Abstract
This study investigated whether vagal nerve stimulation (VNS) leads to improvements in ischemic heart failure via heart rate modulation. At 7 ± 1 days post left anterior descending artery (LAD) ligation, 63 rats with myocardial infarctions (MI) were implanted with ECG transmitters and VNS devices (MI + VNS, N = 44) or just ECG transmitters (MI, N = 17). VNS stimulation was active from 14 ± 1 days to 8 ± 1 weeks post MI. The average left ventricular (LV) end diastolic volumes at 8 ± 1 weeks were MI = 672.40 μl and MI + VNS = 519.35 μl, p = 0.03. The average heart weights, normalized to body weight (±std) at 14 ± 1 weeks were MI = 3.2 ± 0.6 g*kg−1 and MI + VNS = 2.9 ± 0.3 g*kg−1, p = 0.03. The degree of cardiac remodeling was correlated with the magnitude of acute VNS-evoked heart rate (HR) changes. Further research is required to determine if the acute heart rate response to VNS activation is useful as a heart failure biomarker or as a tool for VNS therapy characterization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vagal nerve stimulation (VNS) is being investigated as a potential therapy for heart failure [1–4]. Pre-clinical studies have shown that chronic VNS can blunt heart failure progression [1, 5–7]. However, whether the improvements in myocardial remodeling resulting from chronic VNS are related to the heart rate responses evoked during VNS has not been elucidated. Efferent vagus nerve fibers modulate heart rate directly through innervation of the SA node, while afferent fibers modulate heart rate centrally in the brain stem. The first chronic heart failure study of VNS performed by Li et al. intentionally decreased heart rate by approximately 10 % in rodents and found a 73 % reduction in mortality [7]. Additionally, left ventricular (LV) ejection fraction (EF) was increased, and LV volumes were decreased by VNS applied via a closed loop system that lowered heart rate in a canine microembolization-induced heart failure (HF) model [6]. In contrast, Hamann et al. found similar cardiovascular improvements in a canine microembolization-induced HF model when applying VNS in an open loop manner that did not alter heart rate [5]. The findings of Hamann et al. suggest that acute heart rate reductions during VNS stimulation may not be essential for the amelioration of heart failure progression. However, it is unclear whether the benefits of VNS are enhanced by the presence of VNS-evoked heart rate changes. Using a post myocardial infarction (MI) rat model of heart failure and an implanted VNS system, we further investigated the association between VNS-mediated heart rate changes and heart failure progression.
Methods
Experimental Procedures
All animal experiments were performed in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals and approved by the Boston Scientific Institutional Animal Care and Use Committee.
Male Sprague Dawley rats were randomized to MI (N = 161) or sham (N = 4) procedures. Briefly, an incision was made to create a small opening into the thoracic cavity after which the exposed pericardium was completely opened. A MI was then created by permanently ligating the left anterior descending (LAD) coronary artery. In the sham group, the identical surgical procedures were carried out except for the ligation of the LAD. Fifty-five (34 %) of the rats died during the first week after ligation. At 7 ± 1 days, 63 rats had an infarct affecting >35 % of the LV by visual estimation of echocardiographic images (59 % of rats surviving 1 week) and were randomized in a 13:5 ratio to receiving a VNS device (MI + VNS, N = 44) or not (MI-No Tx, N = 17). Forty-three rats had infarcts <35 % and were euthanized. Two rats were euthanized due to a lack of available devices. Infarct sizes were quantified by off-line measurement of the percentage of the LV with akinesis or dyskinesis at the basal, mid-ventricular (papillary muscle level), and apical levels.
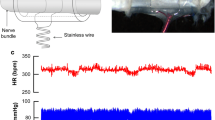
All surviving rats were implanted at 7 ± 1 days post MI with ECG transmitters (TA10CA-F40, Data Sciences International, St. Paul, MN). Rats randomized to receive VNS also received a fully implantable small animal VNS system. The system consisted of two circular platinum electrodes, each 0.75 mm in diameter, set on opposing inner walls of a 5-mm segment of silicone tubing with an inner diameter of 0.75 mm. Each electrode was independently connected to 0.001" coiled wire that was strung through another segment of biluminal silicone tubing and connected to a pulse generator. The VNS cuff was placed around the cervical vagus nerve and secured with a suture. A strain relief cuff was placed around the VNS cuff as well as the carotid artery and secured with a suture. Appropriate lead placement and system functionality were confirmed by an ability to induce respiratory and heart rate responses with stimulation. The VNS system was turned off after implant testing until the start of the therapy window at 14 ± 1 days post MI. The VNS stimulation had the capacity to be programmed with a magnet to no output or to 0.2-ms duration electrical pulses of between 50 and 250 microAmps at 20 Hz for 10 s of every minute (each 60 s period was called a trial; 1440 trials = 1 day). The VNS was activated at 14 ± 1 days post MI and up titrated, with ECG monitoring, until overt changes in heart rate were observed or to 250 microAmps when no heart rate changes were acutely observable.
Data Collection and Analysis
At 7 ± 1 days, 8 ± 1 weeks, and 14 ± 1 weeks post MI, the animals were weighed and echocardiographic images were collected. At 8 ± 1 weeks, VNS stimulation was turned off, and at 14 ± 1 weeks post MI, the rats were euthanized, hearts were weighed, and the length of the right tibia was measured.
Continuous heart rate (HR) signals r(t) were extracted from the ECGs by detecting R-peaks (supplementary materials):
where t i s are R-peak times. r(t) was then sampled at 0.1 s and low pass filtered at 1 Hz (averaging at 1 s) using a two-pole forward-backward Butterworth filter to remove high frequency noise. Each HR value (at every 0.1 s) was then converted into a percentage change by subtracting the mean HR of the corresponding trial (60 s) and normalizing by the mean HR of the corresponding trial then multiplying by 100. A Z-score was produced in similar fashion, by subtracting the mean HR of the corresponding trial and normalizing by the standard deviation of the corresponding trial. Three-dimensional heat maps were then produced by assigning each 0.1-s period to a pixel that was colored according to the percent change or Z-score, with red color indicating a high value and a blue color indicating a low value. Each trial was considered to have a discernible VNS-evoked HR increase or decrease when the 10-s average Z-score for the period surrounding the maximum and minimum Z-scores for that trial was less than −1 or greater than 1. The values exceeding ±1 were considered “significant” because we estimated that noise in Z-scores to be correlated up to 1 s. Therefore, a 10-s averaged Z-score value has a standard deviation of \( \sqrt{\frac{1 \sec }{10 \sec }}=0.32 \).
All results were presented as mean and 95 % confidence interval (95 % CI) unless otherwise indicated in the text. Ninety-five percent confidence intervals were calculated by subtracting and adding two standard errors (±2*SE). t tests (unequal variance) and paired t tests were used for computing statistical significance. All analyses were done in Matlab R2014a (Mathworks, Natick MA).
Results
MI Creation and Device Implantation
Sixty-one rats with significant MIs were enrolled in the study (Fig. 1). The infarct sizes were similar for the MI (30 ± 9 % mean ± std, N = 17) and MI + VNS (32 ± 5 % mean ± std, N = 36) groups (p = 0.43). Six rats died on the table during the VNS system implantation, one died while being monitored post surgery, and one was found dead in its cage 4 days after the surgery. There were no perioperative deaths in the groups receiving the telemetry devices alone (i.e., the MI and sham MI groups). The rats that died perioperative had similar sized infarcts (34 ± 6 % mean ± std, N = 8, p = 0.37) when compared to the survivors. No evidence of myocardial damage was observed in the sham MI group.
VNS Impact on Post-MI Outcomes
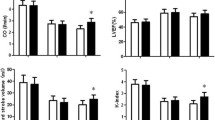
In the MI + VNS arm, there was an 8 % (3/36) mortality for animals from the start of the VNS therapy to the study completion at 14 weeks post MI (Fig. 1). The mortality rate was 6 % (1/17) for the corresponding period in the MI-only group. No sham animals died. There was a significant loss of cardiac function in the rats with MIs relative to the sham controls, with evidence of progressive LV remolding over the 14-week study period (Fig. 2). The average LV end diastolic volumes for the sham and MI groups at 8 ± 1 weeks were 272.2 and 672.4 μl, and at 14 ± 1 weeks, they were 271.2 and 702.7 μl, respectively. Consistent trends for smaller LV volumes suggest that the remodeling response was blunted in the MI + VNS group compared to the MI group. At the end of the VNS stimulation period (8 ± 1 weeks), the MI + VNS group’s average LV end diastolic volume was 519.4 μl (p = 0.03 vs. MI), and at the end of the study (14 ± 1 weeks), it was 586.4 μl (p = 0.06 vs. MI; Fig. 2). This was supported by significantly smaller heart weights when normalized to tibia lengths (Fig. 2d) and to body weights (mean ± std: MI = 3.2 ± 0.6 g*kg−1, MI + VNS = 2.9 ± 0.3 g*kg−1, p = 0.03; sham 2.6 ± 0.1 g*kg−1). Body weights were significantly smaller in the MI + VNS group when compared to the sham and MI groups at baseline, 8, and 14 weeks post MI (Table 1); however, the percent of body weight gain was not significantly different between groups.
Effects of vagal nerve stimulation on heart function and remodeling. (a) End systolic volume (ESV), (b) end diastolic volume (EDV), and (c) ejection fraction (EF) at 1, 8, and 14 weeks after MI for MI + VNS (blue, N = 37,35,32), MI (red, N = 17,17,16), and sham groups (green, N = 4,4,4). (d) Heart weight/tibia length (HW/TbL) at 14 weeks after MI. Error bars indicate ±95 % bounds on mean. p values are calculated only between MI and MI + VNS groups using two sample t tests assuming equal variance. Only values p < 0.1 (†) and p < 0.05 (*) are reported
VNS Impact on Heart Rate
VNS evoked an acute heart rate response that was temporally associated with the stimulation pattern (10 s on 50 s off). The response however was variable, and in the course of minutes to hours, the response to the fixed stimulation pattern appeared to change in magnitude and direction (i.e., the heart rate increased and decreased relative to the mean). In order to better visualize and quantify the HR responses, continuous ECG data was collected from 1 week post MI until the end of the study at 14 weeks. Using the ECG data, R wave peaks were detected, R-R times calculated, and Z-scores were used to transform the individual data points into normalized scores based on the population’s mean and standard deviation. Three-dimensional plots of heart rate changes and Z-scores from representative MI and MI + VNS rats are shown in Fig. 3. From these figures, two types of acute VNS effects are observable. First, there was a lowering of heart rate which lasted for approximately 10 s, and next, there was a transient increase in heart rate.
VNS effects on heart rate. (a–d) Representative heart rate data from a rat receiving VNS (MI + VNS group). VNS was active for 10 s of every minute from day 8 to 51 of the ECG recording. (e–h) Representative heart rate data from a rat not receiving VNS (MI group). (a, e) Visual representation of the percent change in heart rate. Blue color indicates and heart rate that is lower than the mean and red indicates a heart rate above the mean. (b, f) Blue colors indicate a low Z-score with a value below the mean (i.e., heart rate decrease) and red colors indicate a high Z-score with a value above the mean (i.e., heart rate increase). (c, g) Heart rate as a function of time calculated by averaging over the entire 60-s cycle of a trial (green line), averaging during the periods with a low Z-score (blue line) and averaging during periods with a high Z-score (red). (d, h) The magnitude of the acute decreases (blue) and acute increases (red) relative to the average HR over the 60-s cycle of a trial. Note, the lines are layered from top to bottom (red, blue, and green, respectively) and that the bottom lines are obscured by the top lines
VNS-evoked heart rate changes were recognizable in 34 of 35 rats. The magnitude of the acute VNS heart rate lowering effect decreased from an average reduction of 31.5 ± 10.7 bpm after VNS start to a reduction of 13.6 ± 6.3 bpm per trial at the end of the fifth week after the start of VNS (p < 0.001; Fig. 4a). On the other hand, the average magnitude of the acute heart rate increase was smaller but more constant (8.9 ± 2.8 bpm per trial at the start vs. 6.3 ± 2.1 bpm per trial at the end of the fifth week after VNS initiation, p = 0.002; Fig. 4b). When VNS was turned off at the conclusion of the stimulation period, the acute HR effects were immediately lost. The shoulders seen in Fig. 4a, b are due to averaging over VNS ON periods of variable length (43 ± 1.9 days). A discernible heart rate decrease was detected in an average 33.8 ± 7.9 % of the 1-min trials with VNS turned on while a discernible heart rate increase was detected in an average 18.7 ± 2.8 % of the trials with VNS turned on (Fig. 4c). Discernible decreases and increases were detected, but not temporally aligned, in 4.5 to 11 % of the trials in the MI and sham groups (Fig. 4c).
Average VNS Effects. Rolling average magnitude of the intra-trial heart rate decreases (a) and increases (b) for the MI + VNS (blue), MI (red), and sham (green) groups. (c) Percent (mean ± 2SE) of 1-min trials, during the 6-week stimulation period, with discernible HR decreases or increases. Heart rate changes were considered discernible when the 10-s period surrounding the peak decrease or increase had sustained Z-scores that were less than −1 or greater than 1, respectively. (d) Rolling average heart rates. The shaded regions represent the ±95 % confidence bounds of the mean. Note that the circadian rhythms are filtered out
There was no significant difference in the rolling average HR between the MI and sham animals (Fig. 4d). However, the MI + VNS group showed a sharp (52.8 bpm) decrease in the rolling average HR during the first 1.2 days following the start of VNS followed by an increase over 3.9 days. The MI + VNS group’s HR elevation was sustained for the duration of the VNS period (average ΔHR = 13.17 ± 13.51 bpm, p = 0.06 with respect to MI) and then decreased to rates that were similar to the MI and sham groups after discontinuing the VNS. No correlation was found with the MI + VNS group’s rolling average heart rate and the magnitude of the acute changes in HR that were observed when VNS was turned on and off during each trial (rolling average HR and magnitude of decrease r = 0.18, p = 0.29 and rolling average HR and magnitude of decrease r = 0.10, p = 0.54).
Associations Between Acute Heart Rate Response and Chronic Heart Failure Endpoints
Finally, we sought to determine if the degree of anti-remodeling benefit was related to the magnitude of VNS-evoked HR changes. When rats in the MI + VNS group were dichotomized by their degree of pathologic cardiac remodeling (< or >300 μl of end diastolic volume (EDV) increase), significant differences in the magnitude of the evoked responses were seen (Fig. 5). The average VNS-evoked HR changes were greater when the rats remodeled more (25.9 ± 14.6 decrease and 9.8 ± 4.6 bpm increase per trial) and then when the rats remodeled less than 300 μl (11.5 ± 6.9 bpm decrease and 5.5 ± 2.1 bpm increase, mean ± 2*SE). The magnitude of the VNS-evoked HR changes, either an increase or decrease, showed significant positive correlations with the 1 to 14-week change in EDV (Fig. 5c, d). The rolling average heart rates did not differ between those with more or less remodeling (Fig. 5f; r < 0.19, p > 0.29).
Associations between VNS-evoked HR responses and post MI remodeling. Rolling average magnitude of the intra-trial heart rate decreases (a) and increases (b) for the MI rats (red) and MI + VNS rats with more (light blue) or less than (dark blue) a 300-μl change in EDV over 14 weeks. Correlations between the magnitude of the acute VNS-evoked decrease (c) or increase (d) averaged over the 6 weeks of stimulation (y-axis) vs. the change in EDV from 1 to 14 weeks post MI (x-axis), for the MI + VNS (blue), MI (red) and sham (green) rats. MI + VNS group correlations and p values (without correction for the multiple hypotheses) are indicated in the panels. (e) Fraction (mean ± 2SE) of 1-min trials, during the 6-week stimulation period, with discernible HR decreases or increases. Heart rate changes were considered discernible when the 10-s period surrounding the peak decrease or increase had sustained Z-scores that were less than −1 or greater than 1, respectively. (f) Rolling average heart rates. The shaded regions represent the ±95 % confidence bounds of the mean. Note that the circadian rhythms are filtered out
Discussion
The outcomes of this study are consistent with the seminal work of Li et al. who, with a similar experimental design, reduced post-MI remodeling and improved long-term survival with vagal stimulation [7]. Importantly, our disease model was less severe and had less post-MI mortality than the original Li et al. model. This enabled the investigation of VNS-meditated heart rate changes and pathologic cardiac remodeling over a longer period of time. We found that an inhibition of remodeling with VNS was not dependent on a sustained heart rate reduction but was inversely related to the magnitude of the acute VNS-evoked heart rate changes. Future work should be done to determine if VNS-evoked heart rate changes may be used as a heart failure biomarker or as a tool for VNS therapy characterization.
The lack of a mortality benefit in the current study may be due to differences with the experimental model. The post-MI mortality in the current study was lower than that seen in the seminal work of Li et al. [7] Our control group (MI) mortality rate was 6 % (1/17) while the rate reported by Li et al. for the corresponding period was 50 % (15/30) [7]. The mortality rates in the MI + VNS arms were 8 % (3/36) and 15 % (3/22), respectively. Differences in animal vendors, animal husbandry, surgical techniques and/or post-operative care may have led to the mortality differences. The lack of mortality benefit, with VNS, in the current study may have been due to chance associated with the low event rate. Alternatively, the predominate mode of death in the current experimental model may have been different (e.g., pump failure vs. arrhythmic death) and less amendable to VNS treatment [8]. The outcomes with both models are consistent with regard to the impact of VNS on remodeling [7, 9].
A relationship exists between heart rate and outcomes after an MI and for patients with heart failure [10]. Decreased heart rates are also hallmarks of several successful heart failure interventions, such as beta-blockers [10]. Recently, the SHIFT trial demonstrated that Ivabradine, a drug that selectively inhibits the I f ion current in the sinoatrial node and slows heart rate, provides additional benefit to standard of care [11, 12]. Li et al. reported a chronic heart rate reduction of ∼40 bpm with VNS and hypothesized that this improved cardiac efficiency and protected the heart from remodeling [7]. Open-label VNS clinical trials have also shown modest reductions in heart rates and significant improvements from baseline in heart failure status, quality of life, walk distance, and cardiac volumes and function [4, 13]. The randomized controlled neurocardiac therapy for heart failure (NECTAR-HF) did not demonstrate a HR reduction or improvement in remodeling with VNS [2]. Results from pre-clinical experiments, however, suggest that the benefit of VNS is not completely dependent on a heart rate lowering effect. In a pacing induced heart failure model, VNS attenuated LV remodeling despite a constant heart rate that was maintained with ventricular pacing [14]. In addition, Hamann et al. showed that VNS, in a microembolization canine model of heart failure, prevents remodeling in the absence of a VNS-evoked heart rate reduction [5]. The inhibition of pathologic cardiac remodeling by VNS without a sustained heart rate reduction in our current study also suggests a bradycardia-independent benefit. For example, VNS may provide independent benefit in ischemic heart failure by modulating inflammation [15, 16]. It however cannot be ruled out that alternative VNS stimulation parameters that induce sustained heart rate reductions may provide added benefit. Additional research is required to find and test these alternative settings.
Importantly, the application of VNS in the current study produced acute heart rate responses that were temporally consistent with the 10 s ON and 50 s OFF duty cycle. For example, a pattern of decreased heart rate followed by an increased heart rate (presumably reflex mediated) emerged when examining the VNS-evoked responses (Fig. 3). Interestingly, larger heart rate decreases and increases in response to VNS stimulation were associated with less inhibition of adverse cardiac remodeling. This acute HR response may be a surrogate for the intensity of stimulation and reflective of different fiber type recruitment. The vagus nerve is composed of efferent and afferent A, B, and C fibers that are differentially recruited as stimulation amplitude is increased [17]. A-fibers are the largest, carry sensory and motor input, and are the first to be recruited; these are followed by smaller B-fibers which carry sensory input and cardiac efferents; and eventually unmyelinated sensory C-fibers are recruited. Activation thresholds for individual nerve fibers in the canine vagus suggest that the bradycardia threshold coincides with the slow B-fiber threshold (Yoo, P, et al., Manuscript Submitted. Aug 26, 2015). Thus, it is possible that the increased acute HR responses to VNS are reflective of an increased fiber recruitment pattern and that the additional fiber recruitment is also associated with an antagonizing response. The inverse relationship between the magnitude of acute response and remodeling prevention could be further extended to suggest that a VNS dose that does not evoke any acute HR change may produce an even better outcome.
The inverse relationship between the magnitude of acute response and remodeling prevention may also be an example of accentuated antagonism. Early in vivo studies suggest that the sympathetic and parasympathetic autonomic systems can both inhibit and enhance the activity of the other via effects on the release of neurotransmitters at the synaptic junctions [18]. Thus, vagal effects can become progressively stronger with increasing sympathetic background activity. Levy coined the term “accentuated antagonism” to describe this interaction [18]. Although sympathetic tone was not measured and the rolling average HR was not elevated in the animals with the largest magnitude of response, it may still be possible that a provoked HR response is a more sensitive and robust marker of sympathetic tone than average heart rate. If this is true, then quantification of the VNS-evoked heart rate response may be used as a biomarker of sympathetic tone and hence as a tool to monitor heart failure status, independent of any VNS therapy.
The dose of VNS received by patients in the NECTAR-HF clinical trial was limited by side effects (e.g., voice alteration, neck pain, coughing) and no remodeling benefit was seen [2]. Interestingly an analysis of the NECTAR-HF clinical trial Holter data suggests that only a small minority of patients exhibited an acute HR change in response to VNS (unpublished data). This suggests that the nerve fibers captured in this pre-clinical study are different from those captured in the clinical study. Although the fiber type responsible for a blunting of remodeling and/or heart failure benefit is unknown, it may be possible to monitor the motor fiber responses (e.g., voice alteration, neck pain, coughing) and B-fiber responses (e.g., using acute VNS-evoked heart changes) to better define a therapeutic window of stimulation.
Limitations
Eight rats randomized to MI + VNS therapy died either during or shortly after device implantation while no rats randomized to MI-only group died during the ECG telemetery device implantation. This mortality difference may be due to the longer procedure times required for the implantation of the telemetry device and VNS system versus the telemetry device alone. The mortality differences may have introduced a survivor bias to the study. These results must also be translated with caution because pharmacologic interventions could modify the response to VNS. For example, although VNS was tested in the presence of beta-blockers in canine heart failure studies, the full impact of beta-blocker modification on any reflex sympathetic responses is unknown [19]. The combination of VNS and heart-rate-modifying drugs, such as Ivabradine, are also untested.
The NECTAR-HF clinical study applied the same frequency (20 Hz) and duty cycle (10 s ON/50 s OFF) of stimulation as used in this study with heart failure rats but failed to demonstrate a reduction in cardiac remodeling when compared to sham-treated patients [2]. Some notable differences between animal models, including the post-MI rat model presented here, and the patients with heart failure are the duration and context of the heart failure (e.g., acute vs. chronic, otherwise healthy vs. many co-morbidities, no/few co-therapies vs. complex pharmacology and/or CRT, ischemic vs. non-ischemic), possible species specificity (e.g., vagus nerve size and fiber composition), and device differences (e.g., button electrodes vs. helical cuff). The dose of VNS received by patients in the NECTAR-HF clinical trial may also have been limited by side effects that were not appreciated in the animal models [20].
The observed difference in body weight at 1 week post MI is most likely due to chance (randomization); however, the differences seen at subsequent time points may be related to VNS side effects. Numerous studies have suggested an impact of VNS on obesity, and Krolczyl et al. in particular, found weight loss during cervical vagus nerve stimulation in rats [21, 22]. Indexing the heart weight to body weight and tibia length was pre-specified in the study protocol in order to control for body size differences.
The acute and chronic responses to VNS changed over time. The magnitude of the VNS-evoked HR decrease during each ON period decreased over the 6-week stimulation period, and the rolling average HR had a transient decrease that only lasted for approximately 1 day after initiating the VNS therapy. At present, we hypothesize that, the bradycardic effects of VNS change because of neural adaptation or desensitization. The bradycardic response to vagal stimulation is mediated via muscarinic cardiac receptors that are sensitive to acetylcholine. When the vagus nerve is electrically stimulated, acetylcholine is released and then binds to the muscarinic receptors, which leads to the activation of ion channels that slow sinus conduction. With repeated exposure to the same stimulus, however, these muscarinic receptors can translocate to the interior of the cell, resulting in a downregulation of receptor density. This would in turn lead to a loss or attenuation of the bradycardic effect [23]. There is also the possibility that the loss of heart rate response was due to nerve damage or fibrosis. However, previous histological analysis of nerves stimulated with the same cuff used in the present study showed no damage (unpublished data).
Conclusion
This study investigated the association between VNS-mediated heart rate changes and remodeling and found that while VNS blunted the adverse remodeling process, the amount of benefit was independent of chronic heart rate changes but was inversely related to the magnitude of acute heart rate changes evoked by VNS. Further research is required to determine if this acute heart rate response to VNS activation is useful as a heart failure biomarker or as a tool to optimize VNS therapy delivery.
References
De Ferrari, G. M. (2014). Vagal stimulation in heart failure. Journal of Cardiovascular Translational Research, 7, 310–320.
Zannad, F., De Ferrari, G. M., Tuinenburg, A. E., Wright, D., Brugada, J., Butter, C., Klein, H., Stolen, C., Meyer, S., Stein, K. M., Ramuzat, A., Schubert, B., Daum, D., Neuzil, P., Botman, C., Castel, M. A., D'Onofrio, A., Solomon, S. D., Wold, N., & Ruble, S. B. (2015). Chronic vagal stimulation for the treatment of low ejection fraction heart failure: results of the neural cardiac therapy for heart failure (nectar-hf) randomized controlled trial. European Heart Journal, 36, 425–433.
Hauptman, P. J., Schwartz, P. J., Gold, M. R., Borggrefe, M., Van Veldhuisen, D. J., Starling, R. C., & Mann, D. L. (2012). Rationale and study design of the increase of vagal tone in heart failure study: INOVATE-HF. American Heart Journal, 163, 954–962. e951.
Premchand, R. K., Sharma, K., Mittal, S., Monteiro, R., Dixit, S., Libbus, I., DiCarlo, L. A., Ardell, J. L., Rector, T. S., Amurthur, B., KenKnight, B. H., & Anand, I. S. (2014). Autonomic regulation therapy via left or right cervical vagus nerve stimulation in patients with chronic heart failure: results of the ANTHEM-HF trial. Journal of Cardiac Failure, 20, 808–816.
Hamann, J. J., Ruble, S. B., Stolen, C., Wang, M., Gupta, R. C., Rastogi, S., & Sabbah, H. N. (2013). Vagus nerve stimulation improves left ventricular function in a canine model of chronic heart failure. European Journal of Heart Failure, 15, 1319–1326.
Sabbah, H. N., Ilsar, I., Zaretsky, A., Rastogi, S., Wang, M., & Gupta, R. C. (2011). Vagus nerve stimulation in experimental heart failure. Heart Failure Reviews, 16, 171–178.
Li, M., Zheng, C., Sato, T., Kawada, T., Sugimachi, M., & Sunagawa, K. (2004). Vagal nerve stimulation markedly improves long-term survival after chronic heart failure in rats. Circulation, 109, 120–124.
Zheng, C., Li, M., Inagaki, M., Kawada, T., Sunagawa, K., & Sugimachi, M. (2005). Vagal stimulation markedly suppresses arrhythmias in conscious rats with chronic heart failure after myocardial infarction. Conf Proc IEEE Eng Med Biol Soc, 7, 7072–7075.
Li, M., Zheng, C., Inagaki, M., Kawada, T., Sunagawa, K., & Sugimachi, M. (2005). Chronic vagal stimulation decreased vasopressin secretion and sodium ingestion in heart failure rats after myocardial infarction. Conf Proc IEEE Eng Med Biol Soc., 4, 3962–3965.
Fox, K., Borer, J. S., Camm, A. J., Danchin, N., Ferrari, R., Lopez Sendon, J. L., Steg, P. G., Tardif, J. C., Tavazzi, L., & Tendera, M. (2007). Resting heart rate in cardiovascular disease. Journal of the American College of Cardiology, 50, 823–830.
Bohm, M., Swedberg, K., Komajda, M., Borer, J. S., Ford, I., Dubost-Brama, A., Lerebours, G., & Tavazzi, L. (2010). Heart rate as a risk factor in chronic heart failure (shift): the association between heart rate and outcomes in a randomised placebo-controlled trial. Lancet, 376, 886–894.
Bohm, M., Borer, J. S., Camm, J., Ford, I., Lloyd, S. M., Komajda, M., Tavazzi, L., Talajic, M., Lainscak, M., Reil, J. C., Ukena, C., & Swedberg, K. (2015). Twenty-four-hour heart rate lowering with ivabradine in chronic heart failure: insights from the SHIFT Holter substudy. European Journal of Heart Failure, 17, 518–526.
De Ferrari, G. M., Crijns, H. J., Borggrefe, M., Milasinovic, G., Smid, J., Zabel, M., Gavazzi, A., Sanzo, A., Dennert, R., Kuschyk, J., Raspopovic, S., Klein, H., Swedberg, K., & Schwartz, P. J. (2011). Chronic vagus nerve stimulation: a new and promising therapeutic approach for chronic heart failure. European Heart Journal, 32, 847–855.
Zhang, Y., Popovic, Z. B., Bibevski, S., Fakhry, I., Sica, D. A., Van Wagoner, D. R., & Mazgalev, T. N. (2009). Chronic vagus nerve stimulation improves autonomic control and attenuates systemic inflammation and heart failure progression in a canine high-rate pacing model. Circulation. Heart Failure, 2, 692–699.
Olshansky, B. (2015). Vagus nerve modulation of inflammation: cardiovascular implications. Trends in Cardiovascular Medicine.
Andersson, U., & Tracey, K. J. (2012). Neural reflexes in inflammation and immunity. Journal of Experimental Medicine, 209, 1057–1068.
Woodbury, D. M., & Woodbury, J. W. (1990). Effects of vagal stimulation on experimentally induced seizures in rats. Epilepsia, 31(Suppl 2), S7–S19.
Levy, M. N. (1971). Sympathetic-parasympathetic interactions in the heart. Circulation Research, 29, 437–445.
Sabbah, H. N., Imai, M., Zaretsky, A., Rastogi, S., Wang, M., Jiang, A., & Zaca, V. (2007). 509 therapy with vagus nerve electrical stimulation combined with beta-blockade improves left ventricular systolic function in dogs with heart failure beyond that seen with beta-blockade alone. European Journal of Heart Failure Supplements, 6, 114–114.
Barbato, E., Barton, P. J., Bartunek, J., Huber, S., Ibanez, B., Judge, D. P., Lara-Pezzi, E., Stolen, C. M., Taylor, A., & Hall, J. L. (2015). Review and updates in regenerative and personalized medicine, preclinical animal models, and clinical care in cardiovascular medicine. Journal of Cardiovascular Translational Research.
Krolczyk, G., Zurowski, D., Sobocki, J., Slowiaczek, M. P., Laskiewicz, J., Matyja, A., Zaraska, K., Zaraska, W., & Thor, P. J. (2001). Effects of continuous microchip (mc) vagal neuromodulation on gastrointestinal function in rats. Journal of Physiology and Pharmacology, 52, 705–715.
Val-Laillet, D., Aarts, E., Weber, B., Ferrari, M., Quaresima, V., Stoeckel, L. E., Alonso-Alonso, M., Audette, M., Malbert, C. H., & Stice, E. (2015). Neuroimaging and neuromodulation approaches to study eating behavior and prevent and treat eating disorders and obesity. Neuroimage Clin., 8, 1–31.
Shui, Z., Yamanushi, T. T., & Boyett, M. R. (2001). Evidence of involvement of GIRK1/GIRK4 in long-term desensitization of cardiac muscarinic K+ channels. American Journal of Physiology - Heart and Circulatory Physiology, 280, H2554–H2562.
Acknowledgments
We would like to recognize the staff of the Boston Scientific Research and Technology Center and in particular Jason Kilvington, Dennis Werner, Vijay Koya, Amy Townsend, and Holly Coleman for performing the surgeries and echocardiography procedures. We would like to acknowledge Dean Anderlie, Joshua Haarer, Dave Olson, Pete Kelley, and Jason Hamann for designing and manufacturing rodent VNS device. Finally, we would like to recognize Nicholas Wold, Jason Hamann, Stephen Hahn, Scott Meyer, and Juan Hincapie for their critical review of the study protocol and manuscript draft.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All animal experiments were performed in accordance to the National Research Council Guide for the Care and Use of Laboratory Animals and approved by the Boston Scientific Institutional Animal Care and Use Committee.
Funding
This study was funded by Boston Scientific.
Conflict of Interest
Drs. Stolen and Ruble are employees of Boston Scientific, and Drs. Agarwal and Mokelke are former employees of Boston Scientific.
Human Subjects
No human studies were carried out by the authors for this article.
Additional information
Editor-in-Chief Jennifer L. Hall oversaw the review of this article
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 21 kb)
Rights and permissions
About this article
Cite this article
Agarwal, R., Mokelke, E., Ruble, S.B. et al. Vagal Nerve Stimulation Evoked Heart Rate Changes and Protection from Cardiac Remodeling. J. of Cardiovasc. Trans. Res. 9, 67–76 (2016). https://doi.org/10.1007/s12265-015-9668-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-015-9668-7