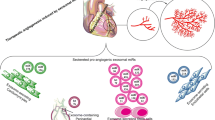
Abstract
Angiogenesis is the process of growing endothelial capillary cells. Exosomes are extracellular vesicles that are rich in miRNAs. Studies have shown that exosomes can carry communication between cells and various tissues by delivering miRNAs to their target organs and cells. It has been repeatedly proven that miRNAs regulate the expression of growth factors and other proteins in endothelial cells through paracrine signalling and participate in the physiological and pathological processes of angiogenesis. In the diagnosis and treatment of diseases, exosome-derived microRNAs can play important roles as biomarkers and drug carriers. In this review, we introduce the characteristics of miRNAs and exosomes and their interactions. Then, we specifically summarize the exosome-derived miRNAs related to angiogenesis, and we discuss the potential uses of exosome-derived miRNAs for diagnosing and treating cardiovascular diseases.

Graphical abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In the early twentieth century, cardiovascular disease (CVD) became the leading cause of death in developed countries. Although advances in medicine have greatly reduced the mortality of patients, CVD is still the main cause of morbidity and mortality worldwide. Therefore, further understanding is needed to help discover the molecular mechanisms of CVD pathogenesis and apply them to prevention and treatment. Angiogenesis is closely related to many CVDs. Regarding ischaemic cardio-cerebrovascular diseases, such as myocardial infarction (MI) and cerebral infarction, angiogenesis is a necessary step in vascular remodelling, and the treatment of angiogenesis through exogenous routes brings new hope for ischaemic disease. Vascular endothelial cells (VECs) are important vascular barriers, and new blood vessels are formed through a highly coordinated blood vessel germination process [1]. Therefore, it is important to understand endothelial cell biology, the angiogenesis process and the molecular mechanisms involved.
microRNAs (miRNAs) are single-stranded noncoding RNAs composed of approximately 22 nucleotides and regulate protein expression at the transcriptional level. For example, miRNAs can regulate the expression of angiogenesis-related factors, regulate endothelial cell proliferation, migration and tube formation, ultimately affecting angiogenesis, and play an important role in the occurrence of tumours and CVDs [2].
Exosomes are small vesicles secreted into the extracellular environment after fusion of multiple vesicles and the cell membrane, with a lipid bilayer membrane structure of 40–100 nm in diameter [3]. Exosomes carry internal bioactive substances such as endogenous proteins, lipids and RNA by internalizing target cells, interacting with receptors and ligands or fusing with lipid membranes and are important mediators for intercellular communication [4]. It was discovered in 2007 that miRNA and mRNA were carried in exosomes [5, 6]. Later, it was reported that exosomes are enriched with a large number of miRNAs, which are contained in exosomes and regulate protein expression in recipient cells through paracrine methods, thereby affecting distant angiogenesis [7].
Therefore, it is very important to study the mechanism and significance of exosome-derived miRNAs for angiogenesis. In this review, we describe the formation mechanism of blood vessels, exosomes and miRNAs, as well as factors that promote and prevent angiogenesis. We also emphasize the roles of exosomes and miRNAs in angiogenesis, the regulatory effect of VECs and clinical applications.
Biogenesis and Characteristics of miRNAs and Exosomes
miRNAs
MiRNA is initially transcribed by RNA polymerase II into a hairpin precursor pri-miRNA, which is then processed by the Drosha complex into a stem-loop pre-RNA of approximately 70 nucleotides; then, the pre-RNA is transferred from the nucleus to the cytoplasm where it fully matures. The pre-RNA binds to the AGO protein, supports the Dicer enzyme and cleaves it into a double-stranded miRNA of 19–24 nucleotides. After the double-strand unwinds, one strand degrades, and one binds to the miRNA-induced silencing complex (miRISC). Generally, a mature miRNA binds to the 3′ end of the target mRNA, which leads to mRNA degradation or incomplete complementary pairing, blocking gene translation and regulating target protein expression (Fig. 1) [8,9,10].
The miRNA genes in the nucleus are transcribed to form hairpin pri-miRNAs, which are processed into stem-loop pre-miRNAs by the Drosha complex. Pre-miRNAs are transferred to the cytoplasm and cleaved into mature double-stranded miRNAs by the AGO protein and Dicer enzyme; then, the miRNAs are denatured, one strand is degraded and the other is combined with RISC to regulate the target gene
Exosomes
Exosomes were first discovered in sheep reticulocytes [11] and were initially considered to be cell waste; however, their function was underestimated. With the development of medical technology, exosomes have been found to be involved in the pathological treatment of various diseases as an important medium for intercellular communication. Earlier, there were various nomenclatures: extracellular bodies, exfoliated vesicles, microparticles, microvesicles (MVs), etc. Exosomes exist in a variety of cells and body fluids, including neurons, platelets, stem cells, tumour cells, urine, saliva and ascites [12]. Exosomes are also separated by many laboratory methods, such as ultracentrifugation, ultrafiltration and affinity purification [13].
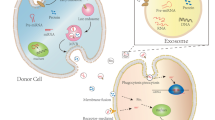
The formation of exosomes can be roughly divided into early endosomes, late endosomes and secretions. The budding process inside of the cell membrane leads to the formation of early endosomes by vesicular endocytosis in the lipid bilayer membrane. Early endosomes have two routes: (1) recycling back to the plasma membrane and (2) the endosomal membrane germinates inward, forming late endosomes or multivesicular bodies (MVBs) [14,15,16]. There are many intraluminal vesicles (ILVs) in MVBs, which are formed by vesicles germinating and breaking inward from the limiting membrane into the internal body cavity. Studies have shown that ALG-2-interacting protein x (Alix) and syndecans contribute to endosomal membrane germination. The endosomal sorting complex required for transport (ESCRT) participates in the budding of the ILV membrane, forms a strong recognition domain and has a high affinity for the endosomal ubiquitinated substrate, leading to membrane germination. ESCRT is a protein family divided into ESCRT, -I, -II, -III and other members. Through ESCRT-dependent or non-dependent mechanisms, it participates in the selective sorting of proteins and isolates the inner body boundary membrane [17,18,19]. After the formation of MVBs, there are two kinds of destinations. One is fusion with lysosomes for degradation, and the other is fusion with the plasma membrane and exocytosis to secrete ILVs, which are secreted into the extracellular matrix and are called exosomes. The study found that the secretion of MVBs depends on KIBRA. KIBRA is a linker-like protein that stabilizes Rab27a, inhibits Rab27a protease degradation and controls exosome secretion. Knocking down Rab27a inhibits exosome secretion (Fig. 2) [20].
The cell membranes of donor cells bud into endocytic vesicles, forming early endosomes. Some recycle and return to the plasma membrane, and some form late endosomes or MVBs. There are many ILVs in MVBs. Cofactors such as ESCRT and Alix contribute to the formation of ILVs. The miRNA formed by the nucleus enters an MVB through hnRNPA2B1. Some MVBs are degraded by lysosomes and some are fused with the plasma membrane, exocytose ILV and secrete exosomes. Rab27a in the cell membrane controls the secretion of MVBs. Exosomes are endocytosed into the interior by receptor cells to release miRNAs and regulate protein expression through various signalling pathways
miRNAs and Exosomes
Many studies have found that exosomes contain a large number of miRNAs, but the mechanism by which miRNAs enter exosomes is rarely reported. In 2013, Carolina et al. [21] reported that miRNAs contain short sequence motifs that control their order in exosomes, guiding miRNA loading into exosomes, and their targeted mutations can regulate the miRNA cargo in vesicles. The protein heterogeneous nuclear ribonucleoprotein A2B1 (hnRNPA2B1) recognizes these motifs, specifically binds exosome miRNAs through hnRNPA2B1 sumoylation and controls their loading into exosomes.
Angiogenic Exosome-Derived miRNAs in CVD
MiRNAs can regulate the expression of proteins related to angiogenesis, target VEGF, eNOS, SCF, etc., and control mitochondrial function. In recent years, many articles have reported that many exosome-derived miRNAs show obvious expression changes during vascular injury and disease, and the expression characteristics are related to ischaemia, tumour angiogenesis, AS, proliferative vascular thickening and obstruction [22]. Exosome sources are divided into types of stem cells, such as cardiac progenitor cells (CPCs), EPCs, MSCs and other cells [23,24,25]. In basic research, some miRNAs can inhibit the development of new blood vessels, i.e. they have anti-angiogenic effects, as in the case of anti-miR92a treatment [26]. Some miRNAs have anti-apoptotic effects and promote angiogenesis, as in the case of miR-210 [27]. In clinical studies, if miRNAs inhibit angiogenesis, anti-miR treatment can improve angiogenesis and arterial formation and reduce the area of local MI. In contrast, the injection of miRNAs that promote angiogenesis can promote blood vessel formation and repair after ischaemia. Therefore, exosome-derived miRNAs can also be divided into those that promote and those that inhibit angiogenesis. Below, we introduce specific miRNAs by research type and effect classification (Table 1; Fig. 3).
Exosome-derived miRNA experiments related to angiogenesis. (1) In vitro experiment: exosomes isolated from stem cells were cocultured with ECs, and miRNA was transfected into ECs. (2) In vivo experiment: exosome-derived miRNAs were injected into the body of an ischaemic mouse model. The miRNAs of the two experiments inhibited related gene expression, which manifested as an increase or inhibition of blood vessel proliferation, migration, tube formation and the improvement or aggravation of cardiac ischaemia, ultimately promoting or inhibiting blood vessel regeneration, respectively
Exosome-Derived microRNAs in Basic Research: Exosome-Derived miRNAs That Promote Angiogenesis
MiR-126
In the past, many studies have reported that miR-126 is the miRNA with the highest content in ECs and regulates angiogenesis and endothelial cell integrity by activating the PI3K/Akt/eNOS signalling pathway [51]. In recent years, the number of studies on cardiovascular exosomes has increased, and there are many reports of miR-126 carried by exosomes in angiogenesis.
In 2013, Sun et al. [28, 52] found that the peripheral blood mononuclear cell (PBMC) subgroup CD34+ had the strongest angiogenic effect, and miR-126 was the most differentially expressed in CD34+. MiR-126 is secreted by PMBCs in the form of microvesicles/exosomes, is taken up by ECs and stimulates angiogenesis. High glucose treatment or diabetes leads to loss of miR-126 expression. In vitro high-glucose treatment of human aortic endothelial cells (HAECs) downregulated miR-126 expression and reduced tube formation. Transfection of the miR-mimic-126 gene can promote angiogenesis. In 2016, Wu et al. [29] cocultured EPCs from healthy patients with EPCs from diabetic patients and found that under high-glucose conditions, miR-126 and VEGFR2 expression were downregulated, EPC migration decreased, and the apoptosis rate and reactive oxygen species (ROS) production increased. However, EPCmiR-mimic-126-MVs can ameliorate adverse effects, reduce vascular oxidative stress and improve EPC function and survival. Both of the above studies have led to therapeutic targets for diabetic vascular complications.
Angiogenic exosome-derived miR-126 is derived from a variety of cells, in addition to PBMCs and EPCs, platelets and MSCs. In 2018, researchers cocultured PLT-EXOs with human umbilical vein endothelial cells (HUVECs) in patients with acute coronary syndrome (ACS). The levels of miR-126 and angiogenic factors in HUVECs activated by PLT-EXOs were increased. The scratch test and proliferation test proved that the proliferation and migration rate of HUVECs are increased [30]. In 2019, Pan et al. [31] isolated miR-126-enriched exosomes from MSCs and incubated them with hypoxia/reoxygenation (H/R)-injured EC. PCR and Western blot (WB) detection revealed that miR-126 and pro-angiogenic factor expression were upregulated. Transfection of overexpressed or silenced miR-126 into MSCs increased the vitality, migration and tube formation of ECs cocultured with MSC-EXOs miR-mimic-126, while those cocultured with MSC-EXOs miR-si-126 had the opposite effect, and apoptosis increased. Afterwards, researchers also confirmed that miR-126 reduces the expression of caspase-3 cleavage and activates angiogenesis by activating the PI3K/Akt/eNOS signalling pathway.
Various studies have shown that miR-126 may be an important target for pro-angiogenesis or anti-angiogenesis therapy and play an important role in diagnosis and treatment in the future.
MiR-21-5P
MiR-21-5P is a widely studied miRNA with cancer-promoting and anti-apoptotic effects. Li et al. [32] found that the increase or decrease in miR-21-5P expression in MSC-EXOs can increase or decrease inflammation in an employed murine lung ischaemia/reperfusion (I/R) model, and in an in vitro hypoxia/reoxygenation (H/R) model, treatment with MSC-EXOs or a miR-21-5P mimic can significantly improve apoptosis and reduce I/R injury (IRI) in mouse lung tissue. MSC-EXOs and miR-21-5P reduce oxidative stress-induced apoptosis by targeting PTEN and PDCD4.
In the same year, Hu et al. [33] cocultured exosomes isolated from EPCs with human microvascular endothelial cells (HUMECs). CCK8, wound healing experiments and tube formation assays demonstrated that HUVECs have increased proliferation, migration and tube formation capabilities. RNA sequencing and PCR analysis confirmed that miR-21-5p is the most abundant miRNA in EPC-EXOs. Through data analysis, the miR-21-5p/THBS1 pathway was screened to play an independent role in EPC-EXO-mediated VECs. Knocking down THBS1 promotes the migration and tube formation of HUVECs. The mRNA and protein levels of THBS1 were reduced, and the inhibition of miR-21-5p could rescue THBS1 expression and counteract the increased effects of EPC-EXOs on proliferation, migration, tube formation and wound healing. In vitro experiments confirmed that the EPC-eXO/miR-21-5p/THBS1 pathway can regulate angiogenesis.
MiR-210
There are many reports in the literature that miR-210 can reduce EC apoptosis and angiogenesis disorders, and its overexpression can increase blood vessel density and angiogenesis [53]. Evidence suggests that miR-210 promotes cell survival by reducing H/R-induced mitochondrial ROS overproduction [54]. Later, Ma et al. [34] found that exosomes isolated from EPCs contained miR-210. Incubation of EPC-EXOsmiR-210 with H/RI EC found that EPC-EXOsmiR-210 effectively inhibited H/R-induced EC apoptosis and ROS overproduction, improved vascular dysfunction, increased tube formation and mobility H/R-induced mitochondrial breakage. Therefore, EPC-EXOs can protect ECs from H/R damage, and miR-210 can improve mitochondrial function to enhance angiogenesis function.
MiR-132
Multiple lines of evidence indicate that miR-132 regulates many processes in ECs [55]. Ma et al. [35] cocultured exosomes isolated from MSCs with HUVECs. PCR detection showed that miR-132 was increased in HUVECs, indicating that exo-miR-132 can be effectively taken up by HUVECs. Increased tube formation and mesh number of HUVECs treated with miR-132 and the effect of miR-132 inhibitors were reversed, indicating that miR-132 can promote angiogenesis in vitro. Dual-luciferase reporter assays showed that the target of miR-132 was RASA1, and its expression was reduced in HUVECs. This result indicated that miR-132 electroexosomes can promote angiogenesis.
MiR-322
Seock-Won et al. [36] transfected miR-322 into CPC-eXOs in vitro and incubated them with HUVECs. The modified Boyden chamber and Matrigel experiments proved that CPC-exo-322 can significantly promote EC migration and tube formation. It was previously reported that the target of miR-322 is CUL2, and HIF-1α mediates miR-322 expression [56, 57]. The test found that CUL2 expression in HUVECs decreased, HIF-1α expression increased, NOX2 was upregulated, and the ROS production increased. The miR-322/CUL2/HIF-1α axis enhances EC angiogenesis. This experiment suggests that miRNA bioengineering of CPCexo carrying angiogenesis-related factors may be a therapeutic method to promote vascularization and regeneration of ischaemic diseases.
MiR-130a
It has been widely reported that the chemical signals of biomaterials can not only promote the osteogenic differentiation of bone marrow stromal cells (BMSCs) but also enhance the pro-angio-genic capacity of ECs. Researchers with the appropriate concentration of Li-incorporated bioactive glass ceramic (Li-BGC) extract can significantly promote the proliferation, migration and tube formation of HUVECs and increase the expression of vascular growth factor (VEGF/ANG1/KDR/HIF-α). The exosomes released by BMSCs were isolated and treated with Li-BGC extracts. The proliferation, migration and tube formation of HUVECs treated with Li-BGC-exos increased, and the pro-angiogenic factors also increased. After PCR and WB detection, it was found that the expression of miR-130a in Li-BGC-exos increased, the miR-130a in HUVECs after treatment increased, the expression of PTEN endogenous protein decreased, and AKT phosphorylation increased [37]. MiR-130a has been reported before and can regulate endothelial cell angiogenesis [58]. Knocking out miR-130a can attenuate Li-BGC-exos-mediated angiogenesis, increase PTEN protein and reduce angiogenic factors.
This experiment proves that the exosome-derived miR-130a/PTEN/AKT axis released by BMSCs may be involved in Li-BGC-mediated angiogenesis, and it also makes it possible for biomaterials to be better used in medical treatment.
MiR-143 and MiR-222
Many studies have shown that cardiomyocytes (CMs) can release exosomes to affect the heart and various tissues of the body [59]. Studies have found that exosomes released by ischaemic CMs (EXOisch) can promote EC proliferation, the survival rate and capillary and germination formation during oxidative stress. Enrichment analysis and PCR screened the two miRNAs with the highest content in exosomes: miR-143 and miR-222. Mouse cardiac endothelial cells (MCECs) were cultured in vitro, and miR-143 increased EC proliferation and migration and promoted capillary-like structure formation and cell germination, while miR-222 had no such changes. Similarly. in HUVECs, miR-222 promotes capillary-like formation and germination, but miR-143 has no such changes. This experiment is the first to show that exosomes released by ischaemic CMs promote angiogenesis [38].
MiR-214
Bas W. et al. [39] isolated exosomes from human microvascular endothelial cells (HMECs) and incubated them with ECs and found that EC migration, tube formation and germination were all increased. PCR detection showed that the miR-124 content of HMEC exosomes is very large, which inhibits the expression of miR-124. EC migration, tube formation and germination will be reduced, indicating that exosome-mediated EC migration and angiogenesis stimulation in vitro depend on the expression of miR-124 in exosomes. The luciferase reporter assay confirmed that ATM was a target gene of miR-124, inhibited exosome-derived miR-124, and increased ATM expression, cell cycle arrest and senescence.
MiR-19a-3p
Can et al. [40] stimulated the release of exosomes in HUVECs through shock wave therapy (SWT) stimulation. When SWT-treated HUVECs were cocultured with ECs, EC proliferation and tube formation increased. Through RNA sequencing, it was found that miR-19a3p was the most enriched in exosomes, and it was previously confirmed that miR-19a has a pro-angiogenic effect in breast cancer [60]. The experiment also proved that SWT-treated exosome miR-19a3p can increase the proliferation and tube formation ability of HUVECs cocultured in vitro. The test found that in cocultured HUVECs, TSP-1 mRNA and protein were reduced, and VEGFR2 expression increased. The experiment shows that exosome-derived miR-19a3p can promote angiogenesis. Stimulating the release of exosomes through physical methods also provides new methods for future exosome treatment.
MiR-125a
Liang et al. [41] treated adipose-derived MSCs (adMSC-Exos) with HUVECs and found that this treatment promoted the formation of HUVECs. Microarray analysis and qPCR detection showed that miR-125 was enriched in adMSC-Exos, and fluorescent substance detection proved that exosomes released miR-125 into HUVECs. Overexpression of miR-125 increases tube formation in HUVECs, increases the expression of Ang1 and Kdr and reduces the anti-angiogenic genes TSP-1 and Vash1. Silencing miR-125 has the opposite effect. miR-125 mediates adMSC-Exo angiogenic activity. Afterwards, a luciferase reporter assay showed that DLL4 was the direct target gene of miR-125. HUVECs were treated with adMSC-Exos. WB showed that DLL4 protein expression was reduced and EC proliferation was increased, while flow cytometry showed that the proportion of CD34+ endothelial tip cells increased, which proved that adMSC-Exos and miR-125 inhibit DLL4 expression, promote endothelial cell formation, and regulate angiogenesis.
MiR-199-3p
Adipose-derived stem cells (ADSCs) are a class of multipotent cells. It has been reported that ADSCs can alleviate ischaemic injury by secreting paracrine factors and exosomes [61]. Researchers co-cultivated ADSC-EXOs with ECs, and by CCK8 and migration assays, EC proliferation and the migration capacity increased significantly. WB results showed that miR-199-3p was highly expressed in exosomes. Overexpression and silencing of miR-199-3p and incubation with ECs increased the expression of miR-199-3p in ECs of the EXO199mimic group, and increased EC proliferation and migration. The EXO199inhibitor group had the opposite effect. Bioinformatics analysis and luciferase reporter assays showed that Sema3A was the target of miR-199-3p. In ADSC-EXOs and EXO199inhibitor, Sema3A expression was reduced, while overexpression of Sema3A inhibited the stimulation of EC proliferation and migration by the EXO199mimic [42]. Therefore, the ADSC-EXO-miR-199-3p/Sema3A axis may be a pathway to promote angiogenesis, and ADSCs are expected to become a treatment tool for CVDs.
MiR-21
An et al. [43] isolated exosomes from ADSCs and cocultured them with HUVECs. The detection found that the level of miR-21 in HUVECs increased, the expression of HIF-1α, VEGF, and SDF-1 increased, and EC tube formation and the network structure increased, indicating that highly expressed ADSC-derived exosome miR-21 can promote HUVEC vascularization. Recent evidence also suggests that miR-21 plays a role in tumour angiogenesis [62, 63]. Through WB detection, it was found that the content of the tumour suppressor gene PTEN in HUVECs decreased, and the expression of the oncogenes AKT and ERK1/2 was upregulated. Studies have shown that miR-21 can be used as a biomarker for tumours and cardiovascular circulation.
MiR-150
Zhang et al. [44] found that exosomes isolated from the plasma of AS patients and those from the purchased human monocyte/macrophage cell line THP-1 have increased miRNA levels, and miR-150 is increased in its exosomes. FITC-labelled miR-150 was transfected into THP-1 cells, and THP-1 cells were incubated with HMECs. Flow cytometry showed that miR-150 was taken up and retained in HMECs, proving that miRNA was transported to the target through exosome cells. THP-1-treated HMECs increased their migration ability. After exogenously adding miR-150, its migration ability increased significantly, and WB showed that c-Myb expression decreased. C-Myb is a transcription factor related to cell proliferation, apoptosis, and tumorigenesis [64]. A luciferase reporter assay showed that knocking down miR-150 and c-Myb activity was normal. In addition, increased levels of miR-150 in plasma exosomes of AS patients reduced c-Myb expression and enhanced cell migration, suggesting that an increase in secreted miR-150 may play a role in regulating endothelial cell function and angiogenesis.
MiR-30b
Gong et al. [45] cultured miR-30b with HUVECs using MSC-derived conditioned medium (CdMMSC), and EC tube formation and globular germination increased, while PCR showed that miR-424, miR-30b, miR-30c and let-7f decreased in CdM and increased in HUVECs, proving that miRs secreted by MSCs were transferred into HUVECs. If the release of exosomes was inhibited, then the miR content in both CdM and HUVECs decreased, indicating that the exosomes released by MSCs mediate miR transport. The researchers chose the highest content of miR-30b in exosomes and then overexpressed and knocked down miR-30b, causing HUVEC tube formation to increase and decrease, respectively, thereby proving that exosome-derived miR-30b promotes angiogenesis.
Let-7b-5p
Cristina et al. [46] performed nanoparticle tracking analyses (NTA) from the pericardial fluid (PF), which showed enrichment of exosomes in human PF and plasma, and microarray analysis of Chinese PCR showed that miRNAs were enriched in exosomes. Exosomes contain Dicer and AGO-2, which also confirms that miRNAs are present in exosomes. PF-derived exosomes were cocultured with HUVECs. Under hypoxic conditions, compared with those in the PBS control group, the exosomes in the treatment group promoted EC proliferation, promoted the formation of surface capillary-like cell networks and inhibited EC apoptosis. PCR found that let-7b-5p was highly expressed in PF-derived exosomes. Overexpressed and silenced let-7b-5p transcripts were transfected into ECs, and it was found that the simulated group promoted EC proliferation and capillary-like formation and reduced TGFBR1 expression, while the inhibited group had the opposite effect. Experiments have shown that PF-derived exosomes can enter ECs through let-7b-5p and mediate angiogenesis.
Exosome-Derived miRNAs That Inhibit Angiogenesis
MiR-320
It has been reported previously that miR-320 can inhibit angiogenesis [65]. W Wang et al. [47] cocultured MCECs with myocytes from Goto-Kakizaki (GK) rats and established MCECs and myocytes from Wistar (WT) rats as a control group. The MTS method detected that GK rat myocytes inhibited EC proliferation and reduced the number of blood vessels, while WT rat myocytes promoted responder cell (RC) proliferation. ECs were cocultured with exosomes of GK and WT rats, and MTS experiments, scratch experiments and tube formation experiments were successively performed. The experiment showed that GK-exo proliferation, migration and tube fulcrum number were all reduced, and the effect of WT-exo was the opposite. The PCR results showed that miR-320 in GK CMs was higher than that in WT CMs. A luciferase reporter assay showed that GK-exo can strongly inhibit Hsp20 gene activity and the protein levels in MCECs, while WT-exo has the opposite effect. WB detection found that GK-exo can reduce the protein levels of the pro-angiogenic factors IGF-1 and Ets2 in MCECs, indicating that the Hsp20 gene is a target gene of miR-320 and that miR-320 can inhibit angiogenic factors. After overexpression of miR-320, the researchers found that the proliferation, migration and tube formation of ECs were inhibited, and the protein levels of Hsp20, IGF-1 and Ets2 were also reduced, indicating that miR-320 can inhibit angiogenesis.
Taken together, miR-320 relies on GK-exo from CMs and ECs. The increased level of miR-320 in diabetic CMs leads to an increase in the level of miR-320 in ECs under high glucose conditions, which leads to the sparseness of diabetic heart microvascularization. This study opens up a new way to treat abnormal angiogenesis caused by diabetes.
MiR-939
The researchers purified the serum of MI patients by ultracentrifugation to obtain exosomes and incubated the exosomes with HUVECs in vitro to internalize them. The ischaemic exosome group had a more significant increase in EC proliferation, migration and tube formation than the normal group, indicating that exosomes derived from coronary serum are regulators of angiogenesis. A model of hindlimb ischaemia in mice was established. Exosomes were injected intramuscularly after the operation. There was no significant difference in blood flow irrigation in the normal group, and scar formation occurred. The density of blood vessels increased significantly, which promoted ischaemic repair of damage. Microarray analysis showed that miR-939-5p was downregulated in ischemic exosomes (isc-exos). A luciferase reporter assay confirmed that iNOS is a target of miR-939-5p. Transfection of overexpressed and inhibited miR-939-5p into HUVECs showed that EC proliferation and tube formation in the simulated group were significantly inhibited, and the mRNA and protein levels of iNOS decreased. The effect of the inhibition group was reversed. Previous studies have also confirmed that miR-939 can destroy blood vessel integrity and inhibit blood vessel regeneration [66]. After incubation of isc-exos with HUVECs, the effect was the same as that of the inhibition group, indicating that inhibition of miR-939-5p can promote blood vessel regeneration, and the lower miR-939-5p level in isc-exos can release miR-939-5p to a large extent. The 5p/iNOS signalling pathway promotes blood vessel regeneration [48].
MiR-106b-5p
Li et al. [49] coincubated exosomes isolated from human cholesteatoma perimatrix fibroblasts (hCPFs) with HUVECs. The presence of hCPFs-exo promoted the formation of HUVECs and increased the expression of pro-angiogenic genes (Angp2, Flk1, HIF-α, etc.). Microarray analysis and PCR showed that miR-106b-5p was significantly downregulated in hCPFs-exo. miR-106b-5p mimics and inhibitors were transfected into cultured HUVECs. Transwell migration assays showed that the inhibition group had higher migration and tube formation forces than the simulated group, indicating that hCPFs-exo regulate angiogenesis through miR-106b-5p. The TargetScan program screened the downstream target of miR-106b-5p as Angp2. A luciferase reporter assay confirmed that miR-106b-5p overexpression can inhibit Angp2 gene activity. Inhibition of Angp2 expression reduced HUVEC migration and tube formation. These results show that miR-106b-5p can inhibit the expression of Angp2 in hCPFs-exo and promote angiogenesis by inhibiting miR-106b-5p expression in hCPFs-exo and increasing Angp2 expression.
MiR-155
Krüppel-like factor 5 (KLF5), a zinc-finger-containing transcription factor, mediates the proliferation and migration of smooth muscle cells and has a central role in CVD [67]. It was previously reported that miR-155 can promote the occurrence and development of AS [68]. In response to the above findings, Zheng et al. [50] exposed human aortic smooth muscle cells (HASMCs) to oxLDL-induced injury and detected increased expression of KLF5 and miR-155 in HASMCs. The vesicles in HASMCs overexpressing KLF5 were isolated, and microarray analysis found that miR-155 was enriched in vesicles. This proves that KLF5 overexpression can upregulate the expression and secretion of miR-155 in HASMCs. After co-cultivation of HASMCs and ECs, KLF5 promoted the transfer of miR-155 in exosomes from SMCs to ECs, and the proliferation, migration and tube formation of ECs were reduced. Inhibition of miR-155 can counteract the above effects. This shows that in SMCs induced by KLF5, overexpressed miR-155 has an anti-angiogenic effect. Taken together, anti-miR-155 therapy is expected to be applied to CVDs, which can effectively prevent AS and restore EC vascular integrity.
Exosome-Derived microRNAs in Clinical Studies: Exosome-Derived miRNAs That Promote Angiogenesis
MiR-21-5P
Hu et al. [33] established a balloon-induced rat vascular injury model. Compared with the control group, the EPC-eXO treatment group showed significantly reduced neointimal hyperplasia, which promoted revascularization after vascular injury.
MiR-132
The researchers injected treated HUVECs into MI mice, and the Matrigel plug was bright red, which showed the promotion of tube formation. The capillary density in the infarcted area increased significantly, except for decreases in the left ventricle ejection fraction (LVEF) and fractional shortening (FS), indicating that miR-132 electroexosomes can effectively protect the cardiac function of MI mice [35].
MiR-322
Seock-Won et al. [36] established a mouse model of MI, and CPCexo-322 was injected into the mouse model. Compared with the control group, the fibrosis area and the infarct area were reduced, and the capillary density was significantly increased, indicating that CPCexo-322 can promote myocardial angiogenesis in the infarct area.
MiR-143 and MiR-222
Researchers established a mouse MI model, and EXOisch was delivered to the hearts of MI mice. It was found that the ejection fraction increased and the number of blood vessels in the infarct area increased, indicating that EXOisch can promote the regeneration of MI blood vessels [38].
MiR-214
Using a migration assay in vivo, the researcher found that in the plug of anti-miR-124 exosomes, the number of ECs decreased, the number of functional blood vessels decreased and the average blood vessel area decreased, indicating that exosomes containing miR-124 can inhibit cell cycle arrest to stimulate blood vessel formation in the body [39].
MiR-19a-3p
Can et al. [40] performed the Matrigel plug experiment. When exosomes released by SWT-treated HUVECs were injected into nude mice, the number of small arteries and capillaries in the emboli increased, fibrosis in the MI area decreased, and the cardiac ejection fraction in the injection group increased significantly compared with those in the control group. This proved that exosomes greatly promote angiogenesis and improve cardiac function after MI.
Let-7b-5p
Cristina et al. [46] established a mouse ischaemia model. When exosomes were injected into the ischaemic area of mice, let-7b-5p expression increased, and TGFBR1 expression decreased. In vivo experiments also proved that PF-source exosomes can promote angiogenesis and improve blood stream recovery.
Exosome-Derived miRNAs That Inhibit Angiogenesis
MiR-155
Experiments confirmed that miR-155 derived from SMCs impaired EC barrier function by inhibiting TJ protein expression. The researchers also injected exosomes secreted by KLF5-transfected HASMCs (miR-155-rich exosomes) into apoE-1-mice fed a high-fat diet and found that the plaque coverage of the aortic area was significantly increased and the endothelium was intact and not damaged. The results proved that miR-155 inhibits the regeneration of blood vessels in the body and promotes the occurrence and development of AS [50].
Angiogenic Exosome-Derived miRNAs in Tumours and Other Organizations
There has always been an inseparable connection between tumours and CVD because tumour metastasis and growth are a vascular-dependent process, and tumour growth mainly depends on the formation of new blood vessels; therefore, biotherapy of tumours is targeted to inhibit angiogenesis and has become a research hotspot in recent years. Many studies have reported that miRNAs in exosomes can regulate tumour angiogenesis and regulate exosome-derived miRNAs in tumours, which can be used as emerging therapies for tumour treatment.
In 2013, Nobuyoshi et al. [69] cocultured metastatic breast cancer cells with HUVECs. The exosomes in cancer cells were transferred to ECs in paracrine form, promoting EC proliferation, migration and tube formation. Microarray analysis showed that many miRNAs are enriched in exosomes in cancer cells, most notably miR-210. Overexpression of miR-210 in exosomes, EC proliferation, migration and tube formation were significantly increased, indicating that exosome miR-210 can enhance angiogenic activity in vitro. This experiment was the first to link cancer metastasis to exosomal miRNA in vitro.
Since then, more cancer diseases related to exosome miRNAs have been reported. In addition to cancer and CVD, some studies have reported that the regeneration of blood vessels in other human tissues is also related to exosome-derived miRNAs (Table 2).
Exosome-Derived miRNAs in the Heart
Although there are few studies on exosomes in angiogenesis, the heart itself has many reports in the literature. Studies have shown that exosomes may provide a basic mechanism by which damaged hearts communicate with other tissues and organs to initiate the repair process and how stem/progenitor cells repair and regenerate the heart muscle [92]. Treatment of heart injury by regulating exosome-derived miRNAs also provides a new reference for the study of angiogenesis (Table 3).
Although some studies have shown that some exosome-derived miRNAs can simultaneously promote angiogenesis in tumours and protect the heart, these effects seem to be contradictory. However, when tumour cells are present in the body, exosome-derived miRNAs are generally derived from tumour cells themselves, and miRNAs act on vascular endothelial cells to promote angiogenesis. When the heart is damaged, exosome-derived miRNAs are derived from MSCs and act on cardiac stem cells or cardiomyocytes to avoid cell damage [69, 102]. Therefore, when the two diseases exist at different times, the inhibition of angiogenesis and anti-tumour effects will not affect the protection of the heart. The two are not contradictory. Of course, if cancer and heart disease exist at the same time, whether the same miRNAs have different effects due to different disease states and exosome sources will need to be confirmed by additional research in the future.
Clinical Application of Exosome-Derived miRNAs
With the in-depth study of exosomes and miRNAs, it has been found that exosome-derived miRNAs have an important role in the regulation of tissues and cells, and there are an increasing number of reports on the diagnosis and treatment of diseases through exosome-derived miRNAs. For example, the miRNAs may be used as diagnostic markers and drug carriers.
Diagnosis of Exosome-Derived miRNAs
Some miRNAs are differentially expressed between tissues or developmental stages, and changes in the levels of several miRNAs in plasma, serum, urine and saliva are associated with different diseases. These miRNAs are expected to serve as diagnostic markers or therapeutic targets for specific diseases in tissues or biological stages. For example, the replication of hepatitis C virus (HCV) requires miR-122, and the detection and regulation of miR-122 levels are helpful for the diagnosis and treatment of HCV [103]. The plasma level of miR-499 (a heart-specific miRNA) in patients with MI showed a perfect correlation with the level of calcitonin [104]. The decreased levels of miR-125a and miR-200a in saliva are related to oral squamous cell carcinoma [105].
The content of exosomes derived from different cells varies. The molecular composition of exosomes reflects the special function of their original cells. Moreover, under different disease states, the expression levels of the exosome contents are also different [106]. The presence of biomarker exosomes in body fluids (plasma, urine, bronchoalveolar) assists in the early detection of disease states [17].
Because exosomes can act as carriers of miRNAs, exosome-derived miRNAs are increasingly regarded as circulating diagnostic markers for intercellular communication, immune regulation, disease diagnosis and prognosis. Exosome-derived miRNAs can perform multiple functions in many pathological and physiological states, such as tumour diagnosis, immune response, CVD, acute organ damage, regenerative medicine and stem cell therapy [107]. For example, circulating exosome-derived miRNAs can appear in the urine through the kidney excretory system and have been identified as a new set of biomarkers [108]. The level of miR-146a in patients with peripartum cardiomyopathy (PPCM) was significantly higher than that in healthy postpartum controls, indicating the diagnostic role of miRNA in cardiovascular medicine [109].
Exosome-Derived miRNA Therapeutics
Many studies have reported the role of miRNAs in angiogenesis, so inhibiting or mimicking miRNA is expected to enable the targeted treatment of CVDs related to miRNA. miRNAs can be delivered into the body through drug delivery systems, such as cholesterol modification, liposomes, viral vectors and synthetic nanocarriers [110, 111]. However, these technologies have potential problems such as toxicity, immunogenicity, manufacturing problems and the inability to cross special barriers [112].
Exosomes can act as effective drug carriers because of their own characteristics. The special lipid composition of exosomes has overall biocompatibility [113]. The size of exosomes can escape the immune system and lack immunogenicity. Exosomes provide RNase-free vesicles, which can protect circulating miRNA from RNase degradation and have the characteristics of preventing drugs from being degraded [7]. For instance, exosomes can be used to package doxorubicin, which targets cancer cells and can penetrate the blood-brain barrier (BBB) to be delivered to glioblastoma cells [113].
Therefore, exosome delivery of miRNA is expected to become an important tool for the treatment of various diseases. Luo et al. [114] introduced miRNA into stem cell-derived exosomes, which has therapeutic value in the reprogramming and enhancement of the acute myocardial infarction (AMI) microenvironment and induces a good prognosis for CVD. Exosomal miR-30c was shown to induce the degradation of microsomal triglyceride transfer protein (MTP) mRNA to interfere with high cholesterol and high LDL concentrations. A feasible treatment procedure can increase the liver miR-30c levels and reduce the risk of high cholesterol, lipids and AMI [115].
miRNA and Circular RNA (circRNA)
CircRNA is a type of noncoding RNA. Many researchers have reported that circRNA can be used as a miRNA sponge to regulate the function of miRNA. CircRNA also exists in exosomes and can be used as a marker for the diagnosis and treatment of diseases.
Wang et al. [116] cultured CMs under hypoxic conditions and isolated exosomes to obtain CM-exo. A cardiovascular endothelial cell (CMVEC) apoptosis model was established after oxidative stress was induced by H2O2. Exosomes were cocultured with CMVECs, PCR showed that the level of circHIPK3 increased, and the anti-apoptotic ability of CMVECs increased. Luciferase reporter assay and Shengxin analysis showed that circHIPK3 is a miR-29a sponge and that IGF-1 is a target of miR-29a in CMVECs. Overexpression of miR-29a can inhibit IGF-1 expression and reverse the anti-apoptotic effect of circHIPK3 on CMVECs. This proves that exosomal circHIPK3 regulates the oxidative damage of CMVECs through the miR-29a/IGF-1 axis.
Linking exosomes, circRNAs and miRNAs together can regulate the function of ECs in multiple ways and provide additional methods to treat angiogenesis.
Conclusion and Perspectives
Exosome-derived miRNAs have been widely reported as diagnostic markers and therapeutic targets. Exosome research has elucidated new mechanisms for communication between cells and between organs. However, as a new technology, it still faces many problems: (1) exosomes are extremely small, and it is difficult to study them under physiological conditions. The dynamic release and uptake of vesicles in tissues and fluids create some practical challenges in exosome research. (2) The selection of exosome sources, the improvement of purification, the use of targeting peptides, pharmacokinetic properties and the role of drugs to modify the surface of exosomes are complex obstacles that must be overcome. (3) The specific downstream molecular mechanism of exosomes promoting angiogenesis is not fully understood, and it is unclear which functional protein or nucleic acid components are involved. These functional components and which signal paths exist in “cross-talk” are also worth studying further. (4) Romain et al. [117] showed that an injection of CDC exosomes into the myocardium 30 min after coronary artery occlusion and reperfusion can significantly reduce infarct size in small pigs, while coronary exosomes have no such effect, and poor delivery and efficacy in the coronary artery may be the main clinical limitation of exosomes.
Although the use of exosome-derived miRNA faces many problems, the advantages of exosomes themselves have become an important basis for the diagnosis and treatment of diseases. (1) The physicochemical stability of exosomes in vivo and their multidimensional packaging are excellent models for therapeutic drugs. (2) Stem cells sometimes pose serious safety risks due to potential tumour formation. Stem cell-derived exosomes do not affect efficacy, have low immunogenicity, and provide safety advantages. (3) According to the established procedures, exosomes can be easily mass produced in a laboratory environment. (4) miRNAs, proteins and other components loaded in exosomes are not prone to cell degradation. (5) The specific biophysical properties of exosomes make them suitable for in vitro research and manipulation.
To overcome the problem of drug delivery, scientists have proposed new technologies. For example, Yang et al. reported the large-scale production of functional mRNA-encapsulated exosomes through cell nanoperforation [112]. The miRNA in exosomes is generally released by itself according to the physiological changes in the body. Researchers use chemical or physical stimulation to release exosomes to achieve treatment. For example, Rick F et al. [118] used the new technology of ultrasonic perforation by ultrasound to destroy the microbubbles triggered by ultrasound and successfully targeted gas-filled albumin shell microbubbles containing antagomiR to the ischaemic myocardium, thereby increasing myocardial localization and the permeability of blood vessels and cells.
In summary, exosome-derived miRNAs play an important role in the diagnosis and treatment of diseases and have attracted increasing attention. Although the downstream mechanism of action of exosome-derived miRNAs is unclear and there are major challenges in drug loading, the development of medical technology, especially for CVDs, has led to the expectation that exosome-derived miRNAs are emerging diagnostic and therapeutic tools for angiogenesis.
References
Krüger-Genge, A., Blocki, A., Franke, R. P., & Jung, F. (2019). Vascular endothelial cell biology: an update. International Journal of Molecular Sciences, 20(18), 4411.
Wojciechowska, A., Braniewska, A., & Kozar-Kamińska, K. (2017). MicroRNA in cardiovascular biology and disease. Advances in Clinical and Experimental Medicine, 26(5), 865–874.
Mathivanan, S., Ji, H., & Simpson, R. J. (2010). Exosomes: extracellular organelles important in intercellular communication. Journal of Proteomics, 73(10), 1907–1920.
Pitt, J. M., Kroemer, G., & Zitvogel, L. (2016). Extracellular vesicles: masters of intercellular communication and potential clinical interventions. The Journal of Clinical Investigation, 126(4), 1139–1143.
Valadi, H., Ekström, K., Bossios, A., Sjöstrand, M., Lee, J. J., & Lötvall, J. O. (2007). Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature Cell Biology, 9(6), 654–659.
Hunter, M. P., Ismail, N., Zhang, X., Aguda, B. D., Lee, E. J., Yu, L., Xiao, T., Schafer, J., Lee, M. L., Schmittgen, T. D., Nana-Sinkam, S. P., Jarjoura, D., & Marsh, C. B. (2008). Detection of microRNA expression in human peripheral blood microvesicles. PLoS One, 3(11), e3694.
Cheng, L., Sharples, R.A., Scicluna, B.J. and Hill, A.F.. Exosomes provide a protective and enriched source of miRNA for biomarker profiling compared to intracellular and cell-free blood. Journal of Extracellular Vesicles, 3.
Schirle, N. T., Sheu-Gruttadauria, J., & MacRae, I. J. (2014). Structural basis for microRNA targeting. Science, 346(6209), 608–613.
Ha, M., & Kim, V. N. (2014). Regulation of microRNA biogenesis. Nature Reviews. Molecular Cell Biology, 15(8), 509–524.
Krol, J., Loedige, I., & Filipowicz, W. (2010). The widespread regulation of microRNA biogenesis, function and decay. Nature Reviews. Genetics, 11(89), 597–610.
Johnstone, R. M., Adam, M., Hammond, J. R., Orr, L., & Turbide, C. (1987). Vesicle formation during reticulocyte maturation. The Journal of Biological Chemistry, 262(19), 9412–9420.
Colombo, M., Raposo, G., & Théry, C. (2014). Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annual Review of Cell and Developmental Biology, 30(1), 255–289.
Shao, H., Im, H., Castro, C. M., Breakefield, X., Weissleder, R., & Lee, H. (2018). New technologies for analysis of extracellular vesicles. Chemical Reviews, 118(4), 1917–1950.
Raposo, G., & Stoorvogel, W. (2013). Extracellular vesicles: exosomes, microvesicles, and friends. The Journal of Cell Biology, 200(4), 373–383.
Mashouri, L., Yousefi, H., Aref, A.R., Ahadi, A.M., Molaei, F. and Alahari, S.K.. (2019) Exosomes: composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Molecular Cancer. 18(1).
Aghabozorgi, A. S., Ahangari, N., Eftekhaari, T. E., Torbati, P. N., Bahiraee, A., Ebrahimi, R., & Pasdar, A. (2019). Circulating exosomal miRNAs in cardiovascular disease pathogenesis: new emerging hopes. Journal of Cellular Physiology, 234(12), 21796–21809.
Niel, G. V., Porto-Carreiro, I., Simoes, S., & Raposo, G. (2006). Exosomes: a common pathway for a specialized function. Journal of Biochemistry, 140(1), 13–21.
Hurley, J. H. (2010). The ESCRT complexes. Critical Reviews in Biochemistry and Molecular, 45(6), 463–487.
McGough, I. J., & Vincent, J.-P. (2016). Exosomes in developmental signalling. Development, 143(14), 2482–2493.
Song, L., Tang,S., Han, X., Jiang, Z., Dong, L., Liu, C., Liang, X., Dong, J., Qiu, C., Wang, Y., and Du, Y.. (2019) KIBRA controls exosome secretion via inhibiting the proteasomal degradation of Rab27a. Nature Communications. 10(1).
Villarroya-Beltri, C., Gutiérrez-Vázquez, C., Sánchez-Cabo, F., Pérez-Hernández, D., Vázquez, J., Martin-Cofreces, N., & Martinez-Herrera, D. J. (2013). Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nature Communications, 4, 2980.
Ji, R., Cheng, Y., Yue, J., Yang, J., Liu, X., Chen, H., Dean, D. B., & Zhang, C. (2007). MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular Neointimal lesion formation. Circulation Research, 100(11), 1579–1588.
Todorova, D., Simoncini, S., Lacroix, R., Sabatier, F., & Dignat-George, F. (2017). Extracellular vesicles in angiogenesis. Circulation Research, 120(10), 1658–1673.
Lazar, E., Benedek, T., Korodi, S., Rat, N., Lo, J., & Benedek, I. (2018). Stem cell-derived exosomes - an emerging tool for myocardial regeneration. World J Stem Cell, 10(8), 106–115.
Barile, L., Lionetti, V., Cervio, E., Matteucci, M., Gherghiceanu, M., Popescu, L. M., & Torre, T. (2014). Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovascular Research, 103(41), 530–541.
Hinkel, R., Penzkofer, D., Zühlke, S., Fischer, A., & Husada, W. (2013). Inhibition of MicroRNA-92a protects against ischemia-reperfusion injury in a large animal model. Circulation, 128(10), 1066–1075.
Yamakuchi, M., Lotterman, C. D., Bao, C., Hruban, R. H., Karim, B., Mendell, J. T., Huso, D., & Lowenstein, C. J. (2010). P53-induced microRNA-107 inhibits HIF-1 and tumor angiogenesis. Proceedings of the National Academy of Sciences of the United States of America, 107(14), 6334–6339.
Mocharla, P., Briand, S., Giannotti, G., Dörries, C., Jakob, P., Paneni, F., Lüscher, T., & Landmesser, U. (2013). AngiomiR-126 expression and secretion from circulating CD34+ and CD14+ PBMCs: role for proangiogenic effects and alterations in type 2 diabetics. Blood, 121(1), 226–236.
Wu, K., Yang, Y., Zhong, Y., Ammar, H. M., Zhang, P., Guo, R., Liu, H., Cheng, C., Koroscil, T. M., Chen, Y., Liu, S., & Bihl, J. C. (2016). The effects of microvesicles on endothelial progenitor cells are compromised in type 2 diabetic patients via downregulation of the miR-126/VEGFR2 pathway. American Journal of Physiology-Endocrinology and Metabolism, 310(10), E828–E837.
Sun, Y., Liu, X.-l., Zhang, D., Liu, F., Cheng, Y.-j., Ma, Y., Zhou, Y.-j., & Zhao, Y.-x. (2019). Platelet-derived exosomes affect the proliferation and migration of human umbilical vein endothelial cells via miR-126. Current Vascular Pharmacology, 17(4), 379–387.
Pan, Q., Wang, Y., Lan, Q., Wu, W., Li, Z., Ma, X., & Yu, L. (2019). Exosomes derived from mesenchymal stem cells ameliorate hypoxia/reoxygenation-injured ECs via transferring MicroRNA-126. Stem Cells International, 2019, 2831756.
Li, J. W., Wei, L., Han, Z., & Chen, Z. (2019). Mesenchymal stromal cells-derived exosomes alleviate ischemia/reperfusion injury in mouse lung by transporting anti-apoptotic miR-21-5p. European Journal of Pharmacology, 852, 68–76.
Hu, H., Wang, B., Jiang, C., Li, R., & Zhao, J. (2019). Endothelial progenitor cell-derived exosomes facilitate vascular endothelial cell repair through shuttling miR-21-5p to modulate Thrombospondin-1 expression. Clinical Science, 133(14), 1629–1644.
Ma, X., Wang, J., Li, J., Ma, C., Chen, S., Lei, W., Yang, Y., & Liu, S. (2018). Loading MiR-210 in endothelial progenitor cells derived exosomes boosts their beneficial effects on hypoxia/reoxygeneation-injured human endothelial cells via protecting mitochondrial function. Cellular Physiology and Biochemistry, 46(2), 664–675.
Ma, T., Chen, Y., Chen, Y., Meng, Q., Sun, J., Shao, L., Yu, Y., Huang, H., Hu, Y., Yang, Z., Yang, J., & Shen, Z. (2018). MicroRNA-132, delivered by mesenchymal stem cell-derived exosomes, promote angiogenesis in myocardial infarction. Stem Cells International, 2018, 3290372.
Youn, S.-W., Li, Y., Kim, Y.-M., Sudhahar, V., Abdelsaid, K., Kim, H., Liu, Y., Fulton, D., Ashraf, M., Tang, Y., Fukai, T., & Ushio-Fukai, M. (2019). Modification of cardiac progenitor cell-derived exosomes by miR-322 provides protection against myocardial infarction through Nox2-dependent angiogenesis. Antioxidants-Basel, 8(1), 18.
Liu, L., Liu, Y., Feng, C., Chang, J., Fu, R., Wu, T., Yu, F., Wang, X., Xia, L., Wu, C., & Fang, B. (2019). Lithium-containing biomaterials stimulate bone marrow stromal cell-derived exosomal miR-130a secretion to promote angiogenesis. Biomaterials, 192, 523–536.
Ribeiro-Rodrigues, T. M., Laundos, T. L., Pereira-Carvalho, R., Batista-Almeida, D., Pereira, R., Coelho-Santos, V., Silva, A. P., Fernandes, R., Zuzarte, M., Enguita, F. J., Costa, M. C., Pinto-do-Ó, P., Pinto, M. T., Gouveia, P., Ferreira, L., Mason, J. C., Pereira, P., Kwak, B. R., Nascimento, D. S., & Girão, H. (2017). Exosomes secreted by cardiomyocytes subjected to ischaemia promote cardiac angiogenesis. Cardiovascular Research, 113(11), 1338–1350.
Balkom, B. W. M. V., Jong, O. G. D., Smits, M., Brummelman, J., Ouden, K. D., Bree, P. M. D., Eijndhoven, M. A. J. V., & Pegtel, D. M. (2013). Endothelial cells require miR-214 to secrete exosomes that suppress senescence and induce angiogenesis in human and mouse endothelial cells. Blood, 121(19), 3997–4006.
Gollmann-Tepeköylü, C., Pölzl, L., Graber, M., Hirsch, J., Nägele, F., & Lobenwein, D. (2020). miR-19a-3p containing exosomes improve function of ischemic myocardium upon shock wave therapy. Cardiovascular Research, 116(6), 1226–1236.
Liang, X., Zhang, L., Wang, S., Han, Q., & Zhao, R. C. (2016). Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR-125a. Journal of Cell Science, 129(11), 2182–2189.
Du, L., Li, G., Yang, Y., Yang, G., Wan, J., Ma, Z., & Hou, Y. (2018). Exosomes from microRNA-199-3p-modified adipose-derived stem cells promote proliferation and migration of endothelial tip cells by downregulation of semaphorin 3A. International Journal of Clinical and Experimental Pathology, 11(10), 4879–4888.
An, Y., J. Zhao, F. Nie, Z. Qin, H. Xue, G. Wang and D. Li. Exosomes from adipose-derived stem cells (ADSCs) overexpressing miR-21 promote vascularization of endothelial cells. Scientific Reports-UK; 2019. 9(1).
Zhang, Y., Liu, D., Chen, X., Li, J., Li, L., Bian, Z., Sun, F., Lu, J., Yin, Y., Cai, X., Sun, Q., Wang, K., Ba, Y., Wang, Q., Wang, D., Yang, J., Liu, P., Xu, T., Yan, Q., Zhang, J., Zen, K., & Zhang, C.-Y. (2010). Secreted monocytic miR-150 enhances targeted endothelial cell migration. Molecular Cell, 39(1), 133–144.
Gong, M., Yu, B., Wang, J., Wang, Y., Liu, M., Paul, C., & Millard, R. W. (2017). Mesenchymal stem cells release exosomes that transfer miRNAs to endothelial cells and promote angiogenesis. Oncotarget, 8(28), 45200–45212.
Beltrami, C., Besnier, M., Shantikumar, S., Shearn, A. I. U., Rajakaruna, C., Laftah, A., Sessa, F., Spinetti, G., Petretto, E., Angelini, G. D., & Emanueli, C. (2017). Human pericardial fluid contains exosomes enriched with cardiovascular-expressed MicroRNAs and promotes therapeutic angiogenesis. Molecular Therapy, 25(3), 679–693.
Wang, X., Huang, W., Liu, G., Cai, W., Millard, R. W., Wang, Y., Chang, J., Peng, T., & Fan, G.-C. (2014). Cardiomyocytes mediate anti-angiogenesis in type 2 diabetic rats through the exosomal transfer of miR-320 into endothelial cells. Journal of Molecular and Cellular Cardiology, 74, 139–150.
Li, H., Liao, Y., Gao, L., Zhuang, T., Huang, Z., Zhu, H., & Ge, J. (2018). Coronary serum exosomes derived from patients with myocardial ischemia regulate angiogenesis through the miR-939-mediated nitric oxide signaling pathway. Theranostics, 8(8), 2079–2093.
Li, Y., Liang, J., Hu, J., Ren, X., & Sheng, Y. (2018). Down-regulation of exosomal miR-106b-5p derived from cholesteatoma perimatrix fibroblasts promotes angiogenesis in endothelial cells by overexpression of angiopoietin 2. Cell Biology International, 42(10), 1300–1310.
Zheng, B., Yin, W.-n., Suzuki, T., Zhang, X.-h., Zhang, Y., Song, L.-l., Jin, L.-s., Zhan, H., Zhang, H., Li, J.-s., & Wen, J.-k. (2017). Exosome-mediated miR-155 transfer from smooth muscle cells to endothelial cells induces endothelial injury and promotes atherosclerosis. Molecular Therapy, 25(6), 1279–1294.
Yang, H.-H., Chen, Y., Gao, C.-Y., Cui, Z.-T., & Yao, J.-M. (2017). Protective effects of MicroRNA-126 on human cardiac microvascular endothelial cells against hypoxia/Reoxygenation-induced injury and inflammatory response by activating PI3K/Akt/eNOS signaling pathway. Cellular Physiology and Biochemistry, 42(2), 506–518.
Fish, J. E., Santoro, M. M., Morton, S. U., Yu, S., Yeh, R.-F., Wythe, J. D., Ivey, K. N., Bruneau, B. G., Stainier, D. Y. R., & Srivastava, D. (2008). miR-126 regulates angiogenic signaling and vascular integrity. Developmental Cell, 15(2), 272–284.
Fasanaro, P., D'Alessandra, Y., Di Stefano, V., Melchionna, R., Romani, S., Pompilio, G., Capogrossi, M. C., & Martelli, F. (2008). MicroRNA-210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand Ephrin-A3. Journal of Biological Chemistry, 283(23), 15878–15883.
Mutharasan, R. K., Nagpal, V., Ichikawa, Y., & Ardehali, H. (2011). microRNA-210 is upregulated in hypoxic cardiomyocytes through Akt- and p53-dependent pathways and exerts cytoprotective effects. American Journal of Physiology. Heart and Circulatory Physiology, 301(4), H1519–H1530.
Katare, R., Riu, F., Mitchell, K., Gubernator, M., Campagnolo, P., Cui, Y., Fortunato, O., & Avolio, E. (2011). Transplantation of human pericyte progenitor cells improves the repair of infarcted heart through activation of an angiogenic program involving micro-RNA-132. Circulation Research, 109(8), 894–906.
Ghosh, G., Subramanian, I. V., Adhikari, N., Zhang, X., Joshi, H. P., & Basi, D. (2010). Hypoxia-induced microRNA-424 expression in human endothelial cells regulates HIF-α isoforms and promotes angiogenesis. The Journal of Clinical Investigation, 120(11), 4141–4154.
Zeng, Y., Liu, H., Kang, K., Wang, Z., Hui, G., Zhang, X., Zhong, J., Peng, W., Ramchandran, R., Usha Raj, J., & Gou, D. (2015). Hypoxia inducible factor-1 mediates expression of miR-322: potential role in proliferation and migration of pulmonary arterial smooth muscle cells. Scientific Reports-UK, 5(1).
Chen, Y., & Gorski, D. H. (2008). Regulation of angiogenesis through a microRNA(miR-130a) that down-regulates antiangiogenic homeobox genes GAX and HOXA5. Blood, 111(3), 217–226.
Dougherty, J. A., Kumar, N., Noor, M., Angelos, M. G., Khan, M., Chen, C.-A., & Khan, M. (2017). Extracellular vesicles released by human induced-pluripotent stem cell-derived cardiomyocytes promote angiogenesis. European Heart Journal, 9, 1794.
Anfossi, S., Giordano, A., Gao, H., Cohen, E. N., Tin, S., & Wu, Q. (2014). High serum miR-19a levels are associated with inflammatory breast cancer and are predictive of favorable clinical outcome in patients with metastatic HER2+ inflammatory breast cancer. PLoS One, 9(1), e83113.
Zhou, F., Gao, S., Wang, L., Sun, C., Chen, L., Yuan, P., Zhao, H., & Yi, Y. (2015). Human adipose-derived stem cells partially rescue the stroke syndromes by promoting spatial learning and memory in mouse middle cerebral artery occlusion model. Stem Cell Research & Therapy, 6(1), 92.
Hermansen, S. K., Nielsen, B. S., Aaberg-Jessen, C., & Kristensen, B. W. (2015). miR-21 is linked to glioma angiogenesis. The Journal of Histochemistry and Cytochemistry, 64(2), 138–148.
Liu, Y., Luo, F., Wang, B., Li, H., Xu, Y., Liu, X., Shi, L., Lu, X., Xu, W., Lu, L., Qin, Y., Xiang, Q., & Liu, Q. (2016). STAT3-regulated exosomal miR-21 promotes angiogenesis and is involved in neoplastic processes of transformed human bronchial epithelial cells. Cancer Letters, 370(1), 125–135.
Xiao, C., Calado, D. P., Galler, G., Thai, T.-H., Patterson, H. C., Wang, J., Rajewsky, N., Bender, T. P., & Rajewsky, K. (2016). MiR-150 controls B cell differentiation by targeting the transcription factor c-Myb. Cell, 165(4), 1027.
Wang, X. H., Qian, R. Z., Zhang, W., Chen, S. F., Jin, H. M., & Hu, R. M. (2009). MicroRNA-320 expression in myocardial microvascular endothelial cells and its relationship with insulin-like growth factor-1 in type 2 diabetic rats. Clinical and Experimental Pharmacology and Physiology, 36(2), 181–188.
Hou, S., Fang, M., Zhu, Q., Liu, Y., Liu, L., & Li, X. (2017). MicroRNA-939 governs vascular integrity and angiogenesis through targeting γ-catenin in endothelial cells. Biochemical and Biophysical Research Communications, 484(1), 27–33.
Shindo, T., Manabe, I., Fukushima, Y., Tobe, K., Aizawa, K., Miyamoto, S., Kawai-Kowase, K., Moriyama, N., Imai, Y., Kawakami, H., Nishimatsu, H., Ishikawa, T., Suzuki, T., Morita, H., Maemura, K., Sata, M., Hirata, Y., Komukai, M., Kagechika, H., Kadowaki, T., Kurabayashi, M., & Nagai, R. (2002). Krüppel-like zinc-finger transcription factor KLF5/BTEB2 is a target for angiotensin II signaling and an essential regulator of cardiovascular remodeling. Nature Medicine, 8(8), 856–863.
Nazari-Jahantigh, M., Wei, Y., Noels, H., Akhtar, S., Zhou, Z., Koenen, R. R., & Heyll, K. (2012). MicroRNA-155 promotes atherosclerosis by repressing Bcl6 in macrophages. The Journal of Clinical Investigation, 122(11), 4190–4202.
Kosaka, N., Iguchi, H., Hagiwara, K., Yoshioka, Y., Takeshita, F., & Ochiya, T. (2013). Neutral sphingomyelinase 2 (nSMase2)-dependent exosomal transfer of angiogenic microRNAs regulate cancer cell metastasis. The Journal of Biological Chemistry, 288(15), 10849–10859.
Umezu, T., Imanishi, S., Azuma, K., Kobayashi, C., Yoshizawa, S., Ohyashiki, K., & Ohyashiki, J. H. (2017). Replenishing exosomes from older bone marrow stromal cells with miR-340 inhibits myeloma-related angiogenesis. Blood Advances, 1(13), 812–823.
Umezu, T., Tadokoro, H., Azuma, K., Yoshizawa, S., Ohyashiki, K., & Ohyashiki, J. H. (2014). Exosomal miR-135b shed from hypoxic multiple myeloma cells enhances angiogenesis by targeting factor-inhibiting HIF-1. Blood, 124(25), 3748–3757.
Lu, J., Liu, Q.-H., Wang, F., Tan, J.-J., Deng, Y.-Q., Peng, X.-H., Liu, X., Zhang, B., Xu, X., & Li, X.-P. (2018). Exosomal miR-9 inhibits angiogenesis by targeting MDK and regulating PDK/AKT pathway in nasopharyngeal carcinoma. Journal of Experimental & Clinical Cancer Research, 37(1), 147.
Bao, L., You, B., Shi, S., Shan, Y., Zhang, Q., Yue, H., Zhang, J., Zhang, W., Shi, Y., Liu, Y., Wang, X., Liu, D., & You, Y. (2018). Metastasis-associated miR-23a from nasopharyngeal carcinoma-derived exosomes mediates angiogenesis by repressing a novel target gene TSGA10. Oncogene, 37(21), 2873–2889.
Zhang, L., Li, H., Yuan, M., Li, M., & Zhang, S. (2019). Cervical cancer cells-secreted exosomal microRNA-221-3p promotes invasion, migration and angiogenesis of microvascular endothelial cells in cervical cancer by down-regulating MAPK10 expression</p>. Cancer Management and Research, 11, 10307–10319.
Wu, X.-G., Zhou, C.-F., Zhang, Y.-M., Yan, R.-M., Wei, W.-F., Chen, X.-J., Yi, H.-Y., Liang, L.-J., Fan, L.-s., Liang, L., Wu, S., & Wang, W. (2019). Cancer-derived exosomal miR-221-3p promotes angiogenesis by targeting THBS2 in cervical squamous cell carcinoma. ANGIOGENESIS, 22(3), 397–410.
Sun, X., Ma, X., Wang, J., Zhao, Y., Wang, Y., Bihl, J. C., Chen, Y., & Jiang, C. (2017). Glioma stem cells-derived exosomes promote the angiogenic ability of endothelial cells through miR-21/VEGF signal. Oncotarget, 8(22), 36137–36148.
Wang, Z.-F., Liao, F., Wu, H. and Dai J.. (2019) Glioma stem cells-derived exosomal miR-26a promotes angiogenesis of microvessel endothelial cells in glioma. Journal of Experimental & Clinical Cancer Research. 38(1).
Pakravan, K., Babashah, S., Sadeghizadeh, M., Mowla, S. J., Mossahebi-Mohammadi, M., Ataei, F., Dana, N., & Javan, M. (2017). MicroRNA-100 shuttled by mesenchymal stem cell-derived exosomes suppresses in vitro angiogenesis through modulating the mTOR/HIF-1α/VEGF signaling axis in breast cancer cells. Cellular Oncology, 40(5), 457–470.
Zhou, Z., Zhang, H., Deng, T., Ning, T., Liu, R., Liu, D., Bai, M., Ying, G., & Ba, Y. (2019). Exosomes carrying MicroRNA-155 target Forkhead box O3 of endothelial cells and promote angiogenesis in gastric cancer. Molecular Therapy - Oncolytics, 15, 223–233.
Deng, T., Zhang, H., Yang, H., Wang, H., Bai, M., Sun, W., Wang, X., Si, Y., Ning, T., Zhang, L., Li, H., Ge, S., Liu, R., Lin, D., Li, S., Ying, G., & Ba, Y. (2020). Exosome miR-155 derived from gastric carcinoma promotes angiogenesis by targeting the c-MYB/VEGF Axis of endothelial cells. Molecular Therapy--Nucleic Acids, 19, 1449–1459.
Bai, M., Li, J., Yang, H., Zhang, H., Zhou, Z., Deng, T., Zhu, K., Ning, T., Fan, Q., Ying, G., & Ba, Y. (2019). miR-135b delivered by gastric tumor exosomes inhibits FOXO1 expression in endothelial cells and promotes angiogenesis. Molecular Therapy, 27(10), 1772–1783.
Yang, H., Zhang, H., Ge, S., Ning, T., Bai, M., Li, J., Li, S., Sun, W., Deng, T., Zhang, L., Ying, G., & Ba, Y. (2018). Exosome-derived miR-130a activates angiogenesis in gastric cancer by targeting C-MYB in vascular endothelial cells. Molecular Therapy, 26(10), 2466–2475.
He, L., Zhu, W., Chen, Q., Yuan, Y., Wang, Y., Wang, J., & Wu, X. (2019). Ovarian cancer cell-secreted exosomal miR-205 promotes metastasis by inducing angiogenesis. Theranostics, 9(26), 8206–8220.
Masoumi-Dehghi, S., Babashah, S. and Sadeghizadeh, M.. (2020) microRNA-141-3p-containing small extracellular vesicles derived from epithelial ovarian cancer cells promote endothelial cell angiogenesis through activating the JAK/STAT3 and NF-κB signaling pathways. Journal of Cell Communication and Signaling .
Matsuura, Y., Wada, H., Eguchi, H., Gotoh, K., Kobayashi, S., Kinoshita, M., Kubo, M., Hayashi, K., Iwagami, Y., Yamada, D., Asaoka, T., Noda, T., Kawamoto, K., Takeda, Y., Tanemura, M., Umeshita, K., Doki, Y., & Mori, M. (2018). Exosomal miR-155 derived from hepatocellular carcinoma cells under hypoxia promotes angiogenesis in endothelial cells. Digestive Diseases and Sciences, 64(3), 792–802.
Hu, H.-Y., Yu, C.-H., Zhang, H.-H., Zhang, S.-Z., Yu, W.-Y., Yang, Y., & Chen, Q. (2019). Exosomal miR-1229 derived from colorectal cancer cells promotes angiogenesis by targeting HIPK2. International Journal of Biological Macromolecules, 132, 470–477.
Shang, D., Xie, C., Hu, J., Tan, J., Yuan, Y., Liu, Z., & Yang, Z. (2020). Pancreatic cancer cell–derived exosomal microRNA-27a promotes angiogenesis of human microvascular endothelial cells in pancreatic cancer via BTG2. Journal of Cellular and Molecular Medicine, 24(1), 588–604.
Xu, H.-J., Liao, W., Liu, X.-Z., Hu, J., Zou, W.-Z., Ning, Y., Yang, Y., & Li, Z.-H. (2019). Down-regulation of exosomal microRNA-224-3p derived from bone marrow–derived mesenchymal stem cells potentiates angiogenesis in traumatic osteonecrosis of the femoral head. The FASEB Journal, 33(7), 8055–8068.
Zuo, R., Kong, L., Wang, M., Wang, W., Xu, J., Chai, Y., Guan, J., & Kang, Q. (2019). Exosomes derived from human CD34+ stem cells transfected with miR-26a prevent glucocorticoid-induced osteonecrosis of the femoral head by promoting angiogenesis and osteogenesis. Stem Cell Research & Therapy, 10(1), 321.
Yang, Y., Cai, Y., Zhang, Y., Liu, J., & Xu, Z. (2018). Exosomes secreted by adipose-derived stem cells contribute to angiogenesis of brain microvascular endothelial cells following oxygen–glucose deprivation in vitro through MicroRNA-181b/TRPM7 Axis. Journal of Molecular Neuroscience, 65(1), 74–83.
Huang, J. H., Xu, Y., Yin, X. M., & Lin, F. Y. (2020). Exosomes derived from miR-126-modified MSCs promote angiogenesis and neurogenesis and attenuate apoptosis after spinal cord injury in rats. Neuroscience, 424, 133–145.
Sahoo, S., & Losordo, D. W. (2014). Exosomes and cardiac repair after myocardial infarction. Circulation Research, 114(2), 333–344.
Khan, M., Nickoloff, E., Abramova, T., Johnson, J., Verma, S. K., Krishnamurthy, P., & Mackie, A. R. (2015). Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circulation Research, 117(11), 52–64.
Ibrahim, A. G., Cheng, K., & Marban, E. (2014). Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Reports, 2(5), 606–619.
Chen, L., Hu, L., Li, Q., Ma, J., & Li, H. (2019). Exosome-encapsulated miR-505 from ox-LDL-treated vascular endothelial cells aggravates atherosclerosis by inducing NET formation. Acta Biochimica et Biophysica Sinica Shanghai, 51(12), 1233–1241.
Hou, Z., Qin, X., Hu, Y., Zhang, X., Li, G., Wu, J., Li, J., Sha, J., Chen, J., Xia, J., Wang, L., & Gao, F. (2019). Longterm exercise-derived exosomal miR-342-5p. Circulation Research, 124(9), 1386–1400.
Chen, Q., Liu, Y., Ding, X., Li, Q., Qiu, F., Wang, M., & Shen, Z. (2020). Bone marrow mesenchymal stem cell-secreted exosomes carrying microRNA-125b protect against myocardial ischemia reperfusion injury via targeting SIRT7. Molecular and Cellular Biochemistry, 465(1–2), 103–114.
Qiao, L., Hu, S., Liu, S., Zhang, H., Ma, H., Huang, K., Li, Z., Su, T., Vandergriff, A., Tang, J., Allen, T., Dinh, P.-U., Cores, J., Yin, Q., Li, Y., & Cheng, K. (2019). microRNA-21-5p dysregulation in exosomes derived from heart failure patients impairs regenerative potential. The Journal of Clinical Investigation, 129(6), 2237–2250.
Halkein, J., Tabruyn, S. P., Ricke-Hoch, M., Haghikia, A., Nguyen, N.-Q.-N., Scherr, M., Castermans, K., Malvaux, L., Lambert, V., Thiry, M., Sliwa, K., Noel, A., Martial, J. A., Hilfiker-Kleiner, D., & Struman, I. (2013). MicroRNA-146a is a therapeutic target and biomarker for peripartum cardiomyopathy. The Journal of Clinical Investigation, 123(5), 2143–2154.
Shi, B., Wang, Y., Zhao, R., Long, X., Deng, W., & Wang, Z. (2018). Bone marrow mesenchymal stem cell-derived exosomal miR-21 protects C-kit+ cardiac stem cells from oxidative injury through the PTEN/ PI3K/Akt axis. PLoS One, 13(2), e0191616.
Bang, C., Batkai, S., Dangwal, S., Gupta, S. K., & Foinquinos, A. (2014). Cardiac fibroblast–derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. The Journal of Clinical Investigation, 124(5), 2136–2146.
Zhu, J., Lu, K., Zhang, N., Zhao, Y., Ma, Q., Shen, J., Lin, Y., Xiang, P., Tang, Y., Hu, X., Chen, J., Zhu, W., Webster, K. A., Wang, J.a., & Yu, H. (2017). Myocardial reparative functions of exosomes from mesenchymal stem cells are enhanced by hypoxia treatment of the cells via transferring microRNA-210 in an nSMase2-dependent way. Artificial Cells, Nanomedicine, and Biotechnology, 1–12.
Jopling, C. L., Yi, M., Lancaster, A. M., Lemon, S. M., & Sarnow, P. (2005). Modulation of hepatitis C virus RNA abundance by a liver-specific MicroRNA. Science, 309(5740), 1577–1581.
Adachi, T., Nakanishi, M., Otsuka, Y., Nishimura, K., Hirokawa, G., Goto, Y., Nonogi, H., & Iwai, N. (2010). Plasma MicroRNA 499 as a biomarker of acute myocardial infarction. Clinical Chemistry, 56(7), 1183–1185.
Park, N. J., Zhou, H., Elashoff, D., Henson, B. S., Kastratovic, D. A., Abemayor, E., & Wong, D. T. (2009). Salivary microRNA: discovery, characterization, and clinical utility for oral cancer detection. Clinical Cancer Research, 15(17), 5473–5477.
Barile, L., Moccetti, T., Marbán, E. and Vassalli, G.. (2016) Roles of exosomes in cardioprotection. EUR HEART J ehw304.
Yang, T., Martin, P., Fogarty, B., Brown, A., Schurman, K., Phipps, R., Yin, V. P., Lockman, P., & Bai, S. (2015). Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio Rerio. Pharmaceutical Research (PHARM RES-DORDR), 32(6), 2003–2014.
Cheng, Y., Wang, X., Yang, J., Duan, X., Yao, Y., Shi, X., Chen, Z., Fan, Z., Liu, X., Qin, S., Tang, X., & Zhang, C. (2012). A translational study of urine miRNAs in acute myocardial infarction. Journal of Molecular and Cellular Cardiology, 53(5), 668–676.
Yang, Y., Rodriguez, J. E., & Kitsis, R. N. (2013). A microRNA links prolactin to peripartum cardiomyopathy. The Journal of Clinical Investigation, 123(5), 1925–1927.
Krützfeldt, J., Rajewsky, N., Braich, R., Rajeev, K. G., Tuschl, T., Manoharan, M., & Stoffel, M. (2005). Silencing of microRNAs in vivo with ‘antagomirs’. Nature, 438(7068), 685–689.
Morrissey, D. V., Lockridge, J. A., Shaw, L., Blanchard, K., Jensen, K., & Breen, W. (2005). Potent and persistent in vivo anti-HBV activity of chemically modified siRNAs. Nature Biotechnology, 23(8), 1002–1007.
Yang, Z., Shi, J., Xie, J., Wang, Y., Sun, J., Liu, T., Zhao, Y., Zhao, X., Wang, X., Ma, Y., Malkoc, V., Chiang, C., Deng, W., Chen, Y., Fu, Y., Kwak, K. J., Fan, Y., Kang, C., Yin, C., Rhee, J., Bertani, P., Otero, J., Lu, W., Yun, K., Lee, A. S., Jiang, W., Teng, L., Kim, B. Y. S., & Lee, L. J. (2019). Large-scale generation of functional mRNA-encapsulating exosomes via cellular nanoporation. Nature Biomedical Engineering, 4(1), 69–83.
Farooqi, A. A., Desai, N. N., Qureshi, M. Z., Librelotto, D. R. N., Gasparri, M. L., Bishayee, A., & Nabavi, S. M. (2018). Exosome biogenesis, bioactivities and functions as new delivery systems of natural compounds. Biotechnology Advances, 36(1), 328–334.
Luo, Q., Guo, D., Liu, G., Chen, G., Hang, M., & Jin, M. (2017). Exosomes from MiR-126-overexpressing Adscs are therapeutic in relieving acute myocardial ischaemic injury. Cellular Physiology and Biochemistry, 44(6), 2105–2116.
Sodi, R., Eastwood, J., Caslake, M., Packard, C. J., & Denby, L. (2017). Relationship between circulating microRNA-30c with total- and LDL-cholesterol, their circulatory transportation and effect of statins. Clinica Chimica Acta, 466, 13–19.
Wang, Y., Zhao, R., Liu, W., Wang, Z., Rong, J., Long, X., Liu, Z., Ge, J., & Shi, B. (2019). Exosomal circHIPK3 released from hypoxia-pretreated cardiomyocytes regulates oxidative damage in cardiac microvascular endothelial cells via the miR-29a/IGF-1 pathway. Oxidative Medicine and Cellular Longevity, 2019, 1–28.
Gallet, R., Dawkins, J., Valle, J., Simsolo, E., Couto, G. D., Middleton, R., Tseliou, E., & Luthringer, D. (2017). Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcinemyocardial infarction. European Heart Journal, 38(3), 201–211.
Kwekkeboom, R. F. J., Sluijter, J. P. G., van Middelaar, B. J., Metz, C. H., Brans, M. A., Kamp, O., Paulus, W. J., & Musters, R. J. P. (2016). Increased local delivery of antagomir therapeutics to the rodent myocardium using ultrasound and microbubbles. Journal of Controlled Release, 222, 18–31.
Funding
The study was supported by the National Natural Science Foundation of China (no. 81760415), the Guangxi Natural Science Foundation (no. 2017GXNSFAA198147) and the Promotional Project of Guangxi Medical and Health Appropriate Technology (no. S201518).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Associate Editor Junjie Xiao oversaw the review of this article
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, TR., Huang, WQ. Angiogenic Exosome-Derived microRNAs: Emerging Roles in Cardiovascular Disease. J. of Cardiovasc. Trans. Res. 14, 824–840 (2021). https://doi.org/10.1007/s12265-020-10082-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-020-10082-9