Abstract
Cancer metastasis is represented by migration and invasion of cancer cells. Cancer cells invade into the blood or lymphatic vessels and this leads to the spread of cancer into the organs in distant sites. For cancer cells to migrate, extracellular matrix (ECM) must be degraded. Cantharidin, a compound derived from blister beetles, is known for its anti-cancer effect in several cancer cells. Here we report that cantharidin inhibits migration and invasion of A549 human lung cancer cell. We found that cantharidin inhibits activation of phosphatidylinositol 3-kinase/Akt signaling pathway. This leads to the selective attenuation of one of the gelatinases, matrix metalloproteinase 2, which can degrade components of ECM, and inhibits migration and invasion of A549 human lung cancer cell.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer metastasis is a process connected with various independent events, such as invasion into adjacent cells, migration to the remote sites through the artery system or the lymphatic system, and angiogenesis (Sahai 2007; Nyberg et al. 2008). Degradation of extracellular matrix (ECM) has grasped attention as one of the most important processes of metastasis. Since ECM blocks cell migration biochemically and/or physically, degradation of ECM may have to be preceded for cancer metastasis (Basset et al. 1997; Johnsen et al. 1998). Matrix metalloproteinases (MMPs) destroy ECM components around the cancer cells allowing cancer cells to invade into the blood vessels and this leads to the spread of cancer into the organs in distant sites (Nelson et al. 2000). There are more than 20 MMPs known so far. Among them, MMPs-2 and -9 are gelatinases which play critical role in metastasis (Deryugina and Quigley 2006). Expressions of these two enzymes are regulated independently by different regulatory elements involved. Contrary to the constitutive expression of MMP-2 in most cells, MMP-9 expression is regulated at the transcription level through the controls of transcription factors such as AP-1, NF-κB and Sp1 (Van den Steen et al. 2002; Sato and Seiki 1993).
Mitogen-activated protein kinases superfamily members (MAPKs) are also well known to be closely related with cell migration and invasion. Among the MAPKs, three MAPKs are known to be the major mammalian MAPKs; extracellular signal regulated kinases 1 and 2 (ERK1/2), c-Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK), and p38 MAPK (Huang et al. 2004). ERK1/2, JNK/SAPK and p38 MAPK are known to play important roles in the regulation of activities and expressions of MMPs (Westermarck and Kähäri 1999).
The phosphatidylinositol 3-kinase (PI3K)/Akt also plays critical role in the cancer development (Carnero et al. 2008). PI3K/Akt signal pathway has been known for its involvement in the regulation of transformation, proliferation and metastasis of various cancer cells including lung cancer (Vivanco and Sawyers 2002). PI3K/Akt pathway has also been known to be involved in the regulation of MMPs and thus, affects metastasis of cancer cells (Bae et al. 2006).
Cantharidin (7-oxabicyclo[2.2.1] heptanes-2,3-dicarboxylic acid derivative) is a terpenoid, derived from Chinese blister (Fig. 1a). It has been reported that cantharidin selectively inhibits protein phosphatase 2A, which is known to be important for cell cycle control and apoptosis (Li et al. 2010). For its possible implications in the oncogenesis, cantharidin has been investigated as a strong candidate for potential anti-cancer agent.
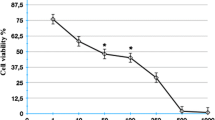
Effect of cantharidin on the viability of A549 lung cancer cell. a Chemical structure of cantharidin. b Viability of A549 cells treated with various dosages of cantharidin (0, 1, 3, 10, 30 and 100 μM) for 24 h. A549 cells were viable with treatment of cantharidin up to 30 μM. Cells were incubated with various concentrations of cantharidin for 24 h as described in “Materials and methods” section
Earlier report indicated that norcantharidin, a derivative of cantharidin, inhibits MMP-9 thus suppresses the metastasis of colorectal cancer cell. Norcantharidin not only inhibited gelatinase activity, but down-regulated the expression of MMP-9 (Chen et al. 2009). In this study, we investigated cantharidin as a strong candidate for the anti-metastatic agent for lung cancer. Cantharidin suppresses the activation of Akt in the A549 lung cancer cell and this resulted in the inhibition of activity of only MMP-2 but not MMP-9. Since degradation of ECM is critical step for the migration and invasion of cancer, metastasis of A549 lung cancer cell may be blocked due to the inhibitory effect of cantharidin on MMP-2 activity. Here we report that cantharidin selectively inhibits the activity of MMP-2, thus blocks migration and invasion of A549 lung cancer cell.
Materials and methods
Chemicals and reagents
Cantharidin was obtained from the Korea Research Institute of Chemical Technology (Daejeon, Korea). Dulbecco’s Modification of Eagle’s Medium (DMEM) with 10 % fetal bovine serum, penicillin–streptomycin and trypsin–EDTA were purchased from Cellgro (Manassas, VA, USA). Antibodies against Akt and p-Akt were purchased from Cell Signaling Technology (Danvers, MA, USA). Antibodies against ERK, p-ERK, JNK, p-JNK, p38, p-p38, PI3K p85α, p-PI3K p85α were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-rabbit IgG-HRP, anti-mouse IgG-HRP and -goat IgG-HRP were from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Cell culture
The human lung cancer A549 cells were provided from the Korea Research Institute of Chemical Technology (Daejeon, Korea). A549 cells were cultured on 100 mm culture dishes with DMEM (Cellgro). The cell medium was adjusted to contain 10 % fetal bovine serum (Cellgro), and 1 % penicillin–streptomycin and grown at 37 °C under a humidified 5 % CO2 atmosphere.
Cell viability assay
Cells were plated at a density of 104 cells per well into 96-well plates in DMEM with 10 % fetal bovine serum. After 24 h of incubation, cells were exposed to different concentrations of cantharidin (0, 1, 3, 10, 30, 100 μM) in 5 % CO2 incubator at 37 °C for 24 h. Then, the viable cell numbers were counted spectrophotometrically using a Cell Counting Kit-8 system (Dojindo Technologies, Kumamoto, Japan) at 450 nm.
Wound-healing assay
Cells were plated in 96-well culture plate and incubated in 5 % CO2 at 37 °C for 24 h. Single layer confluent cells were wounded by scraping using 200P micropipette tip, then washed by DPBS and incubated in DMEM containing 10 % FBS with various concentrations (0, 1, 3, 10 μM) of cantharidin for 16–20 h.
Boyden chamber assay
The invasiveness of A549 cell was tested by the Boyden chamber invasion assay (Attiga et al. 2000). A549 cells were seeded to the upper part of Boyden chamber at a density of 106 cells/ml in 50 μl of 10 % FBS DMEM and 10 % FBS DMEMs treated with various concentrations of cantharidin at various concentrations (0, 1, 3, 10, 30 μM) were loaded at the bottom part of Boyden chamber. A gelatin-coated polycarbonate membrane with 8-μm pore size was placed in between the upper and the bottom parts. After 6 h incubation, the cells that had invaded to the lower surface of the membrane were stained with Diff-Quik stain solution. The numbers of invaded cells were counted in randomly selected fields.
Gelatin zymography
Gelatin zymography was performed to determine the effect of cantharidin on the activity of MMPs-2 and -9 (Chu et al. 2004). A549 cells were treated with various concentrations of cantharidin (0, 1, 3, 10, 30 μM) in 0.1 % FBS DMEM for 24 h, and the supernatants were collected to prepare samples with loading buffer. Proteins were subjected to 8 % SDS-PAGE that had been cast in the presence of 0.15 % gelatin. After electrophoresis, the gels were washed with 2.5 % Triton X-100 solution, and then gels were incubated for 18 h in 50 mM Tris–HCl buffer containing 5 mM CaCl2 at 37 °C. Then, the gels were stained with Coomassie Brilliant Blue R, followed by destaining with 10 % acetic acid and 20 % methanol. The gelatinolytic activities were detected as clear bands against the blue background.
Western blot analysis
A549 cells were treated with 0, 1, 3, 10 μM cantharidin for 24 h. Then the cells were harvested and lysed with RIPA buffer containing 0.1 % Triton X-100 (Sigma), 0.01 % SDS, 5 mM Tris–HCl pH 7.4, 0.1 mM EDTA, 15 mM NaCl, 0.05 % sodium deoxycholate, Complete Mini Protease Inhibitor Cocktail Tablets (Roche), Halt Phosphatase Inhibitor Cocktail (Thermo) and protein extraction reagent. The cell lysates were denatured in a sample buffer and separated in 12 % Tris–glycine–SDS–polyacrylamide gels and transferred to nitrocellulose membranes (Millipore, Bedford, MA, USA). After blocking with skim milk, membranes were incubated with appropriate primary antibodies, secondary antibodies and detected with ECL reagent. For control experiment, anti-β-tubulin antibody (Santa Cruz) was used as a loading control.
Results and discussion
Cytotoxicity of cantharidin on A549 lung cancer cells
Cantharidin has been investigated for its possible roles in the tumorigenesis, especially for cell cycle control and apoptosis (Li et al. 2010). We raised a question if cantharidin also affects migration and invasion of human lung cancer cell, thus possibly inhibits metastasis of human lung cancer. In regard to the possible cytotoxicity of cantharidin to cells reported so far, we tried to investigate the effect of cantharidin on the viability of A549 human lung cancer cells. As indicated in the Fig. 1b, the viability of A549 cells was not critically affected by the treatment of cantharidin up to 30 μM. This may indicate that apoptosis of A549 lung cancer cells may be induced when the concentration of cantharidin reaches to 100 μM concentration.
Cantharidin blocks invasion and migration of A549 cells
To test the effect of cantharidin on migration of human lung cancer cell, we performed wound-healing assay. A549 cells in monolayer were wounded by scraping and ascending dosages of cantharidin were treated to each well. As seen Fig. 2a, cantharidin blocked migration of A549 cells in a dosage dependent manner. The number of cancer cells in the areas scraped on the well is significantly decreased as the dosage of cantharidin treated got increased. This may indicate that cantharidin blocked migration of A549 cells in a dosage dependent manner.
Effects of cantharidin on migration and invasion of A549 lung cancer cell. a Migration of A549 cells treated with various concentrations of cantharidin (0, 1, 3 and 10 μM) for 16–20 h. The number of cells in the scraped area of plates is decreased by cantharidin in a dosage dependent manner. b Invasion of A549 cells treated with various dosages of cantharidin. The number of membrane-associated cells, which represent invading cells, is decreased by cantharidin in a dosage dependent manner (0, 1, 3, 10 and 30 μM)
We employed Boyden chamber invasion assay to test the effect of cantharidin on the invasion of A549 cells. In the upper chamber, equal numbers of A549 cells were seeded and the various dosages (0, 1, 3, 10 and 30 μM) of cantharidin were placed at the bottom chamber. A gelatin-coated polycarbonate membrane with 8-μm pore size was placed in between the upper and the bottom parts, thus invading cells would be associated onto the downside of the membrane. After 6 h, the numbers of invading A549 cells, which are represented by the numbers of membrane-associated cells, were counted under light microscopy. As seen in Fig. 2b, the numbers of invaded cells were reduced by the treatment of cantharidin in a dosage dependent manner, indicating the blockade of invasive ability of A549 cells by cantharidin.
Cantharidin inhibits MMP-2 but not MMP-9
Degradation of ECM must be preceded for the tumor metastasis (Basset et al. 1997; Johnsen et al. 1998). The MMPs are essential enzymes for the degradation of components of ECM (Nelson et al. 2000). Among the various MMPs, which are composed of collagenases, elatases, gelatinases and other enzymes, MMPs-2 and -9 are critically important for the tumor metastasis since they possess gelatinase activity and degrade ECM (Deryugina and Quigley 2006). The expression profiles of the two proteins are different. While MMP-2 is expressed in most cells, the transcription of MMP-9 is tightly regulated by a promoter region of the MMP-9 coding gene. Before, it has been known that norcantharidin, which is a derivative of cantharidin, suppresses the metastasis of colorectal cancer cell by specifically inhibiting MMP-9. Norcantharidin inhibited gelatinase activity of MMP-9. In addition, the expression of MMP-9 is also down-regulated by the transcriptional suppression of MMP-9 coding gene, which is achieved by the inhibition of Sp1 transcription factor activity (Chen et al. 2009).
To investigate whether cantharidin dependent down-regulation of PI3K/Akt affected activities of MMPs, especially gelatinases, thus inhibited migration and invasion of A549 lung cancer cell, we performed gelatin-zymography assay. As seen in the Fig. 3a, b, gelatinase activity of MMP-2 was selectively inhibited by cantharidin in a dosage dependent manner while MMP-9 was not affected. This may indicate the selective attenuation of MMP-2 by cantharidin. The expression levels of MMPs-2 and -9 were not affected by the treatment of various dosages of cantharidin (Fig. 3c). This may indicate that cantharidin only inhibits the gelatinase activity of MMP-2 but not affects the expression of MMP-2.
Selective attenuation of MMP-2 by cantharidin. The effect of cantharidin on activities of MMPs was investigated by gelatin zymography. A549 human lung cancer cells were treated with cantharidin in a dosage dependent manner (0, 1, 3, 10 and 30 μM) and the activities of gelatinases (MMPs-2 and -9) were investigated. a The gelatinase activity of MMP-9 was not affected by cantharidin. b The gelatinase activity of MMP-2 selectively attenuated by cantharidin in a dosage dependent manner. c The expression levels of MMPs-2 and -9 were not affected by the treatment of cantharidin in dosage dependent manner (0, 1, 3 and 10 μM)
Cantharidin affects the PI3K/Akt signal pathway and in A549 cells
MMPs are known to be regulated at multiple steps including transcriptional and/or post-transcriptional levels and the transcriptional regulation has been known to implicate MAPK and/or PI3K/Akt pathways (Westermarck and Kähäri 1999; Bae et al. 2006). Thus, we tested the effect of cantharidin on the expressions and/or the activations of the signaling molecules involved in PI3K/Akt and MAPK signaling. As seen in Fig. 4a, activation of Akt was significantly inhibited by cantharidin. Level of p-Akt, which is an active form of Akt, was decreased as the concentration of cantharidin was increased. This strongly indicates that cantharidin inhibits the PI3K/Akt signaling pathway, and this leads to the suppressed migration and invasion of A549 lung cancer cells. However, cantharidin did not affect the activation of representative MAPKs, ERK, JNK and p38 (Fig. 4b). This may indicate cantharidin dependent inhibition of metastasis of A549 cells are due to the selective down-regulation of PI3K/Akt signaling pathway but not of MAPK signaling pathway.
Effects of cantharidin on PI3K/Akt and MAPK signaling pathways in A549 lung cancer cells. A549 cells were treated with various concentrations (0, 1, 3 and 10 μM) of cantharidin for 24 h, then the cells were harvested and lysed for the analysis of activations of Akt, ERK, JNK and p38, represented by the phosphor-forms of each protein. a The phosphorylation of Akt was down-regulated in a dosage dependent manner of cantharidin indicating inhibition of PI3K/Akt pathway by cantharidin. b The phosphorylations of MAPKs (ERK, JNK and p38) were not affected by cantharidin
Cantharidin has been known for its anti-motility effect of cancer cells. However, it has been reported that cantharidin has toxic side effects such as renal toxicity (Massicot et al. 2005). To minimize the renal toxicity, cantharidin analogs were synthesized and one of them showed to have relatively less toxic effect in vitro while keeping comparable activity to cantharidin (Kok et al. 2006). Also, it was recently reported that the application of semi-synthetic derivative of cantharidin in combination with chemotherapy could reduce side effects of chemotherapy (Zhan et al. 2012). These may indicate that cantharidin deserves to be investigated further.
Here, we report the alternative mechanism of anti-metastatic effect of cantharidin in lung cancer cell. While anti-metastatic effect of norcantharidin in colorectal cancer cell is based on the selective down-regulation of MMP-9, cantharidin selectively attenuates the gelatinase activity of MMP-2 in lung cancer cell by suppressing of PI3K/Akt signaling pathway. This may implicates that cantharidin inhibits metastasis of various cancer cells by affecting different molecules involved in migration and invasion of cancer cells.
References
Attiga, F.A., P.M. Fernandez, A.T. Weeraratna, M.J. Manyak, and S.R. Patierno. 2000. Inhibitors of prostaglandin synthesis inhibit human prostate tumor cell invasiveness and reduce the release of matrix metalloproteinases. Cancer Research 60: 4629–4637.
Bae, I.H., M.J. Park, S.H. Yoon, S.W. Kang, S.S. Lee, K.M. Choi, and H.D. Um. 2006. Bcl-w promotes gastric cancer cell invasion by inducing matrix metalloproteinase-2 expression via phosphoinositide 3-kinase, Akt, and Sp1. Cancer Research 66: 4991–4995.
Basset, P., A. Okada, M.P. Chenard, R. Kannan, I. Stoll, P. Anglard, J.P. Bellocq, and M.C. Rio. 1997. Matrix metalloproteinases as stromal effectors of human carcinoma progression: Therapeutic implications. Matrix Biology 15: 535–541.
Carnero, A., C. Blanco-Aparicio, O. Renner, W. Link, and J.F. Leal. 2008. The PTEN/PI3K/AKT signalling pathway in cancer, therapeutic implications. Current Cancer Drug Targets 8: 187–198.
Chen, Y.J., W.M. Chang, Y.W. Liu, C.Y. Lee, Y.H. Jang, C.D. Kuo, and H.F. Liao. 2009. A small-molecule metastasis inhibitor, norcantharidin, downregulates matrix metalloproteinase-9 expression by inhibiting Sp1 transcriptional activity in colorectal cancer cells. Chemico-Biological Interactions 181: 440–446.
Chu, S.C., D.N. Hu, S.F. Yang, P.Y. Yang, Y.S. Hsieh, S.M. Huang, G. Yu, and S.A. McCormick. 2004. Uveal melanocytes produce matrix metalloproteinases-2 and -9 in vitro. Pigment Cell Research 17: 636–642.
Deryugina, E.I., and J.P. Quigley. 2006. Matrix metalloproteinases and tumor metastasis. Cancer and Metastasis Reviews 25: 9–34.
Huang, C., K. Jacobson, and M.D. Schaller. 2004. MAP kinases and cell migration. Journal of Cell Science 117: 4619–4628.
Johnsen, M., L.R. Lund, J. Rømer, K. Almholt, and K. Danø. 1998. Cancer invasion and tissue remodeling: Common themes in proteolytic matrix degradation. Current Opinion in Cell Biology 10: 667–671.
Kok, S.H., C.H. Chui, W.S. Lam, J. Chen, F.Y. Lau, G.Y. Cheng, R.S. Wong, P.P. Lai, T.W. Leung, J.C. Tang, and A.S. Chan. 2006. Apoptotic activity of a novel synthetic cantharidin analogue on hepatoma cell lines. International Journal of Molecular Medicine 17: 945–949.
Li, W., L. Xie, Z. Chen, Y. Zhu, Y. Sun, Y. Miao, Z. Xu, and X. Han. 2010. Cantharidin, a potent and selective PP2A inhibitor, induces an oxidative stress-independent growth inhibition of pancreatic cancer cells through G2/M cell-cycle arrest and apoptosis. Cancer Science 101: 1226–1233.
Massicot, F., H. Dutertre-Catella, C. Pham-Huy, X.H. Liu, H.T. Duc, and J.M. Warnet. 2005. In vitro assessment of renal toxicity and inflammatory events of two protein phosphatase inhibitors cantharidin and norcantharidin. Basic Clinical Pharmacology and Toxicology 96: 26–32.
Nelson, A.R., B. Fingleton, M.L. Rothenberg, and L.M. Matrisian. 2000. Matrix metalloproteinases: Biologic activity and clinical implications. Journal of Clinical Oncology 18: 1135–1149.
Nyberg, P., T. Salo, and R. Kalluri. 2008. Tumor microenvironment and angiogenesis. Frontiers in Bioscience 13: 6537–6553.
Sahai, E. 2007. Illuminating the metastatic process. Nature Reviews Cancer 7: 737–749.
Sato, H., and M. Seiki. 1993. Regulatory mechanism of 92 kDa type IV collagenase gene expression which is associated with invasiveness of tumor cells. Oncogene 8: 395–405.
Van den Steen, P.E., B. Dubois, I. Nelissen, P.M. Rudd, R.A. Dwek, and G. Opdenakker. 2002. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9). Critical Reviews in Biochemistry and Molecular Biology 37: 375–536.
Vivanco, I., and C.L. Sawyers. 2002. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nature Reviews Cancer 2: 489–501.
Westermarck, J., and V.M. Kähäri. 1999. Regulation of matrix metalloproteinase expression in tumor invasion. FASEB Journal 13: 781–792.
Zhan, Y.P., X.E. Huang, J. Cao, Y.Y. Lu, X.Y. Wu, J. Liu, X. Xu, L. Xu, J. Xiang, and L.H. Ye. 2012. Clinical study on safety and efficacy of quinin® (cantharidin sodium) injection combined with chemotherapy in treating patients with gastric cancer. Asian Pacific Journal of Cancer Prevention 13: 4773–4776.
Acknowledgments
We thank Young Joo Lee for her technical supports. This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology of Korea (2010-0023292).
Author information
Authors and Affiliations
Corresponding author
Additional information
Young Min Kim, Min Jeong Ku have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kim, Y.M., Ku, M.J., Son, YJ. et al. Anti-metastatic effect of cantharidin in A549 human lung cancer cells. Arch. Pharm. Res. 36, 479–484 (2013). https://doi.org/10.1007/s12272-013-0044-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-013-0044-3