Abstract
Caloric restriction prolongs the lifespan of many species. Therefore, investigators have researched the usefulness of caloric restriction for healthy lifespan extension. Sirt1, an NAD+-dependent deacetylase, was identified as a molecule necessary for caloric restriction-related anti-aging strategies. Sirt1 functions as an intracellular energy sensor to detect the concentration of NAD+, and controls in vivo metabolic changes under caloric restriction and starvation through its deacetylase activity to many targets including histones, nuclear transcriptional factors, and enzymes. During the past decade, investigators have reported the relationship between disturbance of Sirt1 activation and the onset of aging- and obesity-associated diseases such as diabetes, cardiovascular disease and neurodegenerative disorders. Consequently, a calorie restriction-mimetic action of Sirt1 is now expected as a new therapy for these diseases. In addition, recent studies have gradually clarified the role of Sirt1 in the onset of kidney disease. Its activation may also become a new target of treatment in the patients with chronic kidney disease including diabetic nephropathy. In this article, we would like to review the role of Sirt1 in the onset of kidney disease based on previous studies, and discuss its possibility as the target of treatment in diabetic nephropathy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
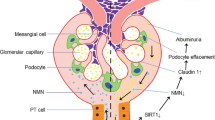
Previous studies on the pathogenesis of diabetic nephropathy have indicated that hyperglycemia-related alterations of intracellular metabolism (Brownlee 2005; Koya and King 1998) and glomerular hypertension with the hyper-activation of the renin-angiotensin system (RAS) influence the deterioration of diabetic nephropathy (Fig. 1). These findings are supported by the results of many large-scale clinical studies: close blood glucose control and the use of RAS inhibitors improve the renal prognosis in the patients with diabetic nephropathy (DCCT 1993; Brenner et al. 2001; UKPDS 1998; Ohkubo et al. 1995). However, the efficacy of RAS inhibitors is not realized in all patients, and there are still a large number of patients in whom blood glucose control is not favorable; therefore, the number of patients with the deterioration of diabetic nephropathy to end-stage of renal disease will increase in the future. As future research issues in diabetic nephropathy, a more potent correction of intracellular metabolic errors, or exploration of a new therapeutic target to inhibit the RAS-independent progression of diabetic nephropathy is urgently required.
Proposed pathogenesis of diabetic nephropathy. Both hyperglycemia-mediated intracellular metabolic alterations, renin-angiotensin II system-dependent hemodynamic changes and renal hypoxia coordinately contribute to the development of diabetic nephropathy. NAD-dependent Sirt1 deacetylase may improve diabetic nephropathy through the amelioration of these metabolic and hemodynamic pathological changes
In 1934, McCay et al. successfully prolonged the lifespan of rats with caloric restriction, and first reported the advantage of caloric restriction for healthy lifespan extension (McCay and Crowell 1934). Subsequently, researches on the relationship between caloric restriction and lifespan prolongation/anti-aging were aggressively promoted. In 2009, its effects were also demonstrated in rhesus monkeys, higher mammals (Colman et al. 2009; Fontana et al. 2010). More than 10 years ago, a molecular mechanism involved in the lifespan-prolonging effects of caloric restriction was clarified, and silent information regulator 2 (a mammalian homolog: Sirt1) was newly identified as a caloric restriction-related anti-aging molecule (Lin et al. 2000). Initially, Sirt1 was identified as an NAD+-dependent histone deacetylase. However, a subsequent study regarding Sirt1 conducted over the decade showed that Sirt1 controls fasted organ-energy metabolism and stress responses by regulating the acetylation of many intra-nuclear transcription factors in an NAD+-concentration-dependent manner (Liang et al. 2009) (Fig. 2). In addition, several studies reported the effects of caloric restriction on renal lesions in animal aging-related renal lesion/glomerular kidney disease models (Cherry 1998; McKiernan et al. 2007). Recently, the involvement of Sirt1 in a caloric restriction-related renoprotective mechanism has been particularly emphasized. Basic research on its involvement in the pathogenesis or its possibility as the target of treatment is being promoted.
Sirt1 Expression in the Kidney
The intra-renal localization of Sirt1 expression in the kidney has not yet been clarified although Sirt1 is ubiquitously expressed in all tissues. A study indicated that Sirt1was strongly expressed in the medullary tubular cells, and it was also confirmed in the cortical proximal tubular cells (He et al. 2010). In another study with kidney tissue, Sirt1 protein expression in an isolated glomerulus, that is, in cultured mesangial, epithelial, and endothelial cells, was confirmed, although its detailed expression pattern in glomerulus was not clarified (Kume et al. 2006). Other studies regarding changes in Sirt1 expression reported that 40 % caloric restriction in rats increased Sirt1 expression in the kidney (Cohen et al. 2004), and Sirt1 expression decreased in the kidneys from streptozotocin-induced diabetic rats and 24-month-old mice (Tikoo et al. 2008; Kume et al. 2010). Deacetylation activity of Sirt1 declined with age in mice and Sirt1 expression decreased in the kidney of type 2 diabetic rats, and these changes were ameliorated by calorie restriction (Kitada et al. 2011; Kume et al. 2010). According to a study (He et al. 2010), Sirt1 expression was enhanced in acute kidney injury model, represented by a unilateral ureteral obstruction (UUO). Sirt1 expression not only in the kidney but also in multiple organs is altered in response to changes in the metabolic state or stress conditions. As a humoral factor influencing such changes in Sirt1 expression, insulin/IGF-1 (Cohen et al. 2004), nitric oxide (NO) (Nisoli et al. 2005), and aldosterone (Fan et al. 2011) have been reported. However, the presence of an evident humoral factor involved in changes in Sirt1 expression and a molecule-regulating mechanism remain to be clarified.
Involvement of Sirt1 in the Pathogenesis of Kidney Disease Models
Sirt1 expression in the kidney depends on each condition. Based on previous studies, we review how this change contributes to the pathogenesis of kidney disease as follows (Table 1):
Role of Sirt1 in Aging-related Renal Lesions
The kidney is one of organs showing aging-related dysfunction, and aging is recognized as a risk factor for the deterioration of diabetic nephropathy. Therefore, the pathogenesis of aging-related renal disorder, a common background factor of patients with diabetic nephropathy, should be clarified. We have previously reported that Sirt1 activity was significantly reduced in the kidneys of 24-month-old wild-type mice, and that its reduction led to mitochondrial dysfunction and enhancement of oxidative stress in the proximal tubular cells, resulting in aging-related renal lesions. On the other hand, it was confirmed that Sirt1 activity was maintained by 40 % caloric restriction for 12 months, starting from 12 months of age, reducing aging-related mitochondrial abnormalities and subsequent renal lesions (Kume et al. 2010). In addition, in the kidneys from Sirt1 hetero-knockout mice (Sirt1+/− mice), the deterioration of aging-related renal lesions was observed in the early phase (12 months of age), as demonstrated in the kidneys from 24-month-old wild-type mice. Interestingly, phenotype of premature renal aging in Sirt1+/− mice was not ameliorated despite long-term caloric restriction. These results suggest that Sirt1 expression and activity reduction are involved in aging of the renal tubular cells, and that restoration of Sirt1 activity is essential for the anti-renal-aging effects of caloric restriction (Kume et al. 2010).
Role of Sirt1 for Renal Hypoxia
The hypoxic state of the renal tubular cells is closely involved in the pathogenesis of diabetic nephropathy (Mimura and Nangaku 2010). Our study with aged mice also showed that the hypoxic state of the renal tubular cells played an important role in the pathogenesis of renal aging. In the cells, a mechanism to induce autophagy against hypoxic stimulation and maintain intracellular homeostasis, including the removal of abnormal mitochondria, is present. Proximal tubular cells also have this mechanism to maintain their homeostasis against hypoxia. However, in kidneys of aged mice, autophagy activity under hypoxia is significantly altered, causing the accumulation of abnormal mitochondria, an increase in oxidative stress, and enhancement of apoptosis (Kume et al. 2010). The activation of a Sirt1-mediated transcription factor, Foxo3, is essential for such hypoxia-induced autophagy control in proximal tubular cells (Fig. 2). Therefore, aging-mediated Sirt1 inactivation leads to inhibition of autophagy activity against hypoxia. Thus, Sirt1 may play a role as a stress response factor under a hypoxic state in the topical area of the kidney (Kume et al. 2010). Currently, the response of autophagy to renal hypoxia observed in a diabetic nephropathy model is unclear. In the future, it may be important to clarify the role of autophagy in diabetic nephropathy, involvement of Sirt1 as an autophagy-controlling mechanism, and association with mitochondrial abnormalities, for developing the target of treatment for proximal tubular cells protection.
The kidney controls systemic oxygen metabolism by producing erythropoietin and regulating the erythrocyte count in response to systemic hypoxic stimulation. A recent study reported that Sirt1 activated a transcription factor involved in erythropoietin production, Hif2α, through its deacetylation, enhancing erythropoietin production in a hypoxic state (Dioum et al. 2009). Collectively, Sirt1 may be responsible for topical renal and systemic oxygen metabolism control.
Role of Sirt1 in Renal Interstitial Fibrosis/tubular Cell Apoptosis
Renal fibrosis is a final histological abnormality in the various kidney diseases including diabetic nephropathy. Furthermore, the importance of tubular cell apoptosis in the deterioration of diabetic nephropathy has also been reported. Recently, the role of Sirt1 in renal fibrosis and apoptosis has been clarified. A mouse UUO model is experimentally used as a renal fibrosis model. Interestingly, UUO in Sirt1+/− mice showed the significant deterioration of fibrosis/apoptosis in comparison with wild-type mice (He et al. 2010). As the molecular mechanism, it was important that Sirt1 positively regulates cyclooxygenase 2 (COX2) expression and subsequent prostaglandin E2 production, leading to renoprotection against oxidative stress (He et al. 2010).
Therapeutic Potency of Sirt1 Activation in Kidney Disease Models
Anti-fibrotic Effect of Sirt1
Several studies reported that Sirt1 activators, resveratrol or SRT1720, reduced UUO-related renal tubular lesions by inhibiting the TGFβ-Smad3 pathway and enhancing COX2 expression (Li et al. 2010; He et al. 2010). In addition, another study indicated that cisplatin-induced renal tubular damage was ameliorated in mice with over-expression of proximal tubule-specific Sirt1 through the protection of the peroxisome function (Hasegawa et al. 2010). The results of a study with cultured proximal tubular cells also showed that Sirt1 induced the expression of an anti-oxidant enzyme, catalase, through the activation of a transcription factor, Foxo3a, inhibiting apoptosis in an oxidative stress (Hasegawa et al. 2008). Recent study has shown that catalase-deficiency in mice significantly exacerbated the development of diabetic nephropathy, suggesting that peroxisomal oxidative stress is strongly associated with the pathogenesis and a therapeutic target in diabetic nephropathy (Hwang et al. 2012). Thus, Sirt1 activation may be applied for new treatment to protect the proximal tubular cells through an anti-oxidant effect via protecting peroxisome as well as a direct anti-fibrotic action.
Anti-inflammatory Effect of Sirt1
As a mechanism involved in the onset of diabetic nephropathy or the target of treatment, microinflammation has recently been emphasized (Shikata 2007). Sirt1 exhibits anti-inflammatory actions in many organs (Yoshizaki et al. 2010). This suggests the involvement of Sirt1 in a caloric restriction-related anti-inflammatory molecular mechanism, as previously reported. As a molecular mechanism involved in the anti-inflammatory actions of Sirt1, this molecule was shown to inhibit the activation of a common pathway of inflammatory signals, NFκB, through deacetylation of an NFκB subunit (p65) (Yeung et al. 2004; Yoshizaki et al. 2010). If this phenomenon is observed in the kidney, especially in the presence of diabetic nephropathy, Sirt1 activation targeting anti-inflammatory actions may become interesting. Actually, with significant reduction of Sirt1 expression, acetylation (activation) of p65 was marked in the kidneys from Wistar fatty rats with type 2 diabetes, showing severe renal lesions with inflammatory cell infiltration (Kitada et al. 2011). Interestingly, caloric restriction in the same rats induced p65 deacetylation, leading to anti-inflammatory actions (Kitada et al. 2011). This finding suggests that the reactivation of Sirt1 is involved in the caloric restriction-mediated anti-inflammatory actions on diabetic nephropathy.
Other Roles of Sirt1 in the Kidney
Role of Sirt1 in Blood Pressure Control
There is a close relationship between energy balance and blood pressure (Uzu et al. 2006). Some studies indicated the influence of Sirt1 on blood pressure control. Sirt1 decreases the number of angiotensin II type 1 receptors in the vascular smooth muscle cells, inhibiting an angiotensin II elevation-related increase in the blood pressure (Miyazaki et al. 2008). Furthermore, Sirt1 increases NO production in the vascular endothelium through endothelial NO synthase (eNOS) activation mediated by deacetylation (Mattagajasingh et al. 2007). In addition, Sirt1 inhibits the expression of the α-subunit of a sodium channel ENaC existing in the collecting tubule cells in the renal medullary region, resulted in suppressing sodium reabsorption in the kidney (Zhang et al. 2009). Thus, Sirt1 may control systemic vascular functions, and influence salt-sensitive hypertension in obese patients with type 2 diabetics by controlling sodium reabsorption in the kidney.
Role of Sirt1 in the Glomerulus
Previously, a few studies with cultured mesangial cells and podocytes reported the role of Sirt1 in the glomerular cells. In cultured mesangial cells, Sirt1 inhibits oxidative stress-related apoptosis through p53 deacetylation (Kume et al. 2006). Furthermore, it suppresses TGFβ-related apoptosis through smad7 deacetylation (Kume et al. 2007). A recent study reported that exposure to high glucose decreased the mesangial cell concentration of NAD+, causing cellular hypertrophy through the reduction of Sirt1 activity (Zhuo et al. 2011). In the cultured podocytes, Sirt1 protects cells from advanced glycated end-products (AGE)-mediated cell death (Chuang et al. 2011). The results suggest that Sirt1 may be involved in the regulation of apoptosis or hypertrophy of the mesangial cells and podocytes in the formation of initial lesions diabetic nephropathy. Its inhibition may contribute to the subsequent prevention of sclerotic lesions. In the future, the role of Sirt1 in the glomerulus must be further examined.
Possibility of Sirt1 as a Nephropathy-associated Gene
Interestingly, gene polymorphism study also indicated that Sirt1 may play a role in susceptibility to diabetic nephropathy in Japanese subjects with type 2 diabetes (Maeda et al. 2011). This study revealed 4 SNPs in Sirt1 that were nominally associated with diabetic nephropathy, and subsequent haplotype analysis revealed that a haplotype consisting of the 11 SNPs within Sirt1 locus had a stronger association with diabetic nephropathy. It would be interesting to clarify the gene polymorphism-related morbid molecular mechanism in diabetic nephropathy.
Conclusion
Investigators in the field of diabetes have focused on Sirt1 research based on the features of Sirt1 which is activated through caloric restriction. As a result, studies on some Sirt1 activators, resveratrol or small molecule activator, have reached the phase of clinical application in type 2 diabetics and other ageing-related diseases. Considering the previously reported role of Sirt1 in kidney disease, Sirt1 may become the therapeutic target of diabetic nephropathy. However, currently, there is little direct evidence regarding nephropathy. Many issues remain to be reviewed: Sirt1 activity/expression in the human kidney tissue, examination with cell-specific-gene-modified mice, and the development of Sirt1 activators. However, we hope that this article will help Sirt1 research development in diabetic nephropathy. In the future, Sirt1 activators will be applied in clinical practice, contributing to improvement in the prognosis of patients with diabetic nephropathy.
References
Brenner, B.M., M.E. Cooper, D. De Zeeuw, W.F. Keane, W.E. Mitch, H.H. Parving, G. Remuzzi, S.M. Snapinn, Z. Zhang, and S. Shahinfar. 2001. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. New England Journal of Medicine 345: 861–869.
Brownlee, M. 2005. The pathobiology of diabetic complications: a unifying mechanism. Diabetes 54: 1615–1625.
Cherry, Engelman. 1998. R. W., Wang, B. Y., Kinjoh, K., El-Badri, N. S., and Good, R. A. Calorie restriction delays the crescentic glomerulonephritis of SCG/Kj mice. Proceedings of the Society for Experimental Biology and Medicine 218: 218–222.
Chuang, P.Y., Y. Dai, R. Liu, H. He, M. Kretzler, B. Jim, C.D. Cohen, and J.C. He. 2011. Alteration of forkhead box O (foxo4) acetylation mediates apoptosis of podocytes in diabetes mellitus. PLoS One 6: e23566.
Cohen, H.Y., C. Miller, K.J. Bitterman, N.R. Wall, B. Hekking, B. Kessler, K.T. Howitz, M. Gorospe, R. De Cabo, and D.A. Sinclair. 2004. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science 305: 390–392.
Colman, R.J., R.M. Anderson, S.C. Johnson, E.K. Kastman, K.J. Kosmatka, T.M. Beasley, D.B. Allison, C. Cruzen, H.A. Simmons, J.W. Kemnitz, and R. Weindruch. 2009. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 325: 201–204.
DCCT Research Group. 1993. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. The New England Journal of Medicine 329: 977–986.
Dioum, E.M., R. Chen, M.S. Alexander, Q. Zhang, R.T. Hogg, R.D. Gerard, and J.A. Garcia. 2009. Regulation of hypoxia-inducible factor 2alpha signaling by the stress-responsive deacetylase sirtuin 1. Science 324: 1289–1293.
Fan, Y.Y., M. Kohno, H. Hitomi, K. Kitada, Y. Fujisawa, J. Yatabe, M. Yatabe, R.A. Felder, H. Ohsaki, K. Rafiq, S.J. Sherajee, T. Noma, A. Nishiyama, and D. Nakano. 2011. Aldosterone/Mineralocorticoid receptor stimulation induces cellular senescence in the kidney. Endocrinology 152: 680–688.
Fontana, L., L. Partridge, and V.D. Longo. 2010. Extending healthy life span–from yeast to humans. Science 328: 321–326.
Hasegawa, K., S. Wakino, K. Yoshioka, S. Tatematsu, Y. Hara, H. Minakuchi, K. Sueyasu, N. Washida, H. Tokuyama, M. Tzukerman, K. Skorecki, K. Hayashi, and H. Itoh. 2010. Kidney-specific overexpression of Sirt1 protects against acute kidney injury by retaining peroxisome function. Journal of Biological Chemistry 285: 13045–13056.
Hasegawa, K., S. Wakino, K. Yoshioka, S. Tatematsu, Y. Hara, H. Minakuchi, N. Washida, H. Tokuyama, K. Hayashi, and H. Itoh. 2008. Sirt1 protects against oxidative stress-induced renal tubular cell apoptosis by the bidirectional regulation of catalase expression. Biochemical and Biophysical Research Communications 372: 51–56.
He, W., Y. Wang, M.Z. Zhang, L. You, L.S. Davis, H. Fan, H.C. Yang, A.B. Fogo, R. Zent, R.C. Harris, M.D. Breyer, and C.M. Hao. 2010. Sirt1 activation protects the mouse renal medulla from oxidative injury. The Journal of Clinical Investigation 120: 1056–1068.
Hwang, I., J. Lee, J.Y. Huh, J. Park, H.B. Lee, Y.S. Ho, and H. Ha. 2012. Catalase deficiency accelerates diabetic renal injury through peroxisomal dysfunction. Diabetes 61: 728–738.
Kitada, M., A. Takeda, T. Nagai, H. Ito, K. Kanasaki, and D. Koya. 2011. Dietary restriction ameliorates diabetic nephropathy through anti-inflammatory effects and regulation of the autophagy via restoration of Sirt1 in diabetic Wistar fatty (fa/fa) rats: a model of type 2 diabetes. Experimental Diabetes Research 2011: 908185.
Koya, D., and G.L. King. 1998. Protein kinase C activation and the development of diabetic complications. Diabetes 47: 859–866.
Kume, S., M. Haneda, K. Kanasaki, T. Sugimoto, S. Araki, M. Isono, K. Isshiki, T. Uzu, A. Kashiwagi, and D. Koya. 2006. Silent information regulator 2 (SIRT1) attenuates oxidative stress-induced mesangial cell apoptosis via p53 deacetylation. Free Radical Biology and Medicine 40: 2175–2182.
Kume, S., M. Haneda, K. Kanasaki, T. Sugimoto, S. Araki, K. Isshiki, M. Isono, T. Uzu, L. Guarente, A. Kashiwagi, and D. Koya. 2007. SIRT1 inhibits transforming growth factor beta-induced apoptosis in glomerular mesangial cells via Smad7 deacetylation. Journal of Biological Chemistry 282: 151–158.
Kume, S., T. Uzu, K. Horiike, M. Chin-Kanasaki, K. Isshiki, S. Araki, T. Sugimoto, M. Haneda, A. Kashiwagi, and D. Koya. 2010. Calorie restriction enhances cell adaptation to hypoxia through Sirt1-dependent mitochondrial autophagy in mouse aged kidney. The Journal of Clinical Investigation 120: 1043–1055.
Li, J., X. Qu, S.D. Ricardo, J.F. Bertram, and D.J. Nikolic-Paterson. 2010. Resveratrol inhibits renal fibrosis in the obstructed kidney: potential role in deacetylation of Smad3. American Journal of Pathology 177: 1065–1071.
Liang, F., S. Kume, and D. Koya. 2009. SIRT1 and insulin resistance. Nature Reviews Endocrinology 5: 367–373.
Lin, S.J., P.A. Defossez, and L. Guarente. 2000. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science 289: 2126–2128.
McCay, C.M., and M.F. Crowell. 1934. Prolonging the life span. The Scientific Monthly 39: 405–414.
Maeda, S., D. Koya, S. Araki, T. Babazono, T. Umezono, M. Toyoda, K. Kawai, M. Imanishi, T. Uzu, D. Suzuki, H. Maegawa, A. Kashiwagi, Y. Iwamoto, and Y. Nakamura. 2011. Association between single nucleotide polymorphisms within genes encoding sirtuin families and diabetic nephropathy in Japanese subjects with type 2 diabetes. Clinical and Experimental Nephrology 15: 381–390.
Mattagajasingh, I., C.S. Kim, A. Naqvi, T. Yamamori, T.A. Hoffman, S.B. Jung, J. Dericco, K. Kasuno, and K. Irani. 2007. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proceedings of the National Academy of Sciences USA 104: 14855–14860.
Mckiernan, S.H., V.C. Tuen, K. Baldwin, J. Wanagat, A. Djamali, and J.M. Aiken. 2007. Adult-onset calorie restriction delays the accumulation of mitochondrial enzyme abnormalities in aging rat kidney tubular epithelial cells. American Journal of Physiology - Renal Physiology 292: F1751–F1760.
Mimura, I., and M. Nangaku. 2010. The suffocating kidney: tubulointerstitial hypoxia in end-stage renal disease. Nature Reviews Nephrology 6: 667–678.
Miyazaki, R., T. Ichiki, T. Hashimoto, K. Inanaga, I. Imayama, J. Sadoshima, and K. Sunagawa. 2008. SIRT1, a longevity gene, downregulates angiotensin II type 1 receptor expression in vascular smooth muscle cells. Arteriosclerosis, Thrombosis, and Vascular Biology 28: 1263–1269.
Nisoli, E., C. Tonello, A. Cardile, V. Cozzi, R. Bracale, L. Tedesco, S. Falcone, A. Valerio, O. Cantoni, E. Clementi, S. Moncada, and M.O. Carruba. 2005. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science 310: 314–317.
Ohkubo, Y., H. Kishikawa, E. Araki, T. Miyata, S. Isami, S. Motoyoshi, Y. Kojima, N. Furuyoshi, and M. Shichiri. 1995. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Research and Clinical Practice 28: 103–117.
Shikata, K. 2007. Microinflammation in the pathogenesis of diabetic nephropathy. Nippon Jinzo Gakkai Shi 49: 474–480.
Tikoo, K., K. Singh, D. Kabra, V. Sharma, and A. Gaikwad. 2008. Change in histone H3 phosphorylation, MAP kinase p38, SIR 2 and p53 expression by resveratrol in preventing streptozotocin induced type I diabetic nephropathy. Free Radical Research 42: 397–404.
UKPDS Group. 1998. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 352: 837–853.
Uzu, T., G. Kimura, A. Yamauchi, M. Kanasaki, K. Isshiki, S. Araki, T. Sugiomoto, Y. Nishio, H. Maegawa, D. Koya, M. Haneda, and A. Kashiwagi. 2006. Enhanced sodium sensitivity and disturbed circadian rhythm of blood pressure in essential hypertension. Journal of Hypertension 24: 1627–1632.
Yeung, F., J.E. Hoberg, C.S. Ramsey, M.D. Keller, D.R. Jones, R.A. Frye, and M.W. Mayo. 2004. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO Journal 23: 2369–2380.
Yoshizaki, T., S. Schenk, T. Imamura, J.L. Babendure, N. Sonoda, E.J. Bae, D.Y. Oh, M. Lu, J.C. Milne, C. Westphal, G. Bandyopadhyay, and J.M. Olefsky. 2010. SIRT1 inhibits inflammatory pathways in macrophages and modulates insulin sensitivity. American Journal of Physiology - Endocrinology and Metabolism 298: E419–E428.
Zhang, D., S. Li, P. Cruz, and B.C. Kone. 2009. Sirtuin 1 functionally and physically interacts with disruptor of telomeric silencing-1 to regulate alpha-ENaC transcription in collecting duct. Journal of Biological Chemistry 284: 20917–20926.
Zhuo, L., B. Fu, X. Bai, B. Zhang, L. Wu, J. Cui, S. Cui, R. Wei, X. Chen, and G. Cai. 2011. NAD Blocks High Glucose Induced Mesangial Hypertrophy via Activation of the Sirtuins-AMPK-mTOR Pathway. Cellular Physiology and Biochemistry 27: 681–690.
Acknowledgments
JSPS KAKENHI (00452235 to S.K.), the Uehara Memorial Foundation (to S.K.), Takeda Science Foundation (to S.K.) and Banyu Life Science Foundation International (to S.K.), The 4th Annual Research Award Grant of Japanese Society of Anti-Aging Medicine (to D.K.), Grant for Collaborative Research from Kanazawa Medical University (C2011-4, C2012-1) and Grant for Specially Promoted Research from Kanazawa Medical University (SR2012-06).
Conflict of interest
All the authors declare no conflict of interests regarding this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kume, S., Kitada, M., Kanasaki, K. et al. Anti-aging molecule, Sirt1: a novel therapeutic target for diabetic nephropathy. Arch. Pharm. Res. 36, 230–236 (2013). https://doi.org/10.1007/s12272-013-0019-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-013-0019-4