum in a humidified 5 % CO2 at 37 °C containing 100 U/ml penicillin, 100 μg/ml streptomycin, and 2.5 μm/ml amphotericin B.
Measurement of reactive species (RS)
The method on measurement of RS scavenging activity was previously reported by Ali et al. (1992). Briefly, H2DCF-DA (2.5 mM) mixed with esterase (1.5 units/ml) was incubated at 37 °C for 20 min and placed on ice in the dark until immediately prior to the study. Phosphate buffer (50 mM) at pH 7.4 was used. H2DCF-DA was deacetylated to nonfluorescent 2,7-dichlorodihydrofluorescein (DCFH) by esterase and subsequently oxidized to highly 2,7-dichlorofluorescein (DCF) by RS. The fluorescence intensity of oxidized DCFH was measured by using the microplate fluorescence GENious (Tecan, Austria) at excitation and emission wavelengths of 485 and 530 nm, respectively, for 30 min with or without the addition of menadione (50 mM) as an RS source.
For intracellular RS measurement, YPEN-1 cells in 96-well plates were pre-incubated for 24 h. After 1 day, the medium was changed to fresh serum free medium. The cells were treated with or without SAL-2, SAL, and 2-SAL and then incubated for 1 h. After treatment with HNE (10 μM) for 1 h, the medium was replaced with fresh serum free medium and H2DCF-DA (125 μM) was added. The fluorescence intensity of DCF was measured for 1 h using the microplate fluorescence GENious with excitation and emission wavelengths of 485 and 535 nm, respectively.
Measurement of peroxynitrite (ONOO−)
Peroxynitrite activity was measured by monitoring the oxidation of DHR 123 by modifying the methods of Kooy et al. (1994). A stock solution of 5 mM DHR 123 in dimethylformamide was purged with nitrogen and stored at −20 °C. The working solution with 5 μM DHR 123 diluted from the stock solution was placed on ice in the dark immediately prior to the study. Then 90 mM NaCl, 50 mM sodium phosphate (pH 7.4), and 5 mM KCl was purged with nitrogen and placed on ice, and 100 μM diethylenetriaminepenta acetic acid (DTPA) was added before use. Peroxynitrite scavenging activity by the oxidation of DHR 123 was measured on the microplate fluorescence reader GENious with excitation and emission wavelengths of 485 and 530 nm, respectively, at room temperature.
Measurement of GSH/GSSG ratio
To assay GSH levels, 1 mM EDTA-50 mM phosphate buffer was added to the supernatant followed by ο-phthaldehyde. After 25 min at room temperature, the fluorescence was measured at excitation wavelength of 360 nm and emission wavelength of 460 nm. And to measure GSSG levels, N-ethylmaleimide was added to the supernatant. After 30 min at room temperature, added to 0.5 N NaOH and ο-phthaldehyde. After 30 min at room temperature the fluorescence was measured at excitation wavelength of 360 nm and emission wavelength of 460 nm (Tauskela et al. 2000).
Cytotoxicity (MTT) assays
Cytotoxicity was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) from Aldrich Chemical Co. (Madison, WI). Cells were seeded into 96-well plates, incubated for overnight to adhere, and then exposed to various concentrations of drugs for 24 h. At the end of the treatment period, MTT reagent dissolved in PBS was added to the medium (final concentration of 0.5 mg/ml) and the plates were incubated in the dark for 2 h. After incubation, the supernatant was removed and formazan crystals were dissolved in 100 μl of DMSO with gentle agitation. The absorbance per well was measured by spectrophotometric absorbance at 570 nm.
Protein analysis by Western blotting analysis
Cells were harvested and washed twice in phosphate buffered saline (PBS) at 4 °C. Total cell lysates were prepared using lysis buffer (40 mM Tris at pH 8.0, 120 mM NaCl, 0.5 % NP-40, 0.1 mM sodium orthovanadate, 2 μg/ml aprotinin, 2 μg/ml leupeptin and 100 μg/ml PMSF). The supernatant was collected and protein concentrations were then measured with protein assay reagents. The same amounts of proteins were subjected to 8–12 % SDS-PAGE, and electrophoretically transferred to a PVDF membrane. The membrane was immediately placed in a blocking solution [5 % non-fat dry milk in TBS-T buffer (10 mM Tris, 100 mM sodium chloride and 0.1 % Tween 20, pH 7.5)] at room temperature for 1 h and then washed with TBS-T buffer, then incubated with primary antibody at room temperature for 2 h. After three times washings with TBS-T buffer, the membrane was incubated with a second antibody at room temperature for 2 h. After washing four times with TBS-T buffer, antibody labeling was detected using ECL per manufacturer’s instructions.
Transfection and luciferase reporter assay for NF-κB activity
NF-κB activity was examined using a luciferase plasmid, pNF-κB-Luc (Clontech, CA) that contains a specific binding sequence for NF-κB. Transfection was carried out using FuGENE 6 Reagent. Briefly, 2 × 104 cells per each well were seeded in 48-well plates. When cultured cells reached about 50 % confluences, cells were treated with 0.1 μg DNA/0.2 μl FuGENE 6 complexes in a total volume of media with 500 μl for 48 h. Subsequently, 3 μM of HNE was treated after the plate was changed with serum-free media, and treatments of SAL-2 (10, 30, 100 μM) were performed 1 h previously. After additional incubation for 6 h, cells were washed with PBS and added with Steady-Glo Luciferase Assay System to the plate. Luciferase activity was measured by a luminometer. Raw luciferase activities were normalized by protein concentration per each well.
Determination of COX activity (PGE2 assay)
COX activity assay (PGE2 assay) was determined by using a commercially available enzyme immunoassay kit (Cayman Chemical). Briefly, 30 μl of homogenized tissue mixed with 10 μl of tested compound in 455 μl of 50 mM potassium phosphate buffer (pH 7.4). After 10 min incubation at room temperature, 5 μl of 3 mM arachidonic acid in 10 % ethanol was added, and then incubated for 30 min at room temperature. Samples were heated for 3 min on 100 °C, and centrifuged for 30 min at 12,000 rpm at 4 °C. Fifty microlitre of this supernatant was used in COX activity measurement. The results are expressed as ng/ml and represent the mean ± SE of three experiments.
Statistical analysis
The results were presented as means ± SE of three, independent triplicate measurements. Statistical significance of the difference between untreated control and treated groups was determined using one-way ANOVA with a post hoc test.
Results
RS/ONOO− scavenging activity of SAL-2, SAL and 2-SAL
Peroxynitrite (ONOO−), one of the products of nitrogen-derived free radicals, is formed by the reaction of two ubiquitous free radical species: superoxide (O2 −) and nitric oxide (NO) (Rubbo et al. 1994; Pryor and Squadrito 1995). To verify the free radical scavenging activity of SAL derivatives, we measured RS and ONOO− levels.
The results are as shown in Table 1, a 50 % inhibition concentration (IC50) was calculated against control group (without SAL-2, SAL and 2-SAL). The RS scavenging activity of SAL-2 (IC50, 29.45 ± 1.26 μM) was highest compared to SAL (>100 μM), 2-SAL (43.02 ± 4.21 μM) and trolox, ROS scavenger, (52.76 ± 0.91 μM). The oxidation of DHR 123 to fluorescent rhodamine 123 by native ONOO− was determined in the presence of the SAL-2, SAL and 2-SAL at increasing concentrations. The comparative data on the potency of the inhibition of DHR 123 oxidation by ONOO− are as shown in Table 1. The ONOO− scavenging activity of SAL-2 was more potent (6.31 ± 0.12 μM) than SAL (>100 μM) and similar to penicillamine, ONOO− scavenger, (6.38 ± 0.13 μM). But 2-SAL showed the most potent effect (1.24 ± 0.11 μM).
RS scavenging activity and up-regulation of GSH/GSSG ratio by SAL-2 in endothelial cells
To investigate the free radical scavenging activity of SAL derivatives in cells, we measured RS levels in endothelial cells with HNE stimulation.
Results indicated in Fig. 2a, HNE-treated cells generated significantly higher RS levels than untreated cells. Up-regulation of intracellular RS generation was decreased by pre-incubation of SAL-2 at concentration with 50 μM. 2-SAL also had RS scavenging activity. However, SAL, a well-known COX inhibitor, has no effect on RS scavenging activity.
Modulation of RS generation and intracellular GSH/GSSG ratio by SAL-2 in YPEN-1 cells. a Cells were treated with 50 μM SAL-2, SAL, and 2-SAL for 1 h, and then treated with 15 μM HNE for 1 h. Intracellular RS level was assayed using H2DCF-DA. b Intracellular GSH/GSSG ratio was assayed by fluorescent method described in “Materials and Methods” section. Each value is the mean ± SE of three experiments. Statistical significance; *p < 0.05, and **p < 0.01 versus vehicle; # p < 0.05, ## p < 0.01, and ### p < 0.001 versus HNE-treated cells
GSH is a cofactor for a number of metabolic and detoxification reactions, including GSH peroxidases and GSH-S-transferases. Thus, HNE is well known to contribute to the cytotoxicity of oxidative stress by depleting cellular GSH levels (Kinter and Roberts 1996). To determine whether SAL derivatives modulate GSH levels, we examined GSH/GSSG ratio in YPEN-1 cells with HNE. Results showed that SAL-2 significantly up-regulated intracellular GSH/GSSG ratio, which decreased by HNE (Fig. 2b). SAL and 2-SAL have also a little effect on up-regulation of GSH/GSSG ratio. As shown in Table 1 and Fig. 2, SAL-2 is more powerful redox regulator than SAL and 2-SAL.
Effects of SAL-2 on production of PGE2 and expression of COX-2 and iNOS
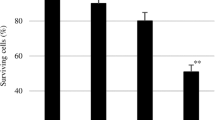
COX-2 catalyzed PG production is important role in the inflammatory process (Sheng et al. 1998; Stolina et al. 2000; Tsujii et al. 1998). The effects of SAL-2, SAL and 2-SAL on PGE2 synthesis were evaluated in the same experimental conditions described above. In Fig. 3a showed that the presence of arachidonic acid (30 μM) increased in PGE2 accumulation. This PGE2 induction was suppressed by SAL-2 but not by SAL and 2-SAL. In view of the results so far achieved, we expect that SAL-2 may exert anti-inflammatory property. Therefore we investigated SAL-2’s anti-inflammatory action by showing the inhibition of COX-2 and iNOS expression, and to gain molecular insights on the underlying mechanisms of SAL-2 by exploring the status of NF-κB levels in YPEN-1 cells that are oxidatively stimulated by HNE. For this, we first tested the cytotoxic effects of SAL and its derivatives, SAL-2 and 2-SAL on YPEN-1 cells. Cells were incubated with various concentrations (10, 50, and 100 μM) of SALs for 24 h and then cell viability was determined by MTT assay. We found that cells with three drugs were showed greater than 90 % cell viability under experimental conditions (Fig. 3b). We next examined weather SAL-2 modulated HNE-induced expression of COX-2 and iNOS. We pretreated cells with various concentration of SAL-2 or SAL before stimulating cells with HNE. The results indicated that SAL-2 abolished HNE-induced COX-2 and iNOS protein expression at 50 and 100 μM concentrations (Fig. 3c). SAL suppressed neither COX-2 nor iNOS protein expression induced by HNE (Fig. 3d). These results suggest that SAL-2 may inhibit HNE-induced NF-κB pathway.
Effect of SAL-2 on arachidonic acid (AA)-induced PGE2 production and HNE-induced COX-2 and iNOS expression. a Production of PGE2 was determined by EIA as described in “Materials and Methods” section. Each bar is the mean ± SE of three experiments. Statistical significance: ***p < 0.001 versus untreated control; ## p < 0.01 and ### p < 0.001 versus AA. b Cell viability was determined by MTT assay described in “Materials and Methods” section. Each bar is the mean ± S.E of three measurements. Statistical significance: **p < 0.01, ***p < 0.001 versus vehicle. c, d Western blotting analysis was probed with antibodies specific for COX-2 and iNOS. Cell lysates (25 μg/lane) were from YPEN-1 cells treated with vehicle, HNE (10 μM), SAL-2 (10, 50, 100 μM) or SAL (10, 50, 100 μM) for 24 h. The data presented is representative at least 3 separate experiments. Each bar is the mean ± SE of three experiments. Statistical significance: ***p < 0.001 versus vehicle; # p < 0.05, ## p < 0.001 and ### p < 0.001 versus HNE-treated cells
Effects of SAL-2 on HNE-induced activation of NF-κB signaling cascade and MAPKs
Because the activation of NF-κB is critical for the induction of both COX-2 and iNOS by HNE (van der Woude et al. 2004), we determined whether SAL-2 might suppress NF-κB activation in HNE-induced YPEN-1 cells. To investigate evident effect of SAL-2 which inhibits nuclear translocation of NF-κB, we used a little high concentration of HNE (15 μM). As shown in Fig. 4a, HNE-induced NF-κB nuclear translocation and IκBα degradation were significantly inhibited by SAL-2. We also examined that activation of NIK and IKK by Western blot analysis using phospho-NIK and phospho-IKKα/β antibodies. The activation of NIK and IKK was detectable within 20 min following 15 μM of HNE treatment (Fig. 4a). These results indicated that HNE may activate NF-κB through activation of NIK and IKKα/β, IκBα phosphorylation and degradation, which, in turn, lead to NF-κB nuclear translocation. And it was determined whether SAL-2 inhibited on HNE-induced phosphorylation of NIK and IKKα/β proteins in YPEN-1 cells. SAL-2 inhibited the HNE-induced activation of NIK and IKKα/β at 50 and 100 μM concentrations (Fig. 4a).
Modulation of NF-κB signaling cascade and MAPKS activation by SAL-2 in YPEN-1 cells. a Cells were treated with vehicle, HNE (15 μM), and/or SAL-2 (10, 50, 100 μM) for 1 h. Nuclear and cytoplasmic extracts (25 μg/lane) were prepared, subjected to SDS-PAGE, and probed with antibodies specific for phospho-NIK, phospho-IKKα/β, NF-κB (p65), and IκBα. β-Actin blot is shown to verify the same amount of protein loaded. b YPEN-1 cells were treated with vehicle, HNE (15 μM) and/or SAL-2 (10, 50, 100 μM) for 20 min. Cytosolic fractions were prepared and performed Western blotting analysis using phospho-JNK, phospho-ERK and phospho-p38 antibodies. Each bar is the mean ± SE of three experiments. Statistical significance: *p < 0.05, **p < 0.01, ***p < 0.001 versus vehicle; # p < 0.05, ## p < 0.01, and ### p < 0.001 versus HNE-treated cells. c NF-κB transcriptional activity was measured by using luciferase assay. Reporter plasmid pNFκB-Luc was transfected to YPEN-1 and co-treated with 3 μM HNE and SAL-2 (10, 30, 100 μM) for 6 h. Each bar is the mean ± SE of three experiments. Statistical significance: **p < 0.01 versus transfected cells; ## p < 0.01 versus HNE-treated cells
Intracellular oxidative stress stimuli can activate both NF-κB and mitogen activated protein kinase (MAPK) modules (Schulze-Osthoff et al. 1997; Ono and Han 2000; Mercurio and Manning 1999). We examined phosphorylation of c-Jun N-terminal kinase (JNK), extracellular signal-regulated kinase (ERK1/2) and p38 by Western blotting analysis using anti-phospho-JNK, anti-phospho-ERK1/2 and anti-phospho-p38. The activation of JNK, ERK1/2 and p38 were detectable within 20 min following 15 μM of HNE treatment (Fig. 4b). We then examined the effects of SAL-2 on HNE-induced activation of JNK, ERK1/2 and p38 in YPEN-1 cells. SAL-2 abolished the HNE-induced activation of these MAPKs at 50 and 100 μM concentrations (Fig. 4b).
To further investigate the importance of HNE and SAL-2 in modulating the activation of NF-κB, transient transfection was performed using pNFκB-Luc plasmid. Treatment with 3 μM HNE led to a fourfold increase in NF-κB transcriptional activities that was inhibited by SAL-2 in a dose-dependent manner (Fig. 4c).
Discussion
In the report, we documented SAL-2 has a potent inhibitory ability on the inflammatory gene expression, including COX-2 and iNOS in YPEN-1 cells. Furthermore, we obtained molecular information that this inhibitory action is mediated through suppression of NF-κB activation. In addition, our data showed that SAL-2 also could attenuate HNE-induced oxidative stress by its scavenging action on RS and upregulation of GSH/GSSG ratio. All these new findings from the current study make clear that SAL-2 is more effective on anti-inflammatory and anti-oxidant effects than SAL or 2-SAL.
SAL is well-known for its selective inhibition of COX with the anti-inflammatory potency. Several reports have shown the suppression of COX-2 protein or mRNA levels by SAL, while others reported no effect or enhancement of COX-2 expressions by SAL (Abate et al. 2000; Tran et al. 2002; Amann et al. 2001). We believed that this discrepancy may be related to the difference in the dosage or concentration was used. Kwon and Chae (2003) and Chung et al. (2000) show that SAL inhibited COX-2 and iNOS only at high concentrations (more than 2 mM) (Chung et al. 2000; Kwon and Chae 2003). Because of these previous findings, we used similar concentration ranges of SAL to test its effects on COX-2 expressions. However, even a concentration as high as 5 mM, SAL showed little inhibitory effect on HNE-induced COX-2 expression (data not shown). We also found that SAL-2 effectively suppressed AA-stimulated PGE2 production in dose-dependent manner; however, SAL did not affect PGE2 production, even at the 100 μM concentration (Fig. 3a). This is agreed to previous findings that SAL as well as its derivate valeroyl SAL showed weak inhibitory effect on PGE2 release at far higher than 100 μM (Fiebich and Chrubasik 2004; Welch et al. 2003). In contrasted with SAL, SAL-2 exhibited high-potency on COX-2 and iNOS inhibition in response to stimulation by HNE in YPEN-1 cells, even as low as at 50 and 100 μM (Fig. 3c). Another derivative of SAL, 2-SAL, has also a little effect on RS scavenging activity and up-regulation of GSH/GSSG ratio. Therefore, we investigated the effect of 2-SAL on HNE-induced protein expression of COX-2 and iNOS. As a result, 2-SAL has a little effect on suppressing COX-2 and iNOS protein levels.
Indeed, we could know that RS levels and GSH/GSSG ratio was most important factor in anti-inflammatory action. Because κB-binding site located in COX-2 and iNOS promoter region, we investigated about suppression of NF-κB by SAL-2. We found that SAL-2 significantly reduced nuclear translocation of NF-κB protein and its DNA-binding activity, as well as IκBα degradation at concentrations higher than 10 μM as show in Fig. 4.
In our study, the molecular modulation of NF-κB by SAL-2 was further revealed. We examined the levels of p-NIK and p-IKKα/β that are the active forms of NIK and IKK in response to SAL-2. Data showed that SAL-2 reduced phosphorylation of NIK and IKKα/β (Fig. 4a). In addition, we found that SAL-2 also inhibits HNE-induced MAPK that is also known to be pro-inflammatory (Fig. 4b). Several lines of evidences showed that MAPK involved in activation of NF-κB and then affected on its dependent gene expressions (Garg and Aggarwal 2002; Chun and Surh 2004). Therefore the expressions of COX-2 and iNOS were diminished by MAPK specific inhibitors (Kamata et al. 2005; Pelletier et al. 2001; Lee et al. 2005; Ho et al. 2004). HNE was reported that it caused the production of RS and lead to NF-κB activation through MAPK kinase pathway (Lee et al. 2010; Rahman 2002). In this study, our data showed that SAL-2 exerted anti-oxidant potential by RS and peroxynitrite scavenging and enhancement of cellular GSH, it is critical mechanism of SAL-2’s role in inhibition of HNE-induced pro-inflammatory mediators through suppression of NF-κB. Based on what is known about the interaction between MAPK and NF-κB, our study indicates that SAL-2 mediated NF-κB through inhibition of MAPK.
Since NF-κB signaling pathway are responsive to oxidative stress (Wheaton et al. 1996; Seo et al. 2002), we expect that it is the SAL-2’s anti-oxidative ability to modulate NF-κB activation. As expected, SAL-2 significantly inhibited RS production as presented in Table 1 and Fig. 2. To clearly confirm the anti-oxidant effects of SAL-2, we investigated the effect of SALs on PG synthesis pathway which RS-generating occurs during the conversion of PGG2 to PGH2, PGE2 that is mediated by the key enzyme, COX (Goppelt-Struebe 1995), which are all potent pro-inflammatory mediators that have been known to implicated in a variety of disease states such as arthritis, and cardiovascular disease (Beharka et al. 1997). Data showed that SAL-2 can also regulate PG synthesis pathway (see Fig. 3a).
The major differences are found in the current study on the actions of SAL-2, SAL and 2-SAL are related to the chemical structural differences among three. One major difference note is the presence of a thiol group in SAL-2 (see Fig. 1). It was recently reported that intracellular thiol concentration is known to be essential in the reduction of chronic inflammation (Santangelo 2003), presumably thiol compounds such as GSH and NAC scavenge RS and prevent oxidative damage in various cells (Topper et al. 1996). As we observed here, SAL-2 was significantly up-regulated GSH/GSSG ratio, and showed more efficient RS scavenging property than 2-SAL. Therefore it is highly likely that SAL-2’s antioxidant and anti-inflammatory effects are due to its thiol group (Fig. 1; Table 1).
In conclusion, our data indicated that SAL-2 suppressed expression of COX-2 and iNOS by inhibition of NF-κB and MAPK activation through RS scavenging and the up-regulation of GSH/GSSG ratio. We propose that SAL-2’s thiol group is essential for its anti-inflammatory function, and up-regulation of GSH/GSSG ratio and RS scavenging effect are the most important factor in inflammatory responses as compared to SAL-2, SAL and 2-SAL (Table 1). Further investigation of the current findings can be expanded to include studies of NF-κB and other signaling pathway involved in inflammatory diseases by conducting the same experiments in vivo.
References
Abate, A., G. Yang, P.A. Dennery, S. Oberle, and H. Schroder. 2000. Synergistic inhibition of cyclooxygenase-2 expression by vitamin E and aspirin. Free Radical Biology and Medicine 29: 1135–1142.
Ali, S.F., C.P. Lebel, and S.C. Bondy. 1992. Reactive oxygen species formation as a biomarker of methylmercury and trimethyltin neurotoxicity. Neurotoxicology 13: 637–648.
Amann, R., T. Egger, R. Schuligoi, A. Heinemann, and B.A. Peskar. 2001. Sodium salicylate enhances the expression of cyclooxygenase-2 in endotoxin-stimulated human mononuclear cells. European Journal of Pharmacology 433: 129–134.
Beharka, A.A., D. Wu, S.N. Han, and S.N. Meydani. 1997. Macrophage prostaglandin production contributes to the age-associated decrease in T cell function which is reversed by the dietary antioxidant vitamin E. Mechanisms of Ageing and Development 93: 59–77.
Chun, K.S., and Y.J. Surh. 2004. Signal transduction pathways regulating cyclooxygenase-2 expression: potential molecular targets for chemoprevention. Biochemical Pharmacology 68: 1089–1100.
Chung, C.K., H.N. Koo, K.Y. Chung, T. Shin, H.R. Kim, H.J. Chae, N.H. An, C.H. Kim, and H.M. Kim. 2000. Inhibitory effect of sodium salicylate on nitric oxide production from TM4 sertoli cells. International Journal of Immunopharmacology 22: 685–692.
Chung, J., H.S. Lee, H.Y. Chung, T.R. Yoon, and H.K. Kim. 2008. Salicylideneamino-2-thiophenol inhibits inflammatory mediator genes (RANTES, MCP-1, IL-8 and HIF-1alpha) expression induced by tert-butyl hydroperoxide via MAPK pathways in rat peritoneal macrophages. Biotechnology Letters 30: 1553–1558.
Crofford, L.J., R.L. Wilder, A.P. Ristimaki, H. Sano, E.F. Remmers, H.R. Epps, and T. Hla. 1994. Cyclooxygenase-1 and -2 expression in rheumatoid synovial tissues. Effects of interleukin-1 beta, phorbol ester, and corticosteroids. Journal of Clinical Investment 93: 1095–1101.
Dong, Z., C. Huang, R.E. Brown, and W.Y. Ma. 1997. Inhibition of activator protein 1 activity and neoplastic transformation by aspirin. Journal of Biological Chemistry 272: 9962–9970.
Esterbauer, H., R.J. Schaur, and H. Zollner. 1991. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radical Biology and Medicine 11: 81–128.
Fiebich, B.L., and S. Chrubasik. 2004. Effects of an ethanolic salix extract on the release of selected inflammatory mediators in vitro. Phytomedicine 11: 135–138.
Garg, A.K., and B.B. Aggarwal. 2002. Reactive oxygen intermediates in TNF signaling. Molecular Immunology 39: 509–517.
Goppelt-Struebe, M. 1995. Regulation of prostaglandin endoperoxide synthase (cyclooxygenase) isozyme expression. Prostaglandins Leukotrienes and Essential Fatty Acids 52: 213–222.
Ho, F.M., C.C. Lai, L.J. Huang, T.C. Kuo, C.M. Chao, and W.W. Lin. 2004. The anti-inflammatory carbazole, LCY-2-CHO, inhibits lipopolysaccharide-induced inflammatory mediator expression through inhibition of the p38 mitogen-activated protein kinase signaling pathway in macrophages. British Journal of Pharmacology 141: 1037–1047.
Inoue, H., C. Yokoyama, S. Hara, Y. Tone, and T. Tanabe. 1995. Transcriptional regulation of human prostaglandin-endoperoxide synthase-2 gene by lipopolysaccharide and phorbol ester in vascular endothelial cells. Involvement of both nuclear factor for interleukin-6 expression site and cAMP response element. Journal of Biological Chemistry 270: 24965–24971.
Kamata, H., S. Honda, S. Maeda, L. Chang, H. Hirata, and M. Karin. 2005. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell 120: 649–661.
Kinter, M., and R.J. Roberts. 1996. Glutathione consumption and glutathione peroxidase inactivation in fibroblast cell lines by 4-hydroxy-2-nonenal. Free Radical Biology and Medicine 21: 457–462.
Kooy, N.W., J.A. Royall, H. Ischiropoulos, and J.S. Beckman. 1994. Peroxynitrite-mediated oxidation of dihydrorhodamine 123. Free Radical Biology and Medicine 16: 149–156.
Kopp, E., and S. Ghosh. 1994. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science 265: 956–959.
Kwon, K.S., and H.J. Chae. 2003. Sodium salicylate inhibits expression of COX-2 through suppression of ERK and subsequent NF-kappaB activation in rat ventricular cardiomyocytes. Archives of Pharmacal Research 26: 545–553.
Lee, D.Y., Y.J. Oh, and B.K. Jin. 2005. Thrombin-activated microglia contribute to death of dopaminergic neurons in rat mesencephalic cultures: dual roles of mitogen-activated protein kinase signaling pathways. Glia 51: 98–110.
Lee, S.J., C.E. Kim, K.W. Seo, and C.D. Kim. 2010. HNE-induced 5-LO expression is regulated by NF-{kappa}B/ERK and Sp1/p38 MAPK pathways via EGF receptor in murine macrophages. Cardiovascular Research 88: 352–359.
Marletta, M.A. 1993. Nitric oxide synthase structure and mechanism. Journal of Biological Chemistry 268: 12231–12234.
Mercurio, F., and A.M. Manning. 1999. NF-kappaB as a primary regulator of the stress response. Oncogene 18: 6163–6171.
Nathan, C. 1992. Nitric oxide as a secretory product of mammalian cells. FASEB Journal 6: 3051–3064.
Nathan, C. 1997. Inducible nitric oxide synthase: what difference does it make? Journal of Clinical Investment 100: 2417–2423.
Ono, K., and J. Han. 2000. The p38 signal transduction pathway: activation and function. Cellular Signalling 12: 1–13.
Pelletier, J.P., J.C. Fernandes, D.V. Jovanovic, P. Reboul, and J. Martel-Pelletier. 2001. Chondrocyte death in experimental osteoarthritis is mediated by MEK 1/2 and p38 pathways: role of cyclooxygenase-2 and inducible nitric oxide synthase. Journal of Rheumatology 28: 2509–2519.
Pryor, W.A., and G.L. Squadrito. 1995. The chemistry of peroxynitrite: a product from the reaction of nitric oxide with superoxide. American Journal of Physiology 268: L699–L722.
Rahman, I. 2002. Oxidative stress and gene transcription in asthma and chronic obstructive pulmonary disease: antioxidant therapeutic targets. Current Drug Targets: Inflammation and Allergy 1: 291–315.
Rubbo, H., R. Radi, M. Trujillo, R. Telleri, B. Kalyanaraman, S. Barnes, M. Kirk, and B.A. Freeman. 1994. Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. Formation of novel nitrogen-containing oxidized lipid derivatives. Journal of Biological Chemistry 269: 26066–26075.
Santangelo, F. 2003. Intracellular thiol concentration modulating inflammatory response: influence on the regulation of cell functions through cysteine prodrug approach. Current Medicinal Chemistry 10: 2599–2610.
Schulze-Osthoff, K., D. Ferrari, K. Riehemann, and S. Wesselborg. 1997. Regulation of NF-kappa B activation by MAP kinase cascades. Immunobiology 198: 35–49.
Seo, J.Y., H. Kim, and K.H. Kim. 2002. Transcriptional regulation by thiol compounds in Helicobacter pylori-induced interleukin-8 production in human gastric epithelial cells. Annals of the New York Academy of Sciences 973: 541–545.
Sheng, H., J. Shao, J.D. Morrow, R.D. Beauchamp, and R.N. Dubois. 1998. Modulation of apoptosis and Bcl-2 expression by prostaglandin E2 in human colon cancer cells. Cancer Research 58: 362–366.
Stolina, M., S. Sharma, Y. Lin, M. Dohadwala, B. Gardner, J. Luo, L. Zhu, M. Kronenberg, P.W. Miller, J. Portanova, J.C. Lee, and S.M. Dubinett. 2000. Specific inhibition of cyclooxygenase 2 restores antitumor reactivity by altering the balance of IL-10 and IL-12 synthesis. Journal of Immunology 164: 361–370.
Tauskela, J.S., K. Hewitt, L.P. Kang, T. Comas, T. Gendron, A. Hakim, M. Hogan, J. Durkin, and P. Morley. 2000. Evaluation of glutathione-sensitive fluorescent dyes in cortical culture. Glia 30: 329–341.
Topper, J.N., J. Cai, D. Falb, and M.A. Gimbrone Jr. 1996. Identification of vascular endothelial genes differentially responsive to fluid mechanical stimuli: cyclooxygenase-2, manganese superoxide dismutase, and endothelial cell nitric oxide synthase are selectively up-regulated by steady laminar shear stress. Proceedings of the National Academy of Sciences USA 93: 10417–10422.
Tran, P.O., C.E. Gleason, and R.P. Robertson. 2002. Inhibition of interleukin-1beta-induced COX-2 and EP3 gene expression by sodium salicylate enhances pancreatic islet beta-cell function. Diabetes 51: 1772–1778.
Tsujii, M., S. Kawano, S. Tsuji, H. Sawaoka, M. Hori, and R.N. Dubois. 1998. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell 93: 705–716.
Van Der Woude, C.J., J.H. Kleibeuker, P.L. Jansen, and H. Moshage. 2004. Chronic inflammation, apoptosis and (pre-)malignant lesions in the gastro-intestinal tract. Apoptosis 9: 123–130.
Welch, B.D., N.G. Carlson, H. Shi, L. Myatt, and B.K. Kishore. 2003. P2Y2 receptor-stimulated release of prostaglandin E2 by rat inner medullary collecting duct preparations. American Journal of Physiology Renal Physiology 285: F711–F721.
Wheaton, K., P. Atadja, and K. Riabowol. 1996. Regulation of transcription factor activity during cellular aging. Biochemistry and Cell Biology 74: 523–534.
Xie, W.L., J.G. Chipman, D.L. Robertson, R.L. Erikson, and D.L. Simmons. 1991. Expression of a mitogen-responsive gene encoding prostaglandin synthase is regulated by mRNA splicing. Proceedings of National Academy of Sciences USA 88: 2692–2696.
Xu, X.M., L. Sansores-Garcia, X.M. Chen, N. Matijevic-Aleksic, M. Du, and K.K. Wu. 1999. Suppression of inducible cyclooxygenase 2 gene transcription by aspirin and sodium salicylate. Proceedings of National Academy of Sciences USA 96: 5292–5297.
Acknowledgments
This work was supported by the Bio-Scientific Research Grant funded by Pusan National University (PNU, Bio-Scientific Research Grant, PNU-2008-101-103).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, S., Sung, B., Jang, E.J. et al. Inhibitory action of salicylideneamino-2-thiophenol on NF-κB signaling cascade and cyclooxygenase-2 in HNE-treated endothelial cells. Arch. Pharm. Res. 36, 880–889 (2013). https://doi.org/10.1007/s12272-013-0116-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-013-0116-4